Abstract
The chemical stress factors for microbial life at deep-sea hydrothermal vents include high concentrations of heavy metals and sulfide. Three hyperthermophilic vent archaea, the sulfur-reducing heterotrophs Thermococcus fumicolans and Pyrococcus strain GB-D and the chemolithoautotrophic methanogen Methanocaldococcus jannaschii, were tested for survival tolerance to heavy metals (Zn, Co, and Cu) and sulfide. The sulfide addition consistently ameliorated the high toxicity of free metal cations by the formation of dissolved metal-sulfide complexes as well as solid precipitates. Thus, chemical speciation of heavy metals with sulfide allows hydrothermal vent archaea to tolerate otherwise toxic metal concentrations in their natural environment.
Hyperthermophilic archaea grow in the steep thermal and chemical gradients in hydrothermal vent chimney rock; in this habitat, they are exposed to metal- and sulfide-rich vent fluid, which is transported through the porous chimney matrix with concomitant precipitation of metal-sulfides and -sulfates and in some chimney silica (15) upon mixing with seawater. Although metal and sulfide concentrations in the rock matrix have not been measured in situ, end-member concentrations represent upper-limit approximations. Sulfide concentrations are typically in the millimolar range and can be higher than 12 mM. Metal concentrations are typically in the range of 10 to 40 μM for Cu, 20 to 220 nM for Co, and 40 to 780 μM for Zn (8, 30). Site-specific peak concentrations can reach 1 to 2 μM for Co (22) and 1,000 to 3,000 μM for Zn (30). The chemical speciation of metals and sulfide, in particular metal-sulfide complex formation, might play a critical role in shaping the environmental niches and survival strategies of hydrothermal vent microorganisms. Metal-sulfide complexes play an important role in biological contexts (23), for example, by relieving cadmium toxicity to amphipods in marine sediments (6) and by influencing the distribution of hydrothermal vent invertebrates such as Riftia pachyptila and Alvinella pompeiana (19).
For hydrothermal vent archaea, efforts to characterize their growth conditions and survival capabilities have focused on extremes of temperature and pH, oxygen sensitivity, and electron acceptor and donor range (13, 27). Genomic information on metal tolerance and metabolism is limited to tentatively identified metal transport proteins (primarily Co, Cu, and Fe transporters) in the genomes of Pyrococcus furiosus, P. abyssi, P. horikoshii (http://www.ncbi.nlm.nih.gov), and Methanocaldococcus jannaschii (4, 26). As a consequence, the tolerances of vent archaea to the high concentrations of metals in their native habitat, their response to the chemical speciation of those metals, and their defense mechanisms against these environmental stress factors have remained largely obscure. Silver (26) has pointed out that a surprisingly small number of detoxicification genes appear to be present in the genome of M. jannaschii given its high-metal habitat in hydrothermal vent chimneys.
In order to define the physiological limits of hyperthermophilic microbial communities in their natural habitat, within and on the chimney rock matrix, we investigated the tolerances of hyperthermophilic hydrothermal vent archaea to extremes of temperature, pH, pressure, sulfide, and potentially toxic metals. In this study, we focus on the effects of metals and sulfide. To understand metal toxicity in the context of metal-sulfide complexation in their habitat, we tested survival of three hyperthermophilic vent archaea, Thermococcus fumicolans (9), Pyrococcus strain GB-D (13), and M. jannaschii (14) in increasing concentrations of three metals (Co, Cu, and Zn) found in hydrothermal vent fluids and in increasing concentrations of sulfide. Copper was chosen due to its known high toxicity for several groups of archaea and bacteria and as a toxic model compound that microorganisms tolerate to only a very limited degree (1, 25). Cobalt and zinc were selected as typical heavy metals in hydrothermal environments that are tolerated in limited measure by vent archaea (16). The three strains were selected as representatives of heterotrophic and autotrophic archaeal genera with cosmopolitan distribution patterns at hydrothermal vents and potentially within hydrothermal subsurface habitats worldwide (15). M. jannaschii was isolated from sedimentary material at the base of a “white smoker” chimney at 21°N East Pacific Rise (14). T. fumicolans was isolated from chimney fragments in the North Fiji Basin (9). These two strains were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). Pyrococcus strain GB-D was isolated from chimney scrapings in the Guaymas Basin and maintained in our laboratory (13).
Anaerobic growth media and culture conditions.
Sulfur-containing half-strength Marine Broth 2216 (Difco) medium (diluted with artificial seawater) was supplemented with 6.93 g of PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer per liter and 0.8 mg of resazurin per liter for the heterotrophs (13). DSMZ medium 282 (http://www.dsmz.de/media/med282.htm), modified to contain 25 g of NaCl per liter, 4.0 g of MgCl2 · 6H2O per liter, and 3.3 mM (final concentration) citrate buffer, was used for M. jannaschii. To reduce metals carried over into the survival medium (see below) from initial inocula, metal amounts in the modified DSMZ medium 282 were decreased by using 0.2 ml of Trace Element 141 solution (http://www.dsmz.de/media/med141.htm) per liter and 0.36 mg of Fe(NH4)2 (SO4)2 · 6H2O per liter, 0.233 mg of NiCl2 · 6H2O per liter, and 0.208 mg of Na2SeO4 · 5H2O per liter. The growth media were reduced with 96 mg of Na2S · 9H20 (0.4 mM) per liter and used for growing all starter cultures for experimental inocula and cell line maintenance. Growth medium tubes for M. jannaschii were overpressurized to 3 atm with 80% H2 and 20% CO2. For regrowth in the dilution series, nitrilotriacetic acid at a nontoxic concentration of 1.91 g/liter was added to chelate metals transferred from survival media. Also, Na2S · 9H20 was increased to 0.48 g/liter. Optimal growth conditions for M. jannaschii included pH 6.0, 82°C, and overpressurization (3 × 105 Pa) with 80% H2 and 20% CO2 (14), and for the heterotrophs, optimal growth conditions included pH 7.5 and 90°C, with N2 headspace. Fresh cell inocula were grown for at least 20 h prior to each experiment.
Anaerobic survival media and survival tests at selected dissolved metal concentrations.
Survival medium (pH 5) for the heterotrophs was prepared by adding 0.82 g of CH3COONa per liter, 0.80 mg of resazurin per liter, and 96 mg of Na2S · 9H2O per liter to Turk's Island artificial seawater (13). For the methanogen, DSMZ medium 282 seawater was supplemented with 1 mg of resazurin per liter, 9.75 g of MES [2-(N-morpholino)ethanesulfonic acid] buffer per liter, and 96 mg of Na2S · 9H2O per liter and set to pH 6. Both survival media were prepared and dispensed anaerobically under N2.
Freshly grown cells of T. fumicolans, Pyrococcus strain GB-D, and M. jannaschii were transferred to survival media (see below) and exposed to dissolved metal concentrations in the range of 1 μM to 10 mM for up to 48 h. The metal survival medium did not contain electron donors (organic substrates in the case of T. fumicolans and Pyrococcus strain GB-D and hydrogen in the case of M. jannaschii) or elemental sulfur, to prevent cell growth and biological production of sulfide. This avoided difficulties in interpreting experiments in growth media where biological sulfide production changes the metal equilibrium chemistry and partial chelation of heavy metal ions occurs. Since changes in pressure complicate metal speciation, experiments were conducted at 1 atm. Glassware was acid washed. The metal survival tests were performed at pH 5.0 and 90°C for T. fumicolans and Pyrococcus strain GB-D and at pH 6.0 and 82°C for M. jannaschii. These pHs approximate the natural acidic pH (range, 3.1 to 5.9) of hydrothermal vent end-member fluids (30) and chimney walls (29) while minimizing precipitation of metals as oxyhydroxides at the highest metal concentrations. Extensive pre-experiments demonstrated that pH 5 for Thermococcales and pH 6 for Methanocaldococcus were the lowest pH values that did not affect survival (data not shown). No difference in survival was found at these pHs compared to tests using published optimal pHs for these archaea.
Overnight growth cultures (cell densities of approximately 3 × 108 cells/ml) were used to inoculate survival media to 106 cells/ml, representing 0.3% of the final volume of survival test media. Survival media containing 106 cells/ml and appropriate metal concentrations were distributed in 15-ml (heterotrophs) and 10-ml (methanogen) aliquots into four Hungate tubes for each combination of metal and sulfide. Zero millimolar sulfide medium was prepared by adding 0.4 mM sulfide to remove oxygen and then bubbling the solution anaerobically with nitrogen to strip off dissolved hydrogen sulfide. The final 0 mM sulfide concentration was confirmed by the Cline assay (5). Metals were added after sulfide concentrations had been established and confirmed. Aqueous anaerobic sulfide solution was added to produce the desired concentrations, and tubes were incubated at optimal temperature and 1 atm.
Survival was assessed by using a decimal dilution series method. After 6, 12, 24, and 48 h, a tube of each cell suspension was diluted into a six-step, 10-fold dilution series in full-growth medium and incubated at optimal temperature and 1 atm for at least 5 days. Tubes were checked periodically for regrowth on the basis of visible turbidity and microscopic examination. For each organism, survival at specific metal concentrations was also confirmed by triplicate most-probable- number dilution series (data not shown).
Metal-sulfide speciation.
For each metal studied, sulfide forms dissolved complexes with divalent metal cations [equation 1; note that metal-sulfide complexes have numerous stoichiometries, e.g., MHS+, M(HS)2, and M2S3] (17, 23) until the solubility product (Ksp) is exceeded (equation 2):
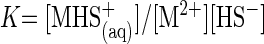 |
(1) |
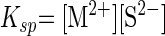 |
(2) |
where K is the stability constant for the aqueous metal-sulfide complex. Metal-sulfide precipitation occurred during medium preparation for higher-metal-concentration treatments, and such precipitates may have partially dissolved during the elevated temperature of experiments (e.g., the solubility product of sphalerite increases with temperature) (3, 10), depending on the kinetics of dissolution. Metal-sulfide clusters likely formed during complexation and precipitation (23); these clusters are a major component of metal speciation prior to condensation and precipitation. For example, Zn sulfide complexes can accumulate in micromolar concentrations as aqueous clusters with stoichiometries of Zn4S64− with the ion activity product in excess of the solubility product (18). Due to the complexity of this metal-sulfide chemistry, we refer to metal-sulfide complexes rather than specific stoichiometries.
Metal toxicity and its amelioration by sulfide.
Metal toxicity was observed in the range of 1 to 100 μM total Cu, Co, and Zn in sulfide-free tests. Although strain- and metal-specific differences were apparent, sulfide always ameliorated metal toxicity if present in concentrations in the same order of magnitude or larger than the metal tested (Fig. 1, 2, and 3). These metal resistances show the effectiveness of metal-sulfide complex formation for Zn, Cu, and Co. Metal toxicity is known to be correlated with free metal concentration (M2+) (28), and the addition of sulfide to the media resulted in the formation of dissolved metal-sulfide complexes at low metal concentrations that greatly reduced free metal concentrations followed by precipitation of metal-sulfides at higher concentrations. These metals differ in their geochemistry, with Zn and Cu forming stronger complexes with sulfides and precipitating at lower free metal concentrations than Co, resulting in extremely low free metal concentrations in the presence of sulfides (Table 1).
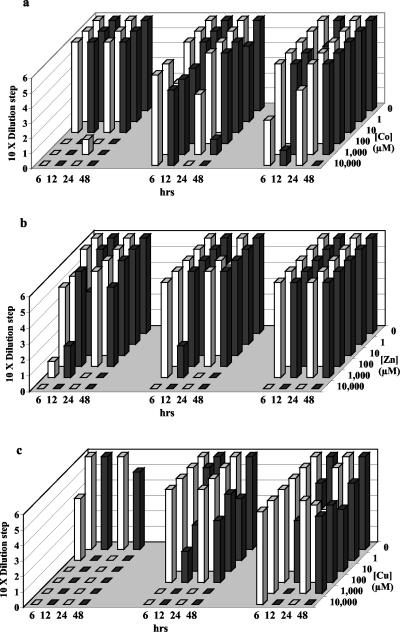
FIG. 1.
Survival of T. fumicolans after exposure to increasing concentrations of total metals for 6, 12, 24, and 48 h. The portion of surviving cells equals the number of 10-fold dilution steps from 1 to 6 (6 equals 100% survival) of the test culture that regrew after exposure. Column diagrams for Co (a), Zn (b), and Cu (c) show three parallel experiments: metal exposure with no sulfide added (left), 0.4 mM sulfide added (center), and 2 mM sulfide added (right).
TABLE 1.
Decreased free metal concentration with sulfide complexationa
| Metale | M2+:Mtotal at sulfide concn of:
|
||
|---|---|---|---|
| 0 mMb | 0.4 mM | 2 mM | |
| Zincc | 0.67 (0.13) | 3.5e−9 (8.7e−4) | 6.9e−10 (3.4e−5) |
| Cobaltd | 0.70 | 0.041 | 0.0086 |
Calculated as a ratio of total dissolved metal (M2+:Mtotal) using MINEQL with verified thermodynamic constants (NIST standard reference database, version 6.0 [http://www.nist.gov.srd]) and literature values. Major ions included Na+ (0.545 M), CI− (0.468 M), Mg2+ (0.0532 M), Ca2+ (0.0102 M), HCO3− (0.0024 M), and CH3COOH (0.01 M). Concentrations of Co- and Zn-acetic acid complexes were small relative to chloro complexes. pHs of 5 and 6 were used for sulfide-free and sulfide treatments based on experimental conditions.
Without sulfide, metal chloro species dominate.
Zinc-free ion concentrations are calculated at room temperature by using stability constants for ZnS and Zn(HS)2 (17, 31) and for chloro complexes (NIST database [see above]). In parentheses, zinc-free ion concentrations are calculated by using stability constants determined at 100°C (3) for Zn chloro complexes and Zn(HS)2 and Zn(HS)3−. The differences in results between temperatures are due to incomplete stability constant data for higher temperatures as well as more sensitive voltammetric methods for determining stability constants at room temperature.
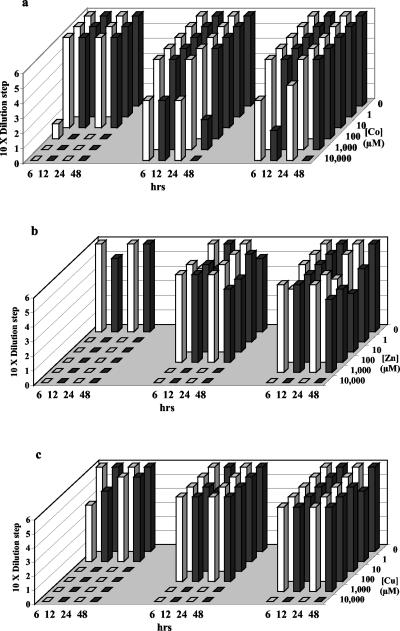
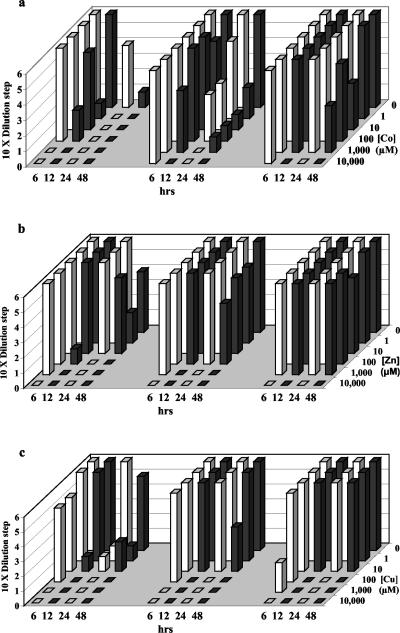
Sulfide alleviated metal toxicity for all three organisms. This occurred when the metals existed primarily as aqueous metal- sulfides and continued when precipitates formed, once concentrations of aqueous metal-sulfides (and corresponding free metal concentrations) exceeded their solubility limit. Sulfide reduced Cu toxicity for T. fumicolans at 1 μM Cu, indicating the importance of soluble CuHS+ complexes. Further, sulfide reduced Cu toxicity at a high Cu concentration (1,000 μM) where solid precipitates were observed (Fig. 1c). CuS precipitates were not observed below 10 μM Cu, while significant CuS precipitates occurred at 100 μM Cu and above. Pyrococcus strain GB-D showed the effectiveness of soluble ZnS(aq) complex formation in ameliorating Zn toxicity (Fig. 2b). While Zn without sulfide was rapidly fatal at 1 μM, Zn tolerance in the presence of sulfide extended to 100 μM, where zinc speciation was dominated by soluble ZnS(aq) complexes, and continued to 1,000 μM in the presence of precipitates (Fig. 2b). Sulfide ameliorated cobalt toxicity for M. jannaschii between 1 and approximately 100 μM cobalt, within the range of soluble CoHS+ complexes, and continued into the range of CoS precipitation above 10 μM (Fig. 3a). CoS precipitates appeared at 10 μM Co, close to calculated values.
FIG. 2.
Survival of a Pyrococcus sp. after exposure to increasing concentrations of total metals for 6, 12, 24, and 48 h. See the legend of Fig. 1 for an explanation of the diagram.
FIG. 3.
Survival of M. jannaschii after exposure to increasing concentrations of total metals for 6, 12, 24, and 48 h. See the legend of Fig. 1 for an explanation of the diagram.
Significance for the hydrothermal vent environment.
Metals in the vent fluids are known to be complexed predominantly by chloride complexes at very high temperatures and low pH values (22). Subsequent mixing with surrounding seawater creates relatively cooler and higher-pH environments favored by extremophiles (∼100°C and pH 5 to 6). During this cooling, a transition from chloride complexation of metals to sulfide occurs (at ∼200°C for Zn species) as the stability of chloride complexes decreases at lower temperatures (3). This metal- sulfide complex formation facilitates the survival of archaea in chimney walls and flanges where they are exposed to high temperatures and high metal and sulfide concentrations. Naturally occurring end-member concentrations of Cu (10 to 44 μM) (8) exceed the range that can be tolerated without soluble sulfide complex formation (Fig. 1c, 2c, and 3c). Zn concentrations (40 to 780 μM) (8, 22) reach the range for which sulfide amelioration is required for survival (Fig. 2b and 3b). However, Co concentrations in end-member fluids (22 to 227 nM) (8) remain below the range required for sulfide amelioration for T. fumicolans and Pyrococcus strain GB-D (Fig. 1a and 2a). In situ chemical speciation data are not available for Cu, Zn, or Co at hydrothermal vents; in situ Fe speciation is dominated by FeS(aq) species (19). Hence, it is also likely that sulfide rather than organic complexes dominates Cu, Zn, and Co speciation at hydrothermal vents.
In previous growth experiments, relatively high metal tolerances were found for members of the order Thermococcales from the Lau Basin hydrothermal sites (0.1 to 2 mM Co and 0.5 to 5 mM Zn) (16). These actively metabolizing cells, growing in media containing organic substrates and cysteine, likely detoxified their media through metal-sulfide complex formation. Sulfide production should be considered when comparing inhibition studies for different microorganisms and metals. In Cu tolerance tests for the nonhydrothermal vent methanogen Methanobacterium thermoautotrophicum, growth was temporarily inhibited at 0.16 μM Cu and totally inhibited at 0.8 μM; similar inhibitory concentrations were found for other heavy metals and autotrophic methanogens (1). The lack of organic compounds in autotrophic media, or the absence of sulfide production by methanogens, may contribute to this high sensitivity relative to the Thermococcales.
Summary.
Classically, metal complexation in surface waters has been shown to involve organic metal complexes that either reduce metal toxicity or supplement nutrition (via iron reductases, siderophores, and metallophores) to unicellular marine organisms (12, 20, 24, 28). In the hydrothermal vent environment, metal complexation appears to play a role in reducing metal toxicity via metal-sulfide complexes. Typically, inorganic complexes are sufficiently weak enough that they are thought to be bioavailable (for example, chloro or hydroxy compounds) (11). However, in this study, inorganic metal-sulfide complexes reduce the toxicity of these metals to the Archaea, most likely by decreasing metal bioavailability. Due to their high stability constants and chemical inertness, metal-sulfide complexes can act as a natural metal buffer that remains stable and oxidation resistant for weeks (23), protecting vent archaea against what would otherwise be toxic concentrations of metals. The decrease in toxicity may be due to a decreased uptake of metals once present as metal-sulfide complexes, or alternatively, it may be due to a lessened toxicity of metal-sulfide complexes once inside the cell. While these data cannot differentiate between these two possibilities, the presence of organic metal complexes (e.g., CoEDTA) is known to greatly reduce metal uptake in cyanobacteria (24). Future studies with Archaea can determine if a similar phenomenon occurs with metal-sulfide complexes.
These results indicate that not only organic ligands but also metal-sulfide complexes have the potential to affect metal bioavailability. Both metal organic and metal-sulfide complexes are sufficiently strong to slow the kinetics of dissociation, which results in lowered exposure of free metal cations to the organism. In the surface ocean, metal speciation has been shown to play an important role in reducing copper toxicity to cyanobacteria by the complexation of copper with organic complexes (12, 21). Similarly, the chemical speciation of metals as metal- sulfide complexes may be important for the survival of archaeal communities at deep-sea hydrothermal vents. The ability of hydrothermal vent archaea to survive extreme metal concentrations may not be due solely to a physiological or genetic capability (26) as has been suggested for protists at vent sites (2). Additionally, metal-sulfide complex formation acts as a chemical defense mechanism that buffers the concentration of bioavailable metals before physiological detoxification reactions set in.
Acknowledgments
We thank Lis Seufke for laboratory and experimental assistance during phases of the methanogen work, Bill Sunda for helpful discussions, and John Waterbury and Meg Tivey for comments on the manuscript.
This work was supported by the National Science Foundation (Life in Extreme Environments [LExEn] grant OCE-0085534 to A.T., S.J.M., K.L, S.B., and C.O.W.), an NSF Postdoctoral Fellowship in Microbial Biology (M.S.A.), the MBL (Environmental Genomes, S/C NCC2-1054) and URI (Subsurface Biospheres) NASA Astrobiology Institute Teams (A.T. and S.J.M.), an NRC Astrobiology postdoctoral fellowship (V.P.E.), and a Princeton Harry Hess postdoctoral fellowship (M.A.S.). We thank the Seaver Foundation for funding equipment and baseline experiments.
Footnotes
Contribution number 10928 of the Woods Hole Oceanographic Institution.
REFERENCES
- 1.Ahring, B., and P. Westermann. 1985. Sensitivity of thermophilic methanogenic bacteria to heavy metals. Curr. Microbiol. 12:273-276. [Google Scholar]
- 2.Atkins, M. S., M. A. Hanna, E. A. Kupetsky, M. A. Saito, C. D. Taylor, and C. O. Wirsen. 2002. Tolerance of flagellated protists to high sulfide and metal concentrations potentially encountered at deep-sea hydrothermal vents. Mar. Ecol. Prog. Ser. 226:63-75. [Google Scholar]
- 3.Bourcier, W. L., and H. L. Barnes. 1987. Ore solution chemistry—VII. Stabilities of chloride and bisulfide complexes of zinc to 350°C. Econ. Geol. 82:1839-1863. [Google Scholar]
- 4.Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. FitzGerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J.-F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. M. Geoghagen, J. F. Weidman, J. L. Fuhrmann, D. Nguyen, T. R. Utterback, J. M. Kelley, J. D. Peterson, P. W. Sadow, M. C. Hanna, M. D. Cotton, K. M. Roberts, M. A. Hurst, B. P. Kaine, M. Borodovsky, H.-P. Klenk, C. M. Fraser, H. O. Smith, C. R. Woese, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058-1073. [DOI] [PubMed] [Google Scholar]
- 5.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 6.Di Toro, D. M., J. D. Mahony, D. J. Hansen, K. J. Scott, M. B. Hicks, S. M. Mayr, and M. S. Redmond. 1990. Toxicity of cadmium in sediments: the role of acid volatile sulfide. Environ. Toxicol. Chem. 9:1487-1502. [Google Scholar]
- 7.Dryssen, D. 1988. Sulfide complexation in surface seawater. Mar. Chem. 24:143-153. [Google Scholar]
- 8.Elderfield, H., and A. Schultz. 1996. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci. 24:191-224. [Google Scholar]
- 9.Godfroy, A., J.-R. Meunier, J. Guezennec, F. Lesongeur, G. Raguénès, A. Rimbault, and G. Barbier. 1996. Thermococcus fumicolans sp. nov., a novel hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent in the North Fiji Basin. Int. J. Syst. Bacteriol. 46:1113-1119. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, K., A. Sugaki, and A. Kitakaze. 1990. Solubility of sphalerite in aqueous sulfide solutions at temperatures between 25 and 240°C. Geochim. Cosmochim. Acta 54:715-725. [Google Scholar]
- 11.Hudson, R. J. M., and F. M. M. Morel. 1990. Iron transport in marine phytoplankton: kinetics of cellular and medium coordination reactions. Limnol. Oceanogr. 35:1002-1020. [Google Scholar]
- 12.Hutchins, D. A., A. E. Witter, A. Butler, and G. W. Luther III. 1999. Competition among marine phytoplankton for different chelated iron species. Nature 400:858-861. [Google Scholar]
- 13.Jannasch, H. W., C. O. Wirsen, S. J. Molyneaux, and T. A. Langworthy. 1992. Comparative physiological studies on hyperthermophilic archaea isolated from deep-sea hot vents with emphasis on Pyrococcus strain GB-D. Appl. Environ. Microbiol. 58:3472-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, W. J., J. A. Leigh, F. Mayer, C. R. Woese, and R. S. Wolfe. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136:254-261. [Google Scholar]
- 15.Kelley, S., J. A. Baross, and J. R. Delaney. 2002. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 30:385-491. [Google Scholar]
- 16.Llanos, J., C. Capasso, E. Parisi, D. Prieur, and C. Jeanthon. 2000. Susceptibility to heavy metals and cadmium accumulation in aerobic and anaerobic thermophilic microorganisms isolated from deep-sea hydrothermal vents. Curr. Microbiol. 41:201-205. [DOI] [PubMed] [Google Scholar]
- 17.Luther, G. W., III, D. T. Rickard, S. Therberge, and A. Olroyd. 1996. Determination of (bi)sulfide stability constants of Mn2+, Fe2+, Co2+, Ni2+, and Zn2+ by voltammetric methods. Environ. Sci. Technol. 30:671-679. [Google Scholar]
- 18.Luther, G. W., III, S. M. Theberge, and D. T. Rickard. 1999. Evidence for aqueous clusters as intermediates during zinc sulphide formation. Geochim. Cosmochim. Acta 63:3159-3169. [Google Scholar]
- 19.Luther, G. W., III, T. F. Rozan, M. Taillefert, D. B. Nuzzio, C. Di Meo, T. M. Shank, R. A. Lutz, and S. C. Carey. 2001. Chemical speciation drives hydrothermal vent ecology. Nature 410:813-816. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado, M. T., and N. M. Price. 2001. Reduction of transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J. Phycol. 37:298-309. [Google Scholar]
- 21.Mann, E. L., N. Ahlgren, J. W. Moffett, and S. W. Chisholm. 2002. Copper toxicity and cyanobacterial ecology in the Sargasso Sea. Limnol. Oceanogr. 47:976-988. [Google Scholar]
- 22.Metz, S., and J. H. Trefry. 2000. Chemical and mineralogical influences on concentrations of trace metals in hydrothermal fluids. Geochim. Cosmochim. Acta 64:2267-2279. [Google Scholar]
- 23.Rozan, T. F., M. E. Lassman, D. P. Ridge, and G. W. Luther III. 2000. Evidence for iron, copper, and zinc complexation as multinuclear sulphide clusters in oxic rivers. Nature 406:879-882. [DOI] [PubMed] [Google Scholar]
- 24.Saito, M. A., J. W. Moffett, S. W. Chisholm, and J. B. Waterbury. 2002. Cobalt limitation and uptake in Prochlorococcus. Limnol. Oceanogr. 47:1629-1636. [Google Scholar]
- 25.Sani, R. K., B. M. Peyton, and L. T. Brown. 2001. Copper-induced inhibition of growth of Desulfovibrio desulfuricans G20: assessment of its toxicity and correlation with those of zinc and lead. Appl. Environ. Microbiol. 67:4765-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver, S. 1997. The bacterial view of the periodic table: specific functions for all elements. Rev. Mineral. 35:345-360. [Google Scholar]
- 27.Stetter, K. O. 1996. Hyperthermophilic prokaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 28.Sunda, W., and R. R. L. Guillard. 1976. The relationship between cupric ion activity and the toxicity of copper to phytoplankton. J. Mar. Res. 34:511-527. [Google Scholar]
- 29.Tivey, M. K. Environmental conditions within active seafloor vent structures: sensitivity to vent fluid composition and fluid flow. In W. Wilcock, C. Cary, E. DeLong, D. Kelley, and J. Baross (ed.), Subseafloor biosphere at mid-ocean ridges. Geophysical monograph, in press. American Geophysical Union, Washington, D.C.
- 30.Von Damm, K. 1995. Controls on the chemistry and temporal variability of seafloor hydrothermal fluids, p. 222-247. In S. E. Humphris, R. A. Zierenberg, L. S. Mullineaux, and R. E. Thomson (ed.), Seafloor hydrothermal systems: physical, chemical, biological and geological interactions. Geophysical monograph 91. American Geophysical Union, Washington, D. C.
- 31.Zhang, J.-Z., and F. J. Millero. 1994. The investigation of the metal sulfide complexes in seawater using cathodic stripping square wave voltammetry. Anal. Chem. Acta 284:497-504. [Google Scholar]