Abstract
Introduction:
Oral Lichen planus (OLP) is chronic, autoimmune, mucocutaneous disease. Numerous etiological factors have been proposed, but an authoritative and exact source of the disease has not been brought forward. Reactive nitrogen species (RNS), mast cell (MC) and stress are considered to play a key role in inflammation-mediated carcinogenesis generating nitric oxide (NO).
Aim:
To evaluate the salivary NO levels, mast cells and stress levels and to correlate them in pathogenesis of OLP.
Materials and Methods:
The study was conducted using saliva samples of patients. The study consisted of two groups: Group-I constituted the subjects with OLP group (n=25) and group II comprised the control group (n=25). The saliva of the patients was evaluated using Griess Reagent and Spectrophotometer, MC count done by using special stains, and stress levels measured using DASS Scale.
Results:
The difference between the means was found to be highly significant (P < 0.05). The intergroup comparison of optical density (OD) values, a mast cell count and stress level was found to be highly significant.
Conclusion:
Salivary NO, increase in mast cell count and stress has a definitive role in OLP pathogenesis.
Keywords: Depression anxiety stress scale, mast cells, oral lichen planus, salivary nitric oxide
INTRODUCTION
Oral lichen planus (OLP) is a common, chronic, autoimmune, mucocutaneous disease affecting the skin, genital mucosa, scalp, nails, and areas in the oral cavity. OLP was first described by British Physician Erasmus Wilson (1869).[1,2,3] Early diagnosis is important as at times a delay can lead to malignant transformation of OLP. The association of chronic inflammation with cancer has been addressed.[4] Reactive nitrogen species (RNS) are considered to play a key role in inflammation-mediated carcinogenesis generating nitric oxide (NO). NO mediate the formation of 8-nitroguanine, a member of nitrative deoxyribonucleic acid (DNA) damage.[5] NO is a noxious chemical in the atmosphere, but in small controlled concentrations it acts as a physiological (vasodilatation) and pathophysiological (chronic inflammation) mediator.[6,7] It is a small (30 Da) uncharged molecule with an unpaired electron which defines it as a radical[8] formed in mammals by the oxidation of L-arginine, an amino acid,[6] through the action of isoenzymes, globally named nitric oxide synthases (NOS) expressed in some tissues as: Brain and neuronal NOS (bNOS) and inducible NOS (iNOS).[7] Conversely, iNOS is identified in several cell types such as macrophages and polymorphonuclear cells (PMNL).[9] Overproduction of NO leads to generation of various RNS such as NOx (refers to NO and NO2) and peroxynitrite (ONOO-).[10] RNS mediate the formation of 8-nitroguanine, a marker of nitrative damage eventually leading to cell death by damaging effects against cellular proteins (DNA and lipids), tissue injury, and organ failure.[11] The possible role of NO as a mediator in the etiopathogenesis of OLP has been advocated.[8,12,13]
Mast cells (MC) are local resident of connective tissue[14] strategically located at neural, epidermal, mucosal, and vascular sites.[15] They play a critical role in allergy and other inflammatory states through the release of multifunctional mediators.[16,17] Several studies have shown the pathological role of MCs in lesions of OLP.[18] MC degranulation in OLP releases a range of proinflammatory mediators such as tumor necrosis factor (TNF)-α, chymase, and tryptase, which together play a role in epithelial basement membrane disruption in OLP, mediated by MC proteases directly or indirectly via activation of T-cell-secreted matrix metalloproteinase (MMP)-9.[15,18] The interaction of NO and MCs is time dependent, requiring several hours and is non-cyclic guanine monophosphate (cGMP) mediated.[15] NO has been suggested to be among the myriads of MC derived mediators with increased levels at the site of inflammation. The increase in NOS-2 and NOS-3 expressions in response to immunoglobulin (Ig) E-mediated reactions supported reports that binding of monomeric IgE to FcR-1 (Fc receptor-1) could initiate signal transductions in MCs even when degranulation was absent. The increased NOS expression implicates that human MCs are capable of increasing the level of NO in the immediate cellular environment following IgE sensitization.[18,19]
The increased levels of NO have also been associated with stress. NO counteracts norepinephrine (NE) activity and sympathetic responsivity. Thus, NO and the stress pathophysiology are closely connected and molecular mechanisms or pathways may be shared under certain conditions. A rise in the levels of NO leads to increased oxidant generation, decreased antioxidant protection and a failure in producing a oxidative repair thus leading to oxidative stress on cells’ RNS, indirectly correlating to OLP.[20,21,22] On activation, NO produces RNS which then produces 8-nitrosoguanine (a marker of nitrative DNA damage) that can result in increased pathogenetic effects of inflammatory cells. Thus, damaging the genetic material, that is, DNA and also lipids; and eventually leading to cell death, tissue injury, and organ failure.[19] Thus, DNA damage especially 8-nitroguanine formation could possibly intensify the action of the various cells, leading to higher epithelial-subepithelial damage. Deeper penetration and increased number of cells will lead to higher NO levels.[23,24,25] Patients with OLP often relate the onset and aggravation of oral symptoms to increased levels of stress. It is possible that the activity of symptoms of OLP in some patients may involve an impaired capacity to suppress an increased immune activity following a stressful event.[26,27,28]
The purpose of the present study were to: Determine the levels of salivary NO in OLP, count the number of MCs in OLP, measure stress levels in OLP, and further correlate the three factors in an attempt to postulate the pathogenesis of OLP.
MATERIALS AND METHODS
The study was conducted in the Department of Oral and Maxillofacial Pathology and Microbiology, using saliva samples of patients who visited the college outpatient department (OPD) from November 2009 to June 2011. The study consisted of two groups: Group I constituted the subjects with OLP group (n = 25) and group II comprised the control group (n = 25). The consent was obtained from all patients and volunteers for examination. The study was approved by the Ethical Committee.
Criteria for selecting the study group (I)
Age group: 20-45 years
Clinically/histopathologically diagnosed cases of OLP [Figure 1].
Figure 1.
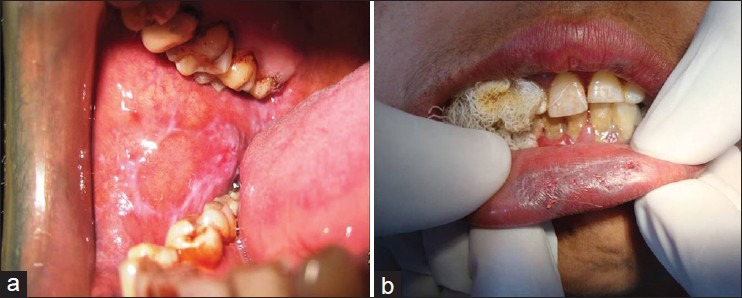
(a) Reticular form of OLP: Showing interlacing- Wickham's striae. (b) Atrophic form of OLP-Linear erosive lesion on the vermilion border of lip. The surrounding mucosa is atrophic and erythematous with very faint reticular striations
The modified World Health Organization (WHO) criterion was used for diagnosis of OLP lesions which includes [Figure 2]: Liquefactive degeneration of basal epithelial cells/basement membrane degeneration, presence of a well-defined band like zone of cellular infiltration that is confined to the superficial part of connective tissue, consisting mainly of lymphocytes, presence of civatte bodies (colloid bodies), and absence of dysplasia.
Figure 2.
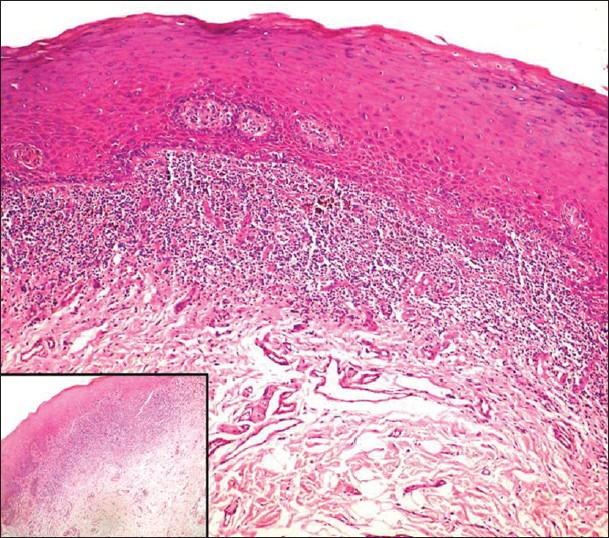
Atrophic lichen planus; showing a dense juxtaepithelial lymphocytic infiltrate (H & E stain, ×100); Inset: Higher magnification (H & E stain ×400)
Criteria for selecting the control group (II)
Age group same as group I subjects (20-45 years), patients undergoing prophylactic removal of tooth: for orthodontic treatment, impacted third molars, free of any inflammation (also excluding gingivitis), and free of any systemic disorders.
The following investigations were done:
Analysis of stress by Depression Anxiety Stress Scale (DASS)
Biochemical analysis of nitric oxide (NO) levels in saliva
Histopathological examination of biopsy specimen.
Analysis of stress by DASS
The DASS[29,30,31,32,33] is a promising 42-item self-report measure of depression, anxiety, and stress (Lovibond and Lovibond, 1995). The DASS is a 42-item self-report inventory that yields three factors: Depression, anxiety, and stress. This measure proposes that physical anxiety (fear symptomatology) and mental stress (nervous tension and nervous energy) factor-out as two distinct domains. This screening and outcome measure reflects the past 7 days. This scale was used to check the levels of stress in individuals with OLP.
Biochemical analysis
Technique for estimation of NO: NO assay protocol
Subjects were asked to rinse the mouth with povidine iodine for 2 min, so as to reduce the bacterial counts. The patients were then made to wait for 1 min, after which freshly secreted unstimulated saliva (1 mL) was collected in a sterile container (Eppendorf tubes). The samples were then diluted with 10 mL of phosphate buffered saline in order to neutralize the pH. After dilution, the mixture was centrifuged for 5 min at 3,000 rpm and the supernatant fluid was stored at −20°C until use/or was used immediately. A standard curve was obtained using known standard solutions of the substance to be determined which were reacted with an appropriate reagent (Griess reagent), so that unknown concentration of the substance to be determined was obtained from the standard curve by a spectrophotometer. The substances to be determined in our study was nitrite and hence known concentrations of sodium nitrite solutions were prepared for evaluation (sodium nitrite concentration of 25-60 μ/L were prepared). These solutions were reacted with Griess reagent (prepared using 1% sulfanilamide, 1% naphthylethylenediamine dihydrochloride (NED), and 2.5% phosphoric acid). Griess reagent is very unstable as it reacts with surface nitrogen. Hence, it was freshly prepared each time for use. 0.5 mL of the prepared standard solutions of sodium nitrite was reacted with equal volumes of Griess reagent in Eppendrof tubes. The prepared solution was incubated for 10 min at room temperature to ensure that complete reaction takes place. The reacted mixture was then transferred onto plastic cuvettes for measurement in the spectrophotometer and the optical densities (ODs) value was recorded. Using these readings taken for the standard solutions, a graph of absorbance versus concentration was plotted, which constituted the standard curve. Then, the calculated ODs were correlated to the standard curve and the corresponding concentration of nitrite was observed. NO was expressed as μmol/L.
Tissue preparation
Biopsies were taken under local anesthesia from the appropriate site. The tissues were allowed to be fixed in 10% formalin. The tissues were then processed in automatic processor and blocks were prepared. Sections of 4 μm thickness were prepared using Leica microtome. Two staining techniques were employed:
Hematoxylin and eosin (H and E) staining [Figure 2]
Toluidine blue staining method (for MCs) [Figure 3].
Figure 3.
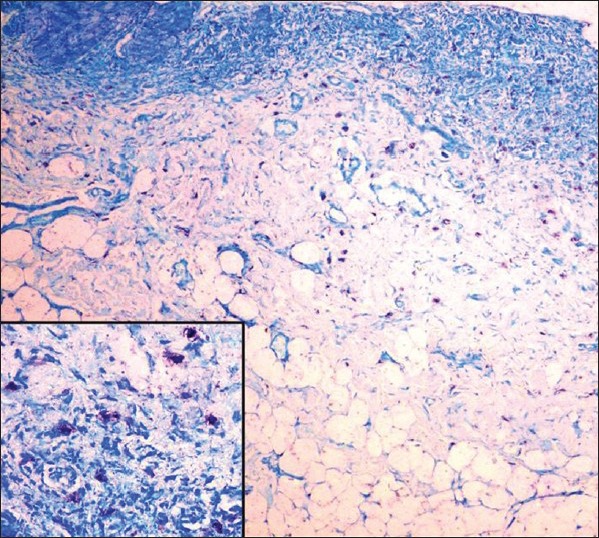
Collection of mast cells near the basement membrane (toluidine blue stain, ×100; inset ×400)
MCs; toluidine blue staining[34,35,36,37]
MCs are spindle to oval shaped and have the same staining characteristics as the fibroblast with H and E staining. Therefore they are difficult to differentiate from fibroblasts. Selective staining with 2% toluidine blue was hence used for MCs. Criteria to identify MCs are seen in results [Figure 4].
Figure 4.
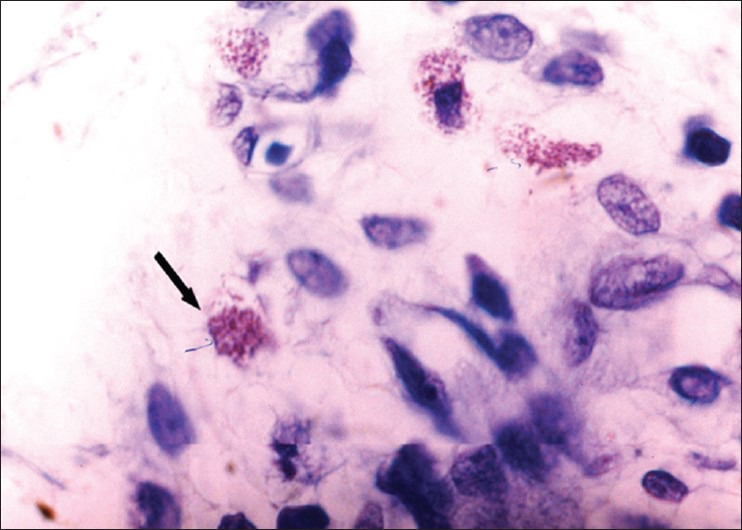
Spreading mast cell; showing mast cell granules. Presence of three or more aggregate granules (toluidine blue stain, ×400)
Results
MCs: Violet/red purple.
Background: Shades of blue.
Counting of MCS (Janardhanan M and Ramesh v)[15]
The total number of mast cells, both intact as well as degranulated MCs, were counted in five high power fields (×400, original magnification) of each section of OLP. Results were expressed as the average number of MCs per high power field. Number of MCs seen in normal oral mucosa = 25.5-30/mm2 (any number >30 was considered as an increase in number).
The data was fed into the computer database and analyzed using the Statistical Package for Social Sciences (SPSS). Mean, standard deviation, and the data was analyzed using Student's paired t-test for comparing the quantitative data between the two groups, the categorical variables were compared using Chi-square test. Besides this, correlation between different factors as age and salivary NO levels was done using Pearson's correlation. These groups were compared by one-way analysis followed by regression analysis.
RESULTS
Twenty-five OLP patients in group I were subgrouped into various clinical forms on the basis of the clinical findings [pie diagram: Figure 5]. The maximum number of patients suffered from reticular type of OLP (n = 12 (48%)). The erosive and the plaque type had 4 cases each (16%), and the atrophic type of lichen planus made up for the remaining 20% of all the cases (n = 5). The mean NO levels in saliva of OLP was observed to be 90.88 ± 12.056 μM/L as compared to mean of 13.84 ± 3.566 μM/L NO levels in healthy controls [Table 1a, Figure 6]. The intergroup comparison of OD values of the two groups was found to be highly significant. Keeping the salivary NO as constant factor, NO was correlated to the increase in age of the patient using Pearson's correlation; however, the results were statistically insignificant. The MC count [Table 1b, Figure 7] in group I came out to be 18.869 ± 0.1237 as compared to mean of 3.637 ± 0.9550 in healthy controls. The difference between the means was found to be highly significant (P < 0.05). The comparison of depression, anxiety, and stress scales between the two groups; group I (OLP) and group II (healthy controls) showed a definitive increase in depression levels between the two groups. Group I with a mean of 12.44 ± 3.709 as compared to mean of 7.20 ± 2.48 in group II, and was statistically significant (P < 0.05); whereas, the anxiety and stress scales of group I and group II were insignificant as well (P > 0.05). [Table 2a,b,c, Figure 8].
Figure 5.
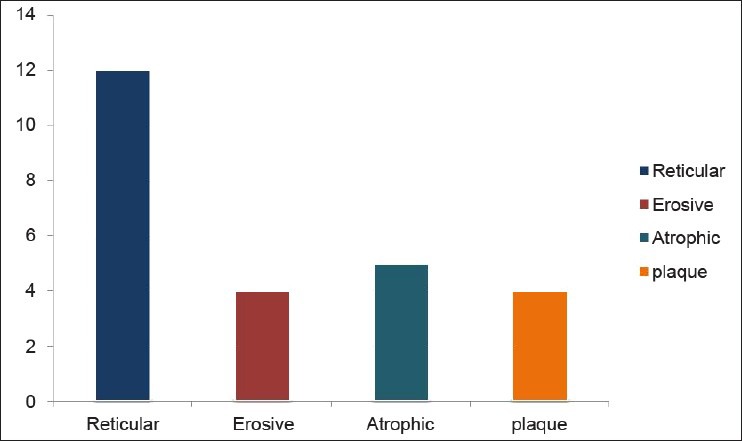
Distribution of various clinical forms of OLP
Table 1a.
Assessment of difference in the mean salivary nitric oxide levels between the two groups
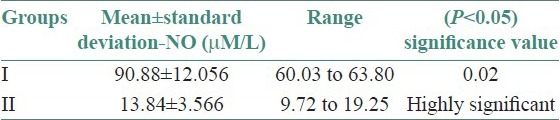
Figure 6.
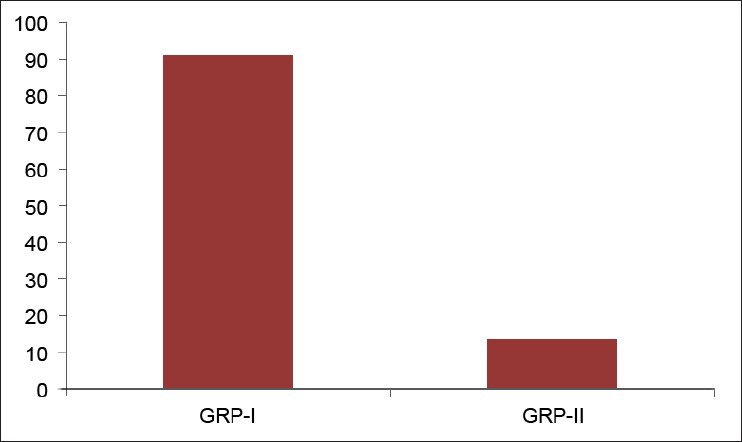
Comparison of mean of salivary NO levels between two groups
Table 1b.
Assessment of the mean mast cell count between the two groups
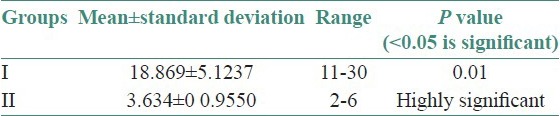
Figure 7.
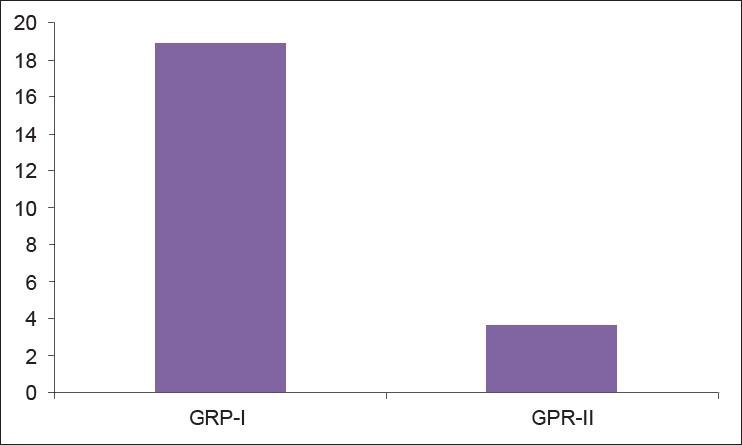
Comparison of mean of mast cells count b/w two groups
Table 2a.
Assessment of mean of depression levels between the two group
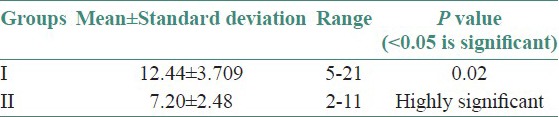
Table 2b.
Assessment of means of anxiety levels between the two groups
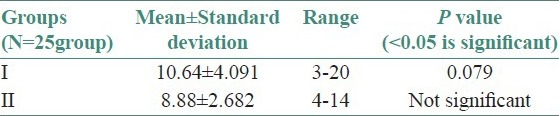
Table 2c.
Assessment of means of stress levels between the two groups
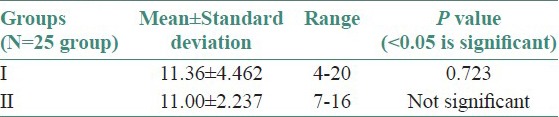
Figure 8.
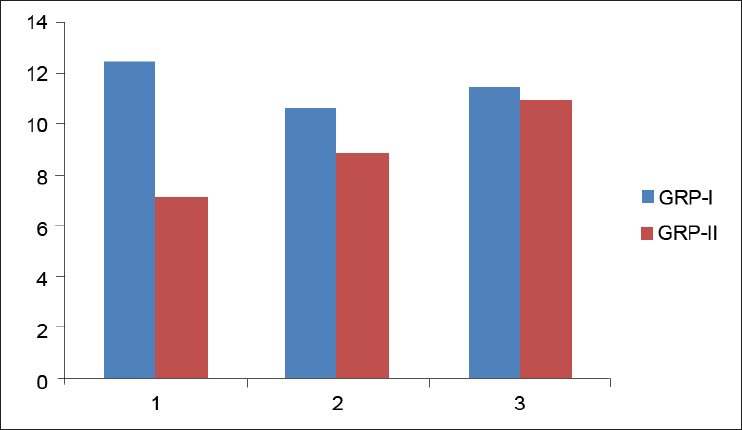
Comparison of means of depression, anxiety and stress b/w Groups I-II
DISCUSSION
Lichen planus has remained an ambiguity from the time of its discovery. OLP is a fairly distinct mucocutaneous disease of oral mucosa presenting as keratotic white reticulate lesions, as well as ulcerative, vesiculobullous, and atrophic-erosive lesions.[1,2,3,4,5,32] On comparison of the salivary NO levels in OLP patients with the control group, in our study we found a significant increase in the levels of salivary NO in OLP patients as compared to the control group (P < 0.05) [Table 1a].[29,30,31,32] We hypothesized that T lymphocytes from OLP tissues produced increased levels of interleukin (IL)-6 and granulocyte macrophage colony stimulating factors (GM-CSF), which further produces more TNF-α, IL-1β, IL-6, and GM-CSF which then leads to increased production of NOS responsible for releasing NO. Increased NOS activity interacts with caspase family of enzymes, thereby promoting apoptosis, which was seen as basal cell degeneration in OLP histopathologically.[32,33,34,35,36,37,38,39,40,41]
Moncad et al., (2008) and Ripetska and Deneha (2008)[10,12] opinioned that increased chronicity (inflammation potential), as well as the release of proinflammatory cytokines (IL-1, TNF-α. etc.) are key activators of NO (iNOS) leading to the production of NO; which in turn mediate DNA damage directly or indirectly through the generation of more persistent RNS. NO when in excess behaves as a key mediator of cell damage, tissue injury, and organ failure. Ohashi et al., (1999)[8] and Brennan et al., (2003)[24] also reported that NO increase caused severe damage to fibroblasts, keratinocytes, and oral epithelial cells in vitro. Hence, the increased salivary NO levels can be attributed in infectious or inflammatory conditions, which leads to the overexpression of iNOS; thus an increased NO could lead to an increased cellular infiltrate seen in different forms of OLP.[7,35] We also carried out a quantitative assessment of MCs in OLP. The mean MC count in OLP patients in the present study was found to be 18.86 cells (mean of five high power fields). This was higher than that of the control group, which showed 3.63 cells (mean of five high power fields). This difference was statistically significant (P < 0.05) [Table 1b]. Increased density of MCs in our study was consistent with that of Jontell et al., (1986);[16] Walsh et al., (1995);[14] and Zhao et al., (2007);[35] and suggested that MC products bring about structural changes in the epithelium and connective tissue in lesions of LP. Secondarily, a close association of MCs with the basement membrane was noted in most of the cases of OLP taken in this study. The lining of MCs along the basement membrane was thought to be a response to external agents or antigenic stimuli, to release histamine. Lodi et al., (2005) have also confirmed that basement membrane disruption in OLP may be mediated by MC proteases directly or indirectly via activation of T-cell secreted MMP-9.[36,37,38]
Stress as a concept describes the effects of psychological and environmental factors on physical and mental well-being.[42] It plays a major role in immunological diseases and immune-related disease processes. Inflammation, infection, autoimmune processes, and perhaps even the onset and development of malignant tumors may occasionally be associated with stress phenomenon.[39,40,41,42,43] Hence, the role of stress in OLP patients was analyzed using DASS. The results showed a significant increase in depression scale (P < 0.05) [Table 2a] in case of OLP patients as compared to the control group. Though the anxiety and stress scales increased in OLP patients as compared to the control group, statistically the values for anxiety and stress levels in OLP patients was not significant (P > 0.05) [Tables 2b and 2c]. Our results were comparable to the findings of Hampf et al., (1987),[37] McCartan[38] studied the psychological factors associated with OLP. Some patients with OLP suffer from depression while some patients have anxiety. Burkhart et al.,[39] have also pointed out that more than half of his patients with OLP related high levels of stress in relation to work, relationship, and losses; before or during the appearance of the condition.
Stress as a pathological factor in OLP can be explained on the hypothesis that glucocorticoids can affect the lymphocyte subsets and induce a shift between Th1/Th2 cytokines; while preferentially inhibiting nonactivated lymphocytes, thus favoring IL-2 expression during clonal expansion. This results in the suppression of cells with little or no affinity for an antigen and favor the clonal expansion of cells with high affinity for antigen.[40,41,42,43] Moreover, major depression has been associated with activation of the inflammatory response as proinflammatory cytokines are potent stimulators of neuroendocrinal response. In particular, IL-6 stimulates production of corticotrophin releasing hormone (CRH) enhancing increased levels of adrenocorticotropic hormone (ACTH) and cortisol levels.[29,30,31]
We further correlated increase in salivary NO with raised number of MCs and DASS. The results showed that, an increase of salivary NO levels, MCs, and DASS individually has a role in etiopathogenesis of OLP; but does not significantly affect the expression or behavior of other factors. Hence, following conclusions were proposed:
Free radical including NO represents one route of pathogenesis and that excess of salivary NO have a pathophysiological implication in OLP. The NO (iNOS) can activate the inflammatory cytokine which has damaging effects against cellular proteins, DNA and lipids eventually leading to cell death, tissue injury and organ failure. Thus, biochemical analysis of patients with OLP can aid to a more improved therapeutic plans, as well as guide in checking the malignant potential of the lesion.[42,43]
Our study points out that the MCs which are not considered as a major component of the cellular infiltrate in OLP has got a definite role in the pathogenesis of OLP.
We emphasize the necessity for additional therapeutic intervention in patients who have stress-induced oral diseases. As an adjunct to conventional therapy for these patients, it may be beneficial to advocate cooperation with psychiatric services to avoid the occurrence of somatization as an aid in possible prevention of disease exacerbations. Additional prospective studies using clinically relevant outcome measures are necessary to further define the impact of psychological stress on immune-based diseases immune dysregulation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 2.Mallaoglu N. Oral lichen planus: A review. Br J Oral Maxillofac Surg. 2000;35:370–7. doi: 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- 3.Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: Etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9:86–122. doi: 10.1177/10454411980090010501. [DOI] [PubMed] [Google Scholar]
- 4.Abbate G, Foscolo AM, Gallotti M, Lancella A, Mingo F. Neoplastic transformation of oral lichen: Case report and review of literature. Acta Otorhinolaryngol Ital. 2006;26:47–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Chaiyarit P, Ma N, Hiraku Y, Pinlaor S, Yongvanti P, Jintakanon D, et al. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Sci. 2005;96:553–9. doi: 10.1111/j.1349-7006.2005.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendall HK, Marshall RI, Bartold PM. Nitric oxide and tissue destruction. Oral Dis. 2001;7:2–10. [PubMed] [Google Scholar]
- 7.Batista AC, Silva TA, Chun JH, Lara VS. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002;8:254–60. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi M, Iwase M, Nagumo M. Elevated production of salivary nitric oxide in oral mucosal diseases. J Oral Pathol Med. 1999;28:355–9. doi: 10.1111/j.1600-0714.1999.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 9.Niu XF, Smith CW, Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res. 1994;74:1133–40. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- 10.Moncad S, Palmer RM, Higgis EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–34. [PubMed] [Google Scholar]
- 11.Sunitha M, Shanmugam S. Evaluation of salivary nitric oxide levels in oral mucosal diseases: A controlled clinical trial. Indian J Dent Res. 2006;17:117–20. doi: 10.4103/0970-9290.29878. [DOI] [PubMed] [Google Scholar]
- 12.Ripetska O, Deneha I. Concentration of Nitrite-anion in saliva of patients at different stages of periodontal diseases. Department of Therapeutic Dentistry. 2008;21:295–8. [Google Scholar]
- 13.Bodies S, Haregewoin A. Evidence for the release and possible neural regulation of nitric oxide in human saliva. Biochem Biophys Res Commun. 1993;194:347–50. doi: 10.1006/bbrc.1993.1826. [DOI] [PubMed] [Google Scholar]
- 14.Walsh LJ, David MF, Xu LJ. Relationship between mast cell degranulation and inflammation in the oral cavity. J Oral Pathol Med. 1995;24:266–72. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 15.Janardhanan M, Ramesh V. Mast cells in oral lichen planus. J Oral Maxillofac Pathol. 2010:1–8. [Google Scholar]
- 16.Jontell M, Hansoon HA, Nygren H. Mast cells in oral lichen planus. J Oral Pathol. 1986;15:273–5. doi: 10.1111/j.1600-0714.1986.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 17.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus--a review. J Oral Pathol Med. 2010;39:729–34. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 18.Crowell JA, Steele VE, Sigman CC, Fay JR. Is inducible nitric oxide synthase a target for chemoprevention? Mol Cancer Ther. 2003;2:815–23. [PubMed] [Google Scholar]
- 19.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist M, Savole M, Nohara O, Wills FL, Wallace JL, Befus AD. Nirtic oxide synthase and nitric oxide production in vivo-derived mast cells. J Leukoc Biol. 2002;71:618–24. [PubMed] [Google Scholar]
- 21.Esch T, Stefeno GB, Fricchione GL, Benson H. Stress-related diseases: A potential role for nitric oxide. Med Sci Monit. 2002;8:RA103–18. [PubMed] [Google Scholar]
- 22.Young JS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;34:176–80. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beevi SS, Raheed AM, Geetha A. Evaluation of oxidative stress and Nitric oxide levels in patients with oral cavity cancer. Jpn J Clin Oncol. 2004;34:379–85. doi: 10.1093/jjco/hyh058. [DOI] [PubMed] [Google Scholar]
- 24.Brennan PA, Umar T, Palacios-Callender M, Speeding AV, Mellor TK, Buckley J, et al. A study to assess inducible nitric oxide synthase expression in oral lichen planus. J Oral Pathol Med. 2000;29:249–54. doi: 10.1034/j.1600-0714.2000.290602.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Kumamto H, Ooya K, Motegi K. Expression of inducible nitric oxide synthase and heat shock proteins in periapical inflammatory lesions. J Oral Pathol Med. 2002;31:488–93. doi: 10.1034/j.1600-0714.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 26.Koray M, Dulger O, Ak G, Horasanli S, Ucok A, Tanyeri H, et al. The evaluation of anxiety and salivary cortisol levels in patients with oral lichen planus. Oral Dis. 2003;9:298–301. doi: 10.1034/j.1601-0825.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 27.Aly DG, Shahin RS. Oxidative stress in lichen planus. Acta Dermatoven Alp Panonica Adriat. 2010;19:3–11. [PubMed] [Google Scholar]
- 28.Neuwenhuijsen K, Boer AG, Vebeek JH, Blonk RW, Vandijk FJ. The Depression Anxiety Stress Scales (DASS): Detecting anxiety disorders and depression in employees absent from work because of mental health problems. Occup Environ Med. 2003;60:77–82. doi: 10.1136/oem.60.suppl_1.i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah B, Ashok L, Sujatha GP. Evaluation of salivary cortisol and psychological factors in patients with oral lichen planus. Indian J Dent Res. 2009;20:288–92. doi: 10.4103/0970-9290.57361. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal SK, Marshall GD., Jr Stress effects on immunity and its application to clinical immunology. Clin Exp Allergy. 2001;31:25–31. [PubMed] [Google Scholar]
- 31.Rad M, Hashemipoor MA, Mojtahedi A, Zarei MR, Chamani G, Kakoei S, et al. Correlation between clinical and histopathological diagnoses of oral lichen planus based on modified WHO diagnostic criteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:796–800. doi: 10.1016/j.tripleo.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Shklar G, Flynn E, Szabo G. Basement membrane alterations in oral lichen planus. J Invest Dermatol. 1978;70:45–50. doi: 10.1111/1523-1747.ep12543477. [DOI] [PubMed] [Google Scholar]
- 33.Simark-Mattssona C, Bergenholtza G, Jontell M, Eklunda C, Seymourb GJ, Sugermanb PB, et al. Distribution of interleukin-2, -4, -10, tumour necrosis factor-α and transforming growth factor-b mRNAs in oral lichen planus. Arch Oral Biol. 1999;44:499–507. doi: 10.1016/s0003-9969(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 34.Madhuri AR, Kale AD, Nayak R. Mast cells are increased in leukoplakia, oral Submucous fibrosis, oral lichen planus and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2007;11:18–22. [Google Scholar]
- 35.Zhao ZZ, Sugerman NB, Zhou XJ, Walsh IJ, Savage NW. Mast cells degranulation and the role of T-cell RANTES in oral lichen planus. Oral Dis. 2001;7:246–51. [PubMed] [Google Scholar]
- 36.Hall WB. Mast cells in desquamative gingivitis, lichen planus and pemphigoid. Oral Surg Oral Med Oral Pathol. 1969;28:646–59. doi: 10.1016/0030-4220(69)90409-5. [DOI] [PubMed] [Google Scholar]
- 37.Hampf BG, Malmstrom MJ, Aalberg VA, Hannula JA, Vikkula J. Psychiatric disturbance in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol. 1987;63:429–2. doi: 10.1016/0030-4220(87)90254-4. [DOI] [PubMed] [Google Scholar]
- 38.McCartan BE. Psychological factors associated with oral lichen planus. J Oral Pathol Med. 1995;24:273–5. doi: 10.1111/j.1600-0714.1995.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 39.Burkhart NW, Burker EJ, Burker EJ, Wolfe L. Assessing the characteristics of patients with oral lichen planus. J Am Dent Assoc. 1996;127:642. doi: 10.14219/jada.archive.1996.0277. 8. [DOI] [PubMed] [Google Scholar]
- 40.Rojo-Monoro JL, Bagan JV, Rojo-Monoro J, Donat JS, Millian MA, Jimenez Y. Psychological factors and oral lichen planus. A psychometric evaluation of 100 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:687–91. doi: 10.1016/s1079-2104(98)90205-0. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhary S. Psychological stressors in oral lichen planus. Aust Dent J. 2004;49:192–5. doi: 10.1111/j.1834-7819.2004.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 42.Ivanovski K, Nakova M, Warburton G, Pesevska S, Filipovska A, Nares S, et al. Psychological profile in oral lichen planus. J Clin Periodontol. 2005;32:1034–40. doi: 10.1111/j.1600-051X.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 43.Soto Araya M, Rojas Alcayaga G, Esguep A. Association between psychological disorders and the presence of oral lichen planus, burning mouth syndrome and recurrent aphthous stomatitis. Med Oral Patol Oral Cir Bucal. 2004;9:1–7. [PubMed] [Google Scholar]


