Abstract
Objectives:
The study evaluated pathognomic histopathological features with the help of light microscopy for detecting the integration of human papillomavirus (HPV) (type 16 and 18) in oral squamous cell carcinoma (OSCC).
Materials and Methods:
Forty-five histopathologically diagnosed cases of OSCC were evaluated for the presence of E6/E7 protein of HPV (16 + 18) with the help of nested multiplex polymerase chain reaction. Both HPV-positive and -negative cases were evaluated for four histological features: Koilocytes, dyskeratosis, invasion, and alteration of collagen.
Results:
Fischer's exact test showed significant difference (P < 0.01%) for the presence of koilocytes and dyskeratosis, whereas no difference was observed for invasion and alteration in collagen between HPV-positive and -negative OSCC.
Conclusion:
The presence of koilocytes and dyskeratosis at light microscopic level can be used as a marker for the presence of HPV (type 16 and 18) in OSCC.
Keywords: Dyskeratosis, human papillomavirus, histopathology, koilocytes, oral squamous cell carcinoma, polymerase chain reaction
INTRODUCTION
Oral squamous cell carcinomas (OSCCs) are characterized by multiphasic and multifactorial etiopathogenesis. Tobacco and alcohol are the most common risk factors for oral malignancy. Other factors, including DNA viruses, especially human papilloma virus (HPV) have been documented to play a role in the initiation or development of these lesions.[1]
HPV involvement in OSCC was first proposed in 1983 by Syrjanen et al. and then supported by others on basis of the fact that HPV shows epitheliotropism and has the ability to immortalize human oral keratinocytes in vitro.[2] High-risk HPV type 16 and 18 were declared as human carcinogens by the International Agency of Research on Cancer.[3]
The HPV family comprises more than 100 genotypes, classified in accordance with the type of epithelial cells infected and the ability to affect cellular transformation. Certain types of HPV such as HPV1 infect cutaneous epithelial cells, whereas HPV6, 11, 16, and 18 infect mucosal epithelial cells of the oral cavity, oropharynx, anogenital tract, and uterine cervix.[4] The genomic HPV DNA has nine open-reading frame sequences present on single strand of DNA. These are divided into seven early (E1–E7) and two late-phase genes (L1–L2). Expression of viral oncoproteins E6 and E7 interferes with crucial cellular mechanisms such as cell cycle regulation and apoptosis.[5]
HPV-positive SCC are clinically and molecularly distinct from HPV-negative SCC and may be associated with different prognostic outcomes.[6]
The prevalence of HPV has been assessed by many techniques, including polymerase chain reaction (PCR), southern blot, dot blotting, and in situ hybridization (ISH). These methods are satisfactory and reliable for detection of HPV, but are neither easily available universally, nor can every patient afford these procedures especially in developing countries such as India.
While histopathological analysis by light microscopy is the most commonly used method for confirming a diagnosis, it is also a useful method for observation of viral particles when molecular biology methods are not available. The purpose of the study was to compare the histopathological features with PCR method to predict the presence of HPV infection in OSCC biopsies as the detection of HPV in patients of OSCC may be of great help in planning targeted therapy and for better prognostication.
MATERIALS AND METHODS
The study was conducted at the Department of Oral Pathology and Microbiology. The study was reviewed and approved by an ethical board of the college.
The study sample comprised of 45 histologically confirmed cases of OSCC. Each case of OSCC was evaluated for the presence of E6 and E7 protein of HPV 16 and HPV 18 with the help of nested multiplex PCR.
Two sets of primers (Bioserve Tech. Hyderabad, India) were used in two successive PCR reactions. In the first reaction, one pair of primers was used to generate DNA products, which besides the intended target, may still consist of non-specifically amplified DNA fragments. The second round of primers (internal) were located within the desired amplification product produced by the first round primers (external) so they specifically amplified required DNA fragment. The amplified products were then gel electrophoresed for band formation. The identification of HPV 16 was done by the presence of a positive band at 457 bp and HPV 18 was identified as positive band at 322 bp [Figure 1].
Figure 1.
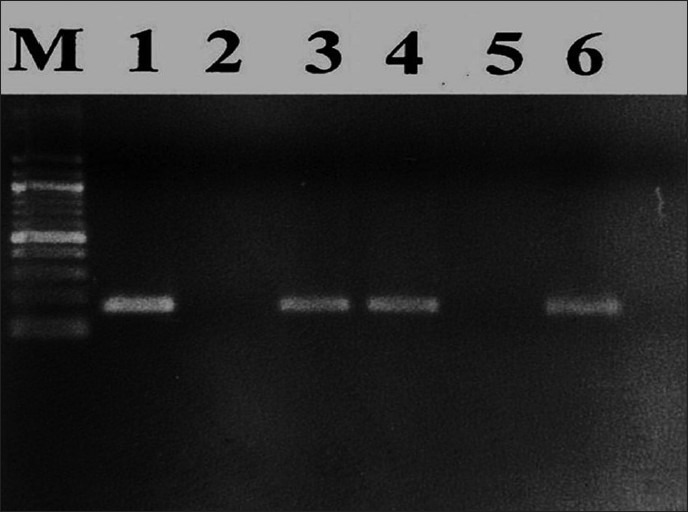
Band analysis: M lane: 100-bp deoxyribonucleic acid marker, lane 1: HPV 16-positive control (457 bp), lane 2: Human papilloma virus-negative control, lane 3: HPV 16-positive specimen, lane 4: HPV 18-positive control (322 bp), lane 5: HPV 16-negative specimen, lane 6: HPV 18-positive specimen
For histological evaluation, 5-μm thick sections were obtained from the same paraffin-embedded squamous cell carcinoma blocks and later stained with hematoxylin and eosin stain. All the 45 cases were evaluated for light microscopy histological features, such as presence of koilocytes, dyskeratosis (individual cell keratinization), invasion and alteration in collagen.
In this study, the presence of koilocytes in tissue was considered significant if more than five koilocytes per 10 high power fields (HPF) were seen in both neoplastic and non neoplastic areas (just surrounding the neoplastic area). Only if koilocytes were present in both the areas, it was considered positive [Figure 2]; positivity for dyskeratosis was considered to be the presence of more than three dyskeratotic cells per 10 HPF [Figure 3]. Invasion was considered to be positive if epithelial cells were found in the connective tissue with clear breach of basement membrane [Figure 4], and collagen alteration was considered when any abnormal pattern of collagen structure in connective tissue was found [Figure 5].
Figure 2.
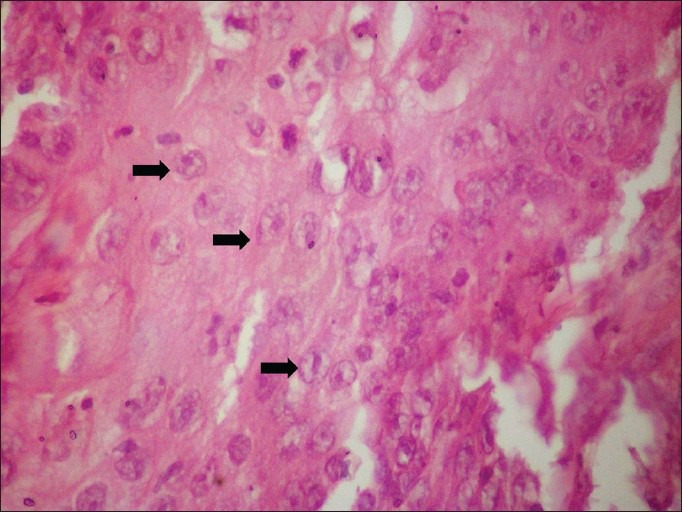
Koilocytes (black arrow) in epithelium (H&E, ×400)
Figure 3.
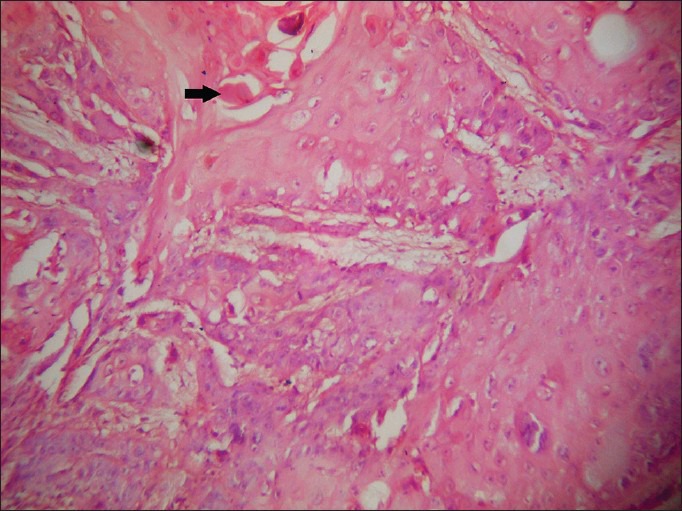
Dyskeratotic cells (black arrow) (H&E, ×400)
Figure 4.
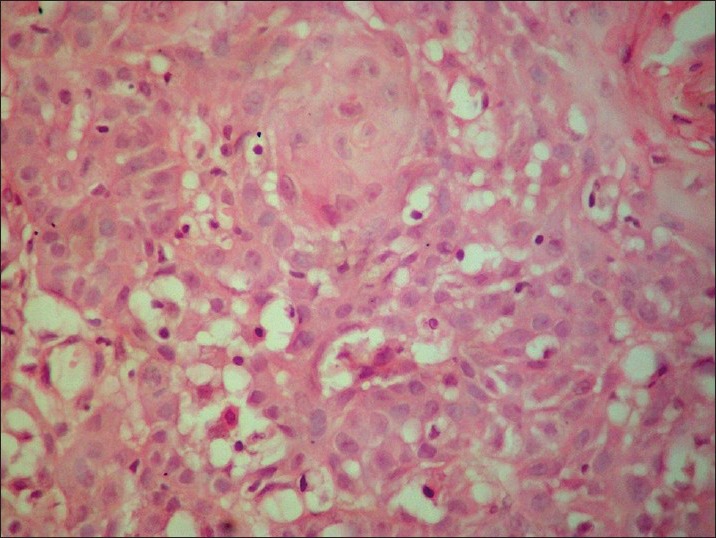
Invasion of epithelial cells in the connective tissue (H&E, ×100)
Figure 5.
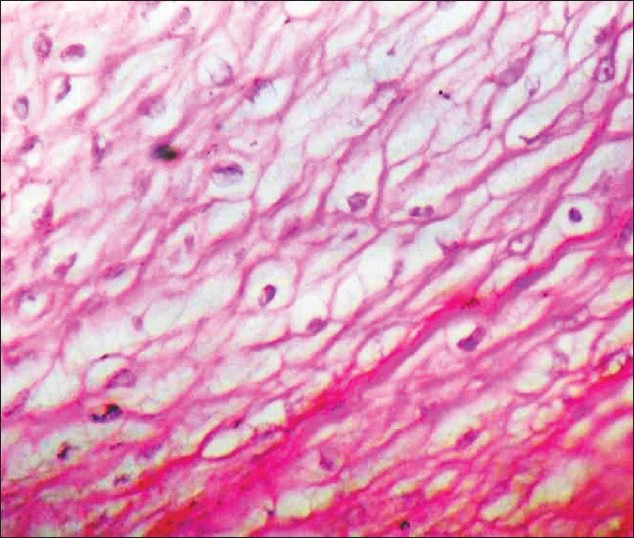
Abnormal pattern of collagen structure (H&E, ×400)
A single pathologist examined all the histological sections for the four features. The results of PCR evaluation for the presence of E6 and E7 protein of HPV 16 and HPV 18 were blinded from the pathologist assessing the histopathological slides in order to remove bias.
Nested multiplex PCR revealed that out of 45 selected cases of OSCC, 22 cases (48.8%) were HPV16 positive, 13 cases (28.8%) were HPV18 positive, and 10 cases (22.2%) were positive for both HPV16 and 18.
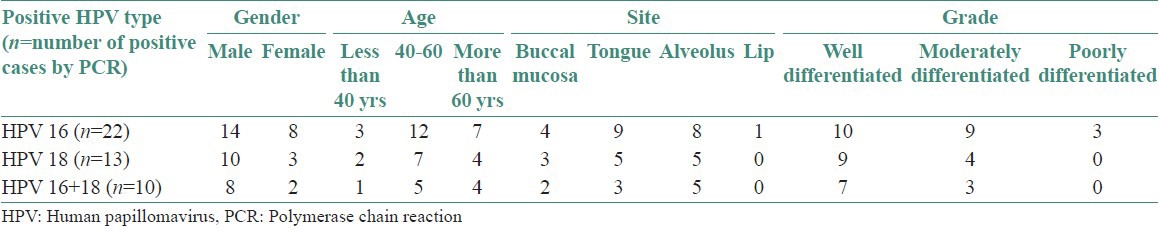
Table 1 shows detailed clinicopathological data of patients’ age, gender, site, and tumor differentiation of OSCC taken up for the study.
Table 1.
Clinicopathological data of patients
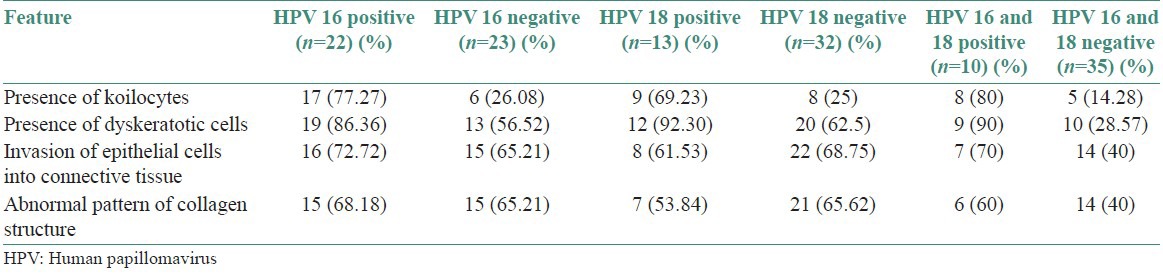
Two groups were made depending on the presence of HPV16, HPV 18, and both HPV16 and 18 separately for the four histological features [Table 2]. The data thus collected was subjected to statistical analysis. SPSS software package version 17 was used for statistical analysis.
Table 2.
Histological features according to HPV type
RESULTS
Two-tailed Fischer's exact test was used for evaluating the difference for the four histological features between HPV positive and negative cases of OSCC.
Table 3 shows statistical evaluation of all four histological features in HPV16, HPV18, and combined, respectively.
Table 3.
Application of Fischer's exact test (two tailed)
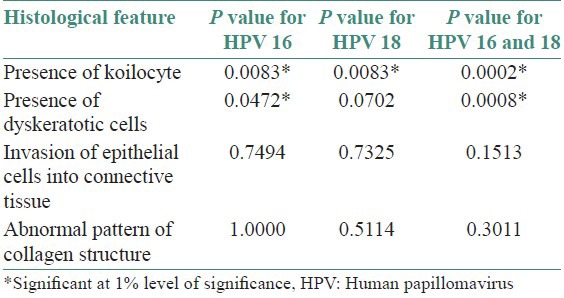
When Fischer's exact test (two tailed) was applied for evaluating the difference in the presence of koilocytes it showed that there was a significant difference in the number of koilocytes in HPV positive and negative cases for HPV16, HPV 18 and combined HPV 16 and 18.
Dyskeratosis was significantly different in HPV 16 and combined HPV 16 and 18, but was not significantly different for HPV18. The other two histological features of invasion and abnormal alteration in collagen showed no significant difference between HPV positive and negative cases HPV16, HPV18, and combined HPV 16 18.
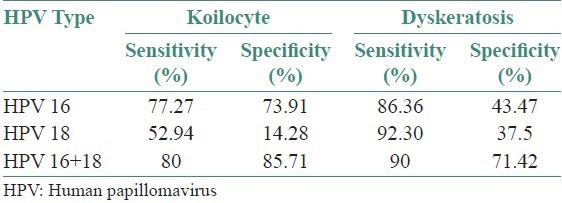
Table 4 shows the sensitivity and specificity of two histological features: Koilocytes and dyskeratosis when nested multiplex PCR is taken as gold standard procedure for identification of HR-HPV.
Table 4.
Sensitivity and specificity of koilocyte and dyskeratosis
DISCUSSION
There have been numerous reports on HPV-DNA detection in HN-SCC with rates varying from 0% to 100% of carcinomas studied.[7,8] These differences in detection rate are due to at least two principal factors: (1) differences in the epidemiological distribution of oncogenic HR-HPVs in the world; and (2) different analytical methods used.[9,10]
The great variation in HPV prevalence found in OSCCs in different studies may be not only due to the differences among the analyzed population, but also due to the differences in the samples tested (ie, formalin-fixed or fresh biopsies, exfoliated fresh cells), the methods of DNA extraction, and most importantly the HPV detection methods used.[11,12] Although HR-HPV detection is important in clinical settings for the OSCC patients, there is no consensus on which to consider the “golden standard” among the numerous detection methods available either as single test or combinations.[13,14,15,16]
A few Indian studies have been done on the prevalence of HPV in OSCC in Indian population. The overall prevalence of HPV in OSCC in India has been reported as ranging from 20% to 50%.[17] The prevalence of HPV in OSCC also shows regional variation. It has been reported as 33.6% in Eastern India, 67% in South India, and 15% in Western India.[18] Chocolatewala et al., reported a prevalence of 17.6%-41.8% for HPV16, and 0%-47.3% for HPV18 in OSCC cases.[17]
Nested amplification of the GP-E6/E7 PCR products with type-specific primers, was chosen to achieve exact typing of the HPV infections. These primers were again selected from sequence alignments of the E6/E7 genes, which were aimed to indicate sequence variation between closely related genotypes. In order to reduce the number of nested PCR for detection of two different genotypes of HPV, multiplex primer cocktails were used in which requirements for both the type-specific primers are met. This strategy allows first, HPV genotyping based on PCR product size; second, extension of assay with multiplex primers cocktails for additional HPV genotypes and also direct detection of the viral oncogenes.[19,20]
Total prevalence of HPV in lesional tissue of oral cavity proper was found to be 55.55%, HPV16 was found to be 48.88%, and HPV18 was found to be 28.88%. The high prevalence of HPV in present study may be due to the fact that most cases were from region of tongue, which is known to show high prevalence of High risk-Human papilloma virus in the oral cavity.
In the present study, the prevalence of HPV16 in SCC of oral cavity proper was found to be 48.88%, which is in accordance with a Mexican patient cohort study done by Anaya-Saavedra et al.,[14] which showed a high frequency of HPV positivity with prevalence of 43.5% in OSCC and Higa et al.,[21] who reported prevalence of 52.2%. Ostwald et al.[22] conducted a study for presence of HPV-DNA in head and neck squamous cell carcinoma and normal mucosa using PCR where they found 45% prevalence of HPV 16. Sugiyama et al.[23] studied the prevalence of HPV by PCR in OSCC, found 35% prevalence of HPV 16. In India, Balaram et al.[18] conducted a study on HPV in OSCC using PCR and found 41.8% prevalence of HPV 16.
The histological diagnosis of HPV presence in a given tissue sample, is based on HPV-related histopathological aspects, such as koilocytes, dyskeratosis, papillomatosis, hyperkeratosis, acanthosis, and parakeratosis. Koilocytes – consisting of the presence of abnormal cells that are vacuolated, with nucleus showing pyknosis and large clear perinuclear halos that usually occupies a greater volume than that of the cytoplasm –is the most common HPV-related cytopathic effect and is considered by pathologists to be the major histopathological feature for determination of HPV infection. It is considered as a pathognomonic sign of HPV-associated lesions.[24] Although some authors suggested an association between cytological and immunohistochemical positivity for HPV, others found no morphological aspects that confirmed the presence of HPV by PCR.[25]
Miyahara et al. (2011)[26] compared the histopathological analysis with PCR to predict the presence of HPV infection in OSCC biopsy tissues and found that the presence of koilocytes is unreliable for the detection of HPV presence in oral and oropharyngeal SCC.
Our study revealed that there was statistically significant differences for koilocytes and dyskeratosis between HPV (16,18) positive and negative cases, which indicated that koilocytosis and dyskeratosis can be reliably used as markers for the diagnosis of HPV in SCC.
This study also revealed that invasion and abnormal alteration in collagen structure showed no histological difference between HPV (16,18) positive and negative cases. Branca et al.[27] studied the difference in invasion pattern between HR-HPV positive and negative cases with the help of important regulators of cancer invasion and metastasis, such as matrix metalloproteinase-2 (MMP-2) and its tissue inhibitor (TIMP-2), which revealed that there was no significant difference between them. Similarly, Garzetti et al.[28] described marked overexpression of MMP-2 in microinvasive carcinomas as compared with cervical intraepithelial neoplasia, but this upregulation was unrelated to the HPV status in these lesions. Hence, even in our study we did not find any difference in invasion pattern between HPV-positive and -negative cases with the help of light microscope.
In the present study, when NMPCR is taken as gold standard, koilocyte shows high sensitivity of 77.27% with 73.91% specificity in detection of OSCC, whereas dyskeratosis shows sensitivity of 86.36% and specificity of 43.47% for HPV 16 alone.
In contrast to present study, Pannone et al.[16] used p16-IHC to demonstrate the presence of HPV and demonstrated that although p16-IHC is a good prognostic indicator when used in combination with HPV-DNA molecular methods, it is not satisfactory when evaluated as HPV detecting test when used alone (specificity less than 75%). They also showed that adding ISH technique, (although it is a method known to be less sensitive than PCR-based ones) has the advantage to preserve the morphological context of HPV-DNA signals in formalin fixed paraffin embedded samples and, unexpectedly, increased the sensitivity of p16/consensus PCR combination.
Thus, it is evident that no single technique can be used as an indicator of HPV testing but it is combination of techniques, which helps in detecting and evaluating prognosis of HPV-related OSCC.
On large community screening examination for detection of OSCC, especially in the developing countries, where molecular detection methods are not easily available, light microscopic features might provide help in selecting patients for further molecular detection methods of HPV as HPV-related OSCC has better prognosis than smoking-related OSCC. Thus the study points the use of light microscopic features, such as koilocyte and dyskeratosis for positive prediction of HPV 16 and 18's presence in OSCC.
CONCLUSION
The study concludes that the presence of koilocytes and dyskeratosis at light microscopic level can be used as an indirect marker for the presence of HPV (16,18) in OSCC. However, features such as invasion and abnormal alteration in collagen cannot be used as predictors for HPV status. Therefore, for economically poor patients of OSCC, indirect determination of the HPV status using koilocytosis and dyskeratosis as indicators, may eliminate the need for more expensive HPV tests using PCR, as HPV-positive patients are reported to have better prognosis than HPV-negative cases.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Vidal L, Gillison ML. Human papillomavirus in HNSCC: Recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22:1125–42. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Syrjanen KJ, Pyrhonen S, Syrjanen SM. Evidence suggesting human papillomavirus (HPV) etiology for the squamous cell papilloma of the paranasal sinus. Arch Geschwulstforsch. 1983;53:77–82. [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses. 2007;90 [PMC free article] [PubMed] [Google Scholar]
- 4.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: A focus on human papilloma virus. J Dent Res. 2007;86:104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 5.Braakhuis BJ, Snijders PJ, Willem-Jan H, Keune CJ, Meijer LM, Henrique J, Ruijter-Schippers, René Leemans C, Ruud H, Brakenhoff J Natl Cancer Inst. 2004;96:16–9. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–54. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: A metaanalysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campisi G, Panzarella V, Giuliani M, Lajolo C, Di Fede O, Falaschini S, et al. Human papillomavirus: Its identity and controversial role in oral oncogenesis, premalignant and malignant lesions (review) Int J Oncol. 2007;30:813–23. [PubMed] [Google Scholar]
- 9.Syrjanen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 10.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs. Head and Neck squamous cell carcinoma biopsies: A meta-analysis (1988-2007) Ann Oncol. 2008;19:1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 11.Kumaraswamy KL, Vidhya M. Human papilloma virus and oral infections: An update. J Cancer Res Ther. 2011;7:120–7. doi: 10.4103/0973-1482.82915. [DOI] [PubMed] [Google Scholar]
- 12.Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, Lin MC. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma–molecular basis and predicting favorable outcome. Mod Pathol. 2008;21:376–86. doi: 10.1038/modpathol.3800979. [DOI] [PubMed] [Google Scholar]
- 13.Turner DO, Williams-Cocks SJ, Bullen R, Catmull J, Falk J, Martin D, et al. High-risk Human papillomavirus (HPV) screening and detection in healthy patient saliva samples: A pilot study. BMC Oral Health. 2011;11:28. doi: 10.1186/1472-6831-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anaya-Saavedra G, Ramírez-Amador V, Irigoyen-Camacho ME, García-Cuellar CM, Guido-Jiménez M, Méndez-Martínez R, et al. High association of human papillomavirus infection with oral cancer: A case-control study. Arch Med Res. 2008;39:189–97. doi: 10.1016/j.arcmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Froberg M, Johansson B, Hjerpe A, Andersson A. Human papillomavirus “reflex” testing as a screening method in cases of minor cytological abnormalities. Br J Cancer. 2008;99:563–8. doi: 10.1038/sj.bjc.6604504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 immunohistochemistry, consensus PCR HPV-DNA, and in situ hybridization. Infect Agents Cancer. 2012;7:4. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chocolatewala NM, Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: An Indian prospective. J Cancer Res Ther. 2009;5:71–7. doi: 10.4103/0973-1482.52788. [DOI] [PubMed] [Google Scholar]
- 18.Balaram P, Nalinakumari KR, Abraham E, Balan A, Hareendran NK, Bernard HU, et al. Human papillomavirus in 91 oral cancers from Indian betel quid chewers-high prevalence and multiplicity of infections. Int J Cancer. 1995;61:450–4. doi: 10.1002/ijc.2910610403. [DOI] [PubMed] [Google Scholar]
- 19.Sotler K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, et al. Detection and typing of Human Papilloma virus by E6 nested multiplex PCR. J Clin Microbiol. 2004;42:3176–84. doi: 10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggelopoulou EP, Skarlos D, Papadimitriou C, Kittas C, Troungos C. Human papilloma virus DNA detection in oral lesions in the Greek population. Anticancer Res. 1999;19:1391–5. [PubMed] [Google Scholar]
- 21.Higa M, Kinjo T, Kamiyama K, Chinen K, Iwamasa T, Arasaki A, et al. Epstein barr virus related oral squamous cell carcinoma in Okinawa, a subtropical island, in southern Japan, simultaneously infected with human papilloma virus. Oral Oncol. 2003;39:405–14. doi: 10.1016/s1368-8375(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 22.Ostwald C, Muller P, Barten M, Rutsatz K, Sonnenberg M, Milde-Langosch K, et al. Human papillomavirus DNA in oral squamous cell carcinomas and normal mucosa. J Oral Pathol Med. 1994;23:220–5. doi: 10.1111/j.1600-0714.1994.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama M, Bhawal UK, Kawamura M, Ishioka Y, Shigeishi H, Higashikawa K, et al. Human papillomavirus-16 in oral squamous cell carcinoma: Clinical correlates and 5-year survival. Br J Oral Maxillofac Surg. 2007;45:116–22. doi: 10.1016/j.bjoms.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Fornatora M, Jones AC, Kerpel S, Freedman P. Human papillomavirus-associated oral epithelial dysplasia (koilicytic dysplasia): An entity of unknown biologic potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:47–56. doi: 10.1016/s1079-2104(96)80377-5. [DOI] [PubMed] [Google Scholar]
- 25.Salvia PN, Bergo SM, Bonesso-Sabadini PI, Tagliarini EB, Hackel C, De Angelo Andrade LA. Correlation between histological criteria and human papillomavirus presence based on PCR assay in cervical biopsies. Int J Gynecol Cancer. 2004;14:126–32. doi: 10.1111/j.1048-891x.2004.014030.x. [DOI] [PubMed] [Google Scholar]
- 26.Miyahara GI, Simonato LE, Mattar NJ, Camilo DJ, Jr, Biasoli ER. Correlation between koilocytes and human papillomavirus detection by PCR in oral and oropharynx squamous cell carcinoma biopsies. Mem Inst Oswaldo Cruz. 2011;106:166–9. doi: 10.1590/s0074-02762011000200008. [DOI] [PubMed] [Google Scholar]
- 27.Branca M, Ciotti M, Giorgi C, Santini D, Di Bonito L, Costa S, et al. Matrix metalloproteinase-2 (MMP-2) and its tissue inhibitor (TIMP-2) are prognostic factors in cervical cancer, related to invasive disease but not to high-risk human papillomavirus (HPV) or virus persistence after treatment of CI. Anticancer Res. 2006;26:1543–56. [PubMed] [Google Scholar]
- 28.Garzetti GG, Ciavattini A, Lucarini G, Goteri G, Romanini C, Biagini G. The 72-kDa metalloproteinase immunostaining in cervical carcinoma: Relationship with lymph nodal involvement. Gynecol Oncol. 1996;60:271–6. doi: 10.1006/gyno.1996.0037. [DOI] [PubMed] [Google Scholar]


