Abstract
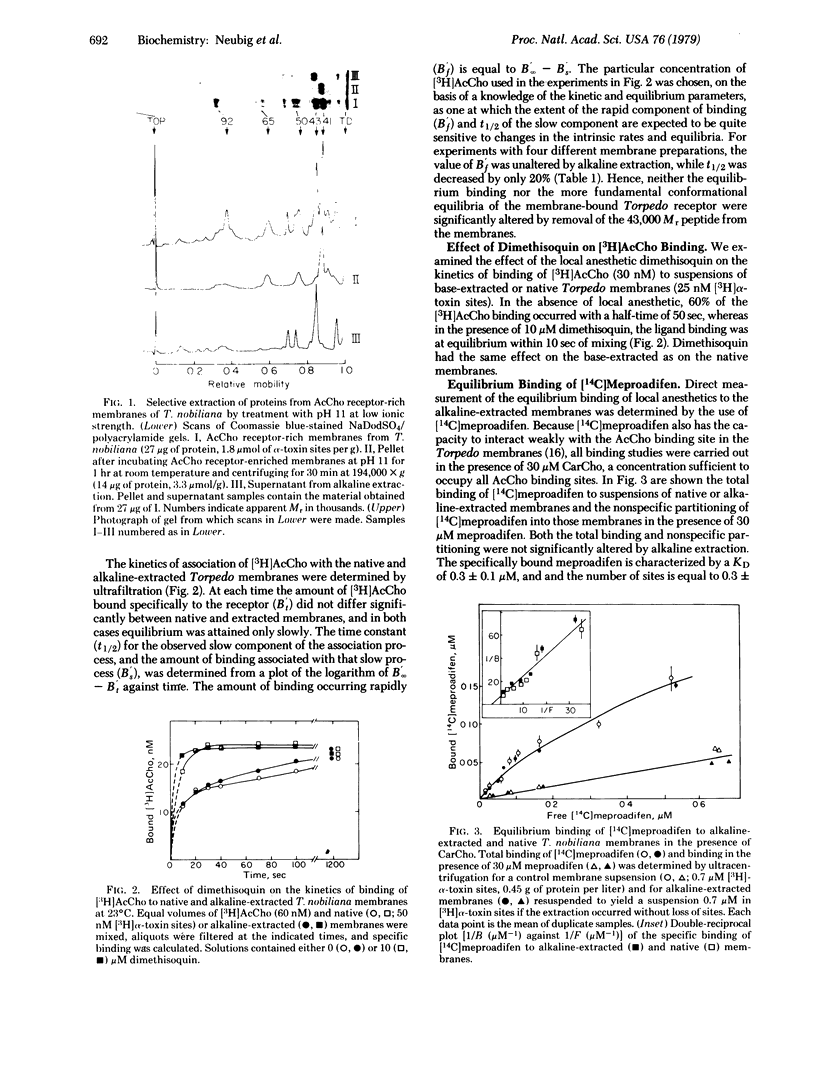
After alkaline extraction, purified subsynaptic fragments isolated from Torpedo electric tissue exhibit on sodium dodecyl sulfate/polyacrylamide gel electrophoresis predominant peptides of apparent Mr 41,000, 50,000, and 65,000 (i.e., the peptides characteristic of the nicotinic receptor purified and isolated in detergent solutions). The peptide of Mr 43,000 that is also found in the isolated postsynaptic membranes is recovered in the supernatant after alkaline extraction. The alkaline-extracted membranes were functionally intact, as demonstrated by the following criteria. The kinetics of binding of [3H]acetylcholine in the presence and absence of 30 micron carbamoylcholine to occupy acetylcholine binding sites, [14C]-meproadifen [2-(diethylmethylaminoethyl)-2,2-diphenylvalerate iodide ] was bound with a dissociation constant, KD, of 0.3 +/- 0.1 micron to 0.3 +/- 0.1 site per [3H]alpha-toxin site. This binding was displaced by perhydrohistrionicotoxin. The carbamoylcholine-stimulated efflux of 22Na+ from the Torpedo vesicles were preserved after alkaline extraction. It is concluded that not only the acetylcholine binding site, but also the local anesthetic binding site, must be associated with the peptides of the cholinergic receptor itself and not that of Mr 43,000. Those peptides remaining after alkaline extraction are also sufficient for permeability control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Karle I., Myers C. W., Tokuyama T., Waters J. A., Witkop B. Histrionicotoxins: roentgen-ray analysis of the novel allenic and acetylenie spiroalkaloids isolated from a Colombian frog, Dendrobates histrionicus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1870–1875. doi: 10.1073/pnas.68.8.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi A. T., Eldefrawi M. E., Albuquerque E. X., Oliveira A. C., Mansour N., Adler M., Daly J. W., Brown G. B., Burgermeister W., Witkop B. Perhydrohistrionicotoxin: a potential ligand for the ion conductance modulator of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1977 May;74(5):2172–2176. doi: 10.1073/pnas.74.5.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot J., Raftery M. A. Interactions of perhydrohistrionicotoxin with postsynaptic membranes. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1347–1353. doi: 10.1016/s0006-291x(77)80127-7. [DOI] [PubMed] [Google Scholar]
- Gasko O. D., Knowles A. F., Shertzer H. G., Suolinna E. M., Racker E. The use of ion-exchange resins for studying ion transport in biological systems. Anal Biochem. 1976 May 7;72:57–65. doi: 10.1016/0003-2697(76)90506-6. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Disulfide bond cross-linked dimer in acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1977 Dec 7;79(3):692–699. doi: 10.1016/0006-291x(77)91167-6. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Iwatsubo M., Changeux J. P. Etude par une méthode de mélange rapide de l'interaction d'un agoniste cholinergique fluorescent avec des fragments de membrane riches en récepteur cholinergique de Torpedo marmorata. C R Acad Sci Hebd Seances Acad Sci D. 1977 Feb 28;284(9):771–774. [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E., Goombs S. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane preparations. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4371–4375. doi: 10.1073/pnas.72.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G., Changeux J. P. Studies on the effect of histrionicotoxin on the monocellular electroplax from Electrophorus electricus and on the binding of (3H)acetylcholine to membrane fragments from Torpedo marmorata. Mol Pharmacol. 1976 Jan;12(1):92–100. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U., Schimerlik M., Lee T., Witzemann T. L., Blanchard S., Raftery M. A. Ligand-induced conformation changes in Torpedo californica membrane-bound acetylcholine receptor. Biochemistry. 1978 Jun 13;17(12):2405–2414. doi: 10.1021/bi00605a024. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F., Czech M. P. Partial purification of the D-glucose transport system in rat adipocyte plasma membranes. J Biol Chem. 1977 Sep 25;252(18):6554–6561. [PubMed] [Google Scholar]
- Sobel A., Heidmann T., Hofler J., Changeux J. P. Distinct protein components from Torpedo marmorata membranes carry the acetylcholine receptor site and the binding site for local anesthetics and histrionicotoxin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):510–514. doi: 10.1073/pnas.75.1.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Popot J. L., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. III. Pharmocological desensitization in vitro of the receptor-rich membrane fragments by cholinergic agonists. J Mol Biol. 1976 Sep 25;106(3):485–496. doi: 10.1016/0022-2836(76)90248-5. [DOI] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J Biol Chem. 1977 Nov 10;252(21):7648–7656. [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Raftery M. A. Selective photoaffinity labeling of acetylcholine receptor using a cholinergic analogue. Biochemistry. 1977 Dec 27;16(26):5862–5868. doi: 10.1021/bi00645a034. [DOI] [PubMed] [Google Scholar]



