Abstract
Treatment planning for oral squamous cell carcinoma (OSCC) is based on the clinical TNM (Tumor, Node and Metastasis) classification. This system operates on the assumption that small tumours without clinical spread have a better prognosis than larger tumours with metastases. However, it is a well-known fact that some tumours with the same clinical staging show different growth patterns and clinical behaviour. This makes the prognosis for patients with OSCC difficult to predict on the basis of clinical staging alone. Although many histopathological characteristics of OSCC have been identified as prognostic factors, none is believed to be completely infallible. Therefore, a great need exists for more reliable prognostic markers, which will assist in treatment decisions. It is now well documented that several molecular events of significance for tumour spread, such as gain and loss of adhesion molecules, secretion of proteolytic enzymes, increased cell proliferation and initiation of angiogenesis occur at the tumour–host interface or invasive front, where the deepest and presumably most aggressive cells reside. This review describes the various molecular events and interactions, which take place in the invasive front of the OSCC, and elucidates their role as prognostic markers.
Keywords: Head and neck, invasive front, molecular markers, squamous cell carcinoma
INTRODUCTION
Treatment planning in oral squamous cell carcinoma (OSCC) is based on the assessment of the size of the primary lesion (T), the number of regional lymph node metastases (N) and the presence of distant metastases (M).[1] This system operates on the lines of omission or commission, as small tumours are assumed to have a better prognosis and the larger tumours tend to metastasize. However, this generalization is often dangerous as it is well known that tumours with similar clinical staging have differing growth patterns and clinical behaviour.[2] Thus, the prognosis of patients with OSCC is difficult to predict accurately on the basis of clinical staging alone.
Regional lymph node involvement is a critical factor in the assessment of tumour behaviour in OSCC, because its frequency is reportedly the same for both small and large tumours.[3] Furthermore, it was illustrated in a series of 60 patients with cancer of the mouth and oropharynx that the risk of nodal metastasis is not related to tumour size but to histological grade.[4] As definitive correlation has not been established between the stage of the tumour and its histological grade,[5] grading alone may also not be an unequivocal indicator of tumour prognosis.[6] This is particularly true in the context of OSCC exhibiting varying histological features at different areas within the same tumour. It is thus important to realize that the cells at the invasive margins of OSCC and other cancers often show characteristics other than those at the superficial part of the tumour.[7,8,9] This review attempts to explain the various molecular events and interactions that take place at the invasive front of the OSCC, along with their role as prognostic markers.
INVASIVE TUMOUR FRONT AND ITS ROLE IN ORAL CARCINOGENESIS
The invasive tumour front (ITF) has been defined as the most progressed, three to six tumour cell layers or detached tumour cell groups at the advancing edge of the OSCCs.[7] The ITF frequently shows a lower degree of differentiation and a higher grade of cellular dissociation in comparison with other parts of the tumour. It is believed that integral prognostic information about the tumour's invasive and metastatic capacity[10] can be deduced from the ITF, where the deepest and presumably most aggressive cells reside.[8,9] Several studies have reported the prognostic significance of ITF in OSCCs.[7,11,12,13] Numerous molecular events of significance for tumour spread, such as gain and loss of adhesion molecules, secretion of proteolytic enzymes, increased cell proliferation, and initiation of angiogenesis occur at the tumour–host interface or invasive front.[8,9]
Changes in the extracellular matrix at the invasive front
Tumour progression in OSCC is associated with a reorganization of extracellular matrix (ECM). Components resulting from the degradation of the basement membrane may play a role in tumour cell invasion.[14] A decrease in the expression of the basement membrane components such as laminin-5 (Ln-5), collagen IV, decorin, heparan-sulfate proteoglycan along the tumour–stroma borderline has been reported.[14,15,16,17,18] However, increased expression of fibronectin, tenascin, and Ln-5γ2 chain has been reported in the associated tumour–stroma[14,15,16,17,18] and is further enhanced by the presence of fibroblasts. These findings indicate that the enhanced expression of basement membrane proteins such as fibronectin during human oral cancer progression is dependent on the epithelial–mesenchymal environment, especially the existence of fibroblasts.[19]
Laminin
Laminins are important autocrine factors produced by cancers to promote tumorigenesis. OSCCs cells have been shown to contain 32/67 kDa laminin receptors, which operate as accessory integrin molecules to stabilize tumour cells upon their adherence to laminin. After establishing this bond, the tumour begins to secrete enzymes[20,21] that cause membrane rupture by destroying type IV collagen and laminin. Tumour cells are then able to penetrate the connective tissue and start the invasion process.[20,22]
The importance of laminin-5 (Ln-5) has been demonstrated in squamous cell carcinoma (SCC).[23] Ln-5 plays a vital role in tumour migration and shows an increased expression in areas of direct tumour–stroma interactions in OSCCs.[17] Invasion of OSCCs is associated with Ln-5 synthesis, focal loss from the basement membrane, and deposition in the stroma beneath invading carcinoma cell complexes.[24] Overexpression of Ln-5γ2 chain in head and neck squamous cell carcinoma (HNSCC), particularly at the invasive front of tumours, has been reported.[18,25]
Stromal spot-like Ln-5 deposits have been shown to occur at the invasive front in the vicinity of mesenchymal cells and vessel structures. In fact, it has been demonstrated that mesenchymal cells may also express the Ln-5γ2 chain mRNA, thus contributing to the promotion of tumour cell migration as well as vessel formation in OSCC by providing and organizing promigratory Ln-5 fragments.[17,24] Moreover, stromal Ln-5 deposits show a spatial association with transforming growth factor – beta 1 (TGF-β1) as well as with matrix metalloproteinase (MMP)-14 and bone morphogenetic protein (BMP)-1.
Co-localization of Ln-5 and unspliced tenascin-C (Tn-C) at tumour cell–fibroblast interface may have a supportive role for invasive tumour behaviour.[26] Different patterns of co-localization have been implicated for its formation in vivo. A ribbon-like co-localization has been detected in subepithelial basement membranes around well-differentiated OSCC and tumour clusters.[27] Furthermore, a fibrillar Ln-5γ2/Tn-CL co-localization is seen to occur in the stroma beneath the tumour clusters.[26,27] Additionally, at the site of ruptured basement membranes, dot-like or strand like co-deposits of both molecules have been observed but their co-localizations are only rarely detectable. These different patterns may reflect a sequential modulation and reorganization of the ECM in the tumour–stroma interface as they occur in different stages of OSCC invasion.[27]
Tenascin
Tenascin (Tn) is an ECM glycoprotein that shows a site restricted expression, especially in areas of cell proliferation, cell motility, and tissue modeling at the epithelial–mesenchymal junction during embryogenesis.[28] When this protein is produced by malignant cells, there seems to be an increase in proliferation and migration, probably owing to the fact that it blocks binding of fibronectin to cells and thus has antiadhesive properties.[29] In normal tissue specimens, Tn immunoreaction appears as a linear continuous thin line purely in the basement membrane region.[30,31]
Hyperkeratosis without dysplasia shows a distinct zone of enhanced Tn immunoreactivity immediately beneath the epithelium,[30,32] which correlates with the degree of hyperplasia.[31] In dysplasia of various degrees, enhancement of the stromal Tn content can be observed, being most conspicuous in carcinoma in situ lesions.[30,31,32] In SCC, the Tn reactivity was shown to be most intense in the underlying stroma, often covering it in its entirety. Notably, the strongest immunoreaction has been noted at the advancing edges of the tumour.[30,31,32] Thus, enhanced expression of tenascin may play an important role during active phases of tumour cell proliferation and stromal changes in both premalignant and malignant lesions of the oral mucosa. Carcinoma cells may be the trigger for tenascin expression from mesenchymal cells,[30] however, tenascin may also be produced by carcinoma cells at the invasive fronts.[32]
The inclusion or omission of the alternatively spliced region in the tenascin-C (Tn-C) mRNA gives rise to the large (Tn-CL) or small (Tn-CS) variants, respectively. Tn-CL protein has been demonstrated within the whole stromal compartment, regardless of the grade of malignancy. The majority of the Tn-CL mRNA signal-bearing cells are carcinoma cells. Some stromal myofibroblasts have been shown to synthesize Tn-CL. In well-differentiated carcinomas, the Tn-CL-synthesizing carcinoma cells are localized to a single positive cell layer in the tumour–stroma interface, particularly in invasive areas. Higher grades of malignancy have been associated with a significantly increased number of Tn-CL-synthesizing carcinoma cells randomly distributed within the invading tumor areas. These cells are capable of organizing and depositing a three-dimensional Tn-CL matrix.[33]
Syndecan
Syndecans form a family of cell surface proteoglycans, which can interact with various effector molecules, such as ECM molecules and growth factors. Syndecan-1 is the most extensively studied member of the syndecan family. It is found mainly in epithelial cells, but its expression is developmentally regulated during embryonic development. It has been shown to mediate cell adhesion to several ECM molecules and to act as a coreceptor for fibroblast growth factors and potent angiogenic growth factors, which are involved in differentiation. Syndecan-1 expression is reduced during malignant transformation of various carcinomas, including HNSCCs.[34] Ro et al.,[35] in 2006, reported that a reduction of syndecan-1 expression correlated with tumour size and invasion in SCCs of the tongue. Kurokawa et al.,[36] 2006, analyzed the correlation between the intensity of syndecan-1 immunostaining and clinicopathological factors, especially the histological grade of malignancy at the deep invasive front of OSCCs. Significant differences were noted between syndecan-1 expression and prognosis, differentiation and pattern of invasion at the deep invasive front. Patients with intermediate or strong intensity for syndecan-1 had significantly better prognoses than those with negative or weak intensity. Reduced expression of syndecan-1 is considered to be a useful marker of histological malignancy at the deep tumour invasive front and may be a valuable prognostic factor.
Galectin
Galectins are a family of animal lectins that are characterized by conserved carbohydrate recognition domains and binding affinity for β-galactosidases. Galectins bind to a wide array of glycoproteins and glycolipids on the cell surface and in the ECM, including laminins, fibronectin, and integrins. By binding to these conjugates, galectins can deliver signals intracellularly as well as mediate cell–cell and cell–matrix adhesion.[37]
Galectin-1 (Gal-1), a prototype of the galectin family, performs significant functions in cancer regulation, including modulation of apoptosis, cell migration and adhesion and immune response. Overexpression of Gal-1 at the ITF is associated with poor prognosis in OSCCs.[21,38] Gal-1 has been shown to promote invasion in OSCC by upregulating MMP-9 and MMP-2, and by reorganizing the actin cytoskeleton through enhanced activation of Cdc42, a small GTPase and member of the Rho family.[21] It has been shown that Cdc42 is the major contributor to filopodia actin structure, and the activation of Cdc42 promotes cancer cell migration and invasion.[39,40] Thus, upregulation of Gal-1 may increase the number and length of filopodia on tumour cells, which in turn may promote OSCC migration and invasion[21] [Figure 1].
Figure 1.
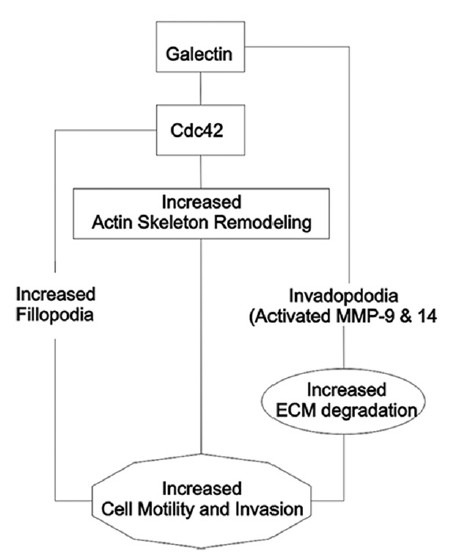
Role of galectin at the invasive front of oral cancer
Fibronectin
Fibronectin (FN) is a high-molecular mass adhesive glycoprotein synthesized and secreted by numerous cell types, such as endothelial cells of neovasculature, stromal fibro/myofibroblasts and tumour cells.[16] It is involved in numerous functions including cell adhesion, migration, homeostasis, wound healing and oncogenic transformation.[41] Its loss during carcinogenesis may allow free migration of neoplastic cells.[16] Kosmehl et al., 1999, demonstrated that expression of oncofetal fibronectins, type-III connecting segment (IIICS), fibronectin, de novo glycosylated fibronectin, and extradomain-B (ED-B) fibronectin could be demonstrated throughout the stromal compartment. ED-B fibronectin-synthesizing cells are confined to small stromal areas and to single stromal and inflammatory cells at the invasion front.[16,17]
Cortactin
Cortactin is an actin-binding protein that plays a role in cell motility and invasion by promoting actin-related protein 2/3 complex actin nucleation and by stabilizing the newly formed actin branch points.[42] Overexpression of cortactin has been reported to enhance MMP-9 secretion and promote MMP-14 surface expression in HNSCC cell lines, resulting in enhanced ECM degradation at plasma membrane structures known as invadopodia.[42,43] Indeed, cortactin overexpression has been consistently associated with more aggressive and invasive tumours, lymph node metastasis, and poor clinical outcome in HNSCC studied by Williams et al., 1993.[44]
Cortactin overexpression is seen more frequently in OSCC than in normal epithelium, and appears to be localized to the ITF. Yamada et al.,[45] 2010, reported that it has also been found more frequently in tumours with high T and N classification and significantly correlates with regional invasion [Figure 2].
Figure 2.
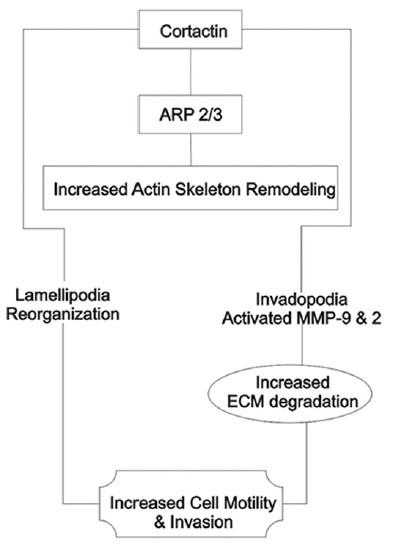
Role of cortactin at the invasive front of oral cancer
Changes in the cell adhesion molecules
ECM contains a variety of cell adhesion molecules, including integrins, laminins, selectins, cadherins, catenins, dystroglycans, and the CD44 receptor. In OSCCs, a striking accumulation of CD44s, v3, v4, v9 and a loss of E-cadherin/β-catenin has been observed at the ITF by Bánkfalvi et al., 2002.[46]
A study on immunoexpression of CD44 isoforms in OSCCs revealed downregulation of CD44v3, CD44v4-5 and CD44v6, which was found to correlate with cell differentiation, tumour grade and the pattern of neoplastic invasion.[47] Furthermore, CD44 serves as a docking site for MMP-9, which allows its retention and the resultant localization of proteolytic activity on the cell surface.[48,49]
The expression of E-cadherin at the ITF is reported to be lower than that at the central/superficial part for most OSCCs and is statistically associated with invasive front grading (IFG) score, tumour size, tumour thickness, and poor survival of OSCC patients studied by Wang et al.,[50] 2009. Loss of expression of β-catenin and γ-catenin[51] at the ITF of OSCCs has also been correlated with aggressive behaviour.
Integrin expression by cancer cells at the invasive front has been related to the mode of invasion and prognosis in OSCC. OSCC patients with higher expression levels of α3, α6a and β1 integrins have significantly better prognosis than those with lower expression levels. In addition, β1 integrin expression showed the highest correlation with clinical and pathological characteristics.[52] Triggering of the αvβ6 integrin, an adhesion molecule expressed by oral cancer cells, upregulates the production of MMP-2 and MMP-9, which cleave basement membrane proteins and thus facilitate oral cancer penetration of the epithelial basement membrane.[53] Upregulation of αvβ6 expression in OSCCs has been consistently demonstrated in vivo, where expression is often strongest at the invasive front of the tumour.[54]
Role of proteases at tumour invasive front
Once the tumour cells adhere to the ECM, proteolytic degradation of the ECM must occur to create space for invading tumour cells to move and disseminate. This process involves proteases from several families, which are classified as: serine proteases, which include the plasminogen activators, cysteine proteases, aspartic proteases, metalloproteinases, and their inhibitors.[55,56,57] Proteolytic activity is especially evident at the invasive front, demonstrating a close relationship with tumour invasiveness. Both invasiveness and activity of tumour-associated proteolytic enzymes are more dependent on the tumour microenvironment than the inherent properties of the cancer cell.[55,56,57] Role of MMPs at the invasive tumour front in HNSCC and OSCC have been summarized in Table 1.
Table 1.
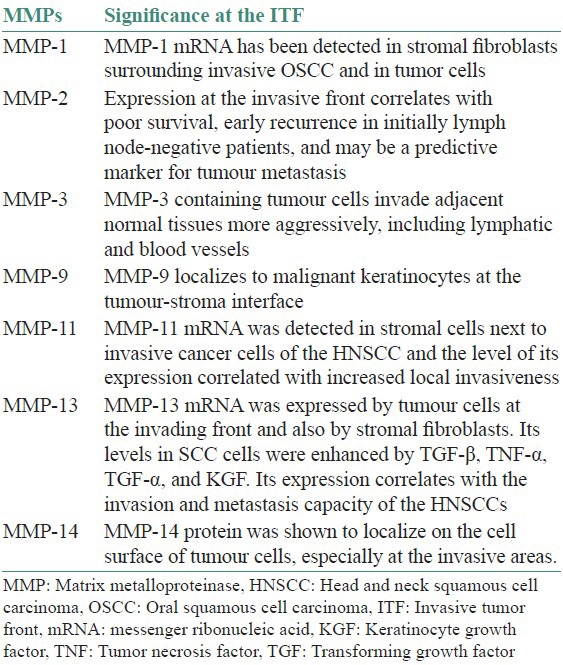
Role of tissue inhibitors of MMPs
Tissue inhibitors of MMPs (TIMPs) are naturally occurring proteins primarily responsible for inhibition of MMPs in various tissues. Thus, theoretically, TIMPs should block the proteolytic activity of MMPs, and tissue invasion by tumour cells should be reduced in their presence.[56] However, Sutinen et al.,[58] O-Charoenrat et al.[59] and other authors have demonstrated high levels of TIMPs in HNSCC correlating with poor prognosis. It probably reflects the fact that there are other cell mechanisms operating, which modulate the activities of these enzymes.[60]
Increased TIMP-1 expression has been associated with poor prognosis in HNSCC. This can be explained by the growth-promoting activity of TIMPs on a variety of cell types or the induction of TIMPs by secreted MMPs (or vice versa) from tumour–host interaction in the extracellular milieu.[59] Similarly, higher expression of TIMP-2 was reported to be the most independent factor for worse prognosis in early-stage OSCC studied by Katayama et al.,[60] 2004. This has been explained by the following mechanism: Pro-MMP-2 binds to TIMP-2, in combination with MT1-MMP on the cell surface, forming a ternary complex. Then, pro-MMP-2 in the complex is activated by adjacent MT1-MMP that is free from TIMP-2.[60] Elevated TIMP-2 in patients with OSCC significantly correlates with TNM stage, local recurrence, and poor survival, as reported by de Vicente et al.,[61] 2005. Additionally, Ondruschka et al., affirmed that the expression of TIMP-2 at the invasive front correlates with local tumour invasion in OSCC.[62]
Role of genomic changes
Noguchi et al., who studied the DNA content of cells at the ITF, considered it essential in measuring tumour aggressiveness, and suggested that this could influence disease-specific survival, especially in conjunction with clinical findings.[12] Allelic imbalance (AI)/loss of heterozygosity (LOH) have been demonstrated in OSCC.[63,64,65] In a study of LOH at 2q, 3p, and 21q by Yamamoto et al., it was shown that allelic loss in these regions is associated with the progression of OSCC and correlates with poor prognosis, particularly regarding 2q.[63] Partridge et al. observed that AI/LOH correlate with higher recurrence and less survival in OSCC patients and served as a better prognosticator than the TNM staging system.[64] Wang et al. demonstrated that cells at the ITF, central/superficial areas, and stroma show a high frequency of LOH and microsatellite instability on chromosomes 17p13 (TP53) and 9p21 (RPS6).[65] The frequency of RPS6 and TP53 aberration in the epithelial compartment, both at ITF and the center was significantly higher than in the stroma. The overall frequency of these two markers was statistically higher at the ITF than at the center/superficial part. The molecular study of these loci at the invasive front may help select patients who should undergo more aggressive therapies.
Marked cyclooxygenase (COX)-2 expressions was found in the cytoplasm of cells, both at the tumour invasive front and in the surrounding stroma and vessels, indicating a putative role in tumour invasion and development of metastases.[66]
Vascular endothelial growth factor (VEGF) subtypes C and D are associated with the risk of nodal metastases of OSCCs and are frequently upregulated at the ITF, indicating their possible role in the process of tumour invasion and development of metastases.[67]
TGFβ1 and epidermal growth factor (EGF) co-stimulation have been shown to induce phenotype transition in OSCC cells, which fulfils the criteria of epithelial–mesenchymal transition (EMT) in terms of vimentin upregulation and E-cadherin downregulation on protein level as well as cell scattering. Furthermore, cells display a strongly enhanced invasiveness and adhesion to type I–IV collagens. Phenotype transition is accompanied by an enhanced expression of Ln-5, especially its γ2 chain.[68]
The presence of p53 mutation indicates the most anaplastic fields in the invasive areas of the tumours, which may signify a poor prognosis.[69] Kato et al., measured p53 labeling index (p53-LI) at the invasive front of OSCC and have shown that patients with low-scoring p53-LI had a significantly worse prognosis than those with high-scoring p53-LI.[70] Horta et al., demonstrated both p53 and p21 (WAF1/CIP1) to be overexpressed at the ITF of lower lip SCC when compared with the tumour as a whole.[71] Consequently, the measurement of p53-LI at the invasive front of OSCC may be a significant indicator of prognosis. However, Piffkò et al. could not demonstrate the prognostic impact on OSCC with regard to p53 alterations at the ITF. However, the detection of disparate p53 aberrations between primary and second primary carcinomas in some patients may provide evidence for their independent origin, with possible impact on prevention and therapy.[72]
Role of proliferative cells at the ITF
The expression of proliferation-related molecules seems to be upregulated at the invasive zone when compared with other areas. Tumuluri et al., reported that cell proliferation at the invasive tumour front has a strong positive correlation with histological grade of malignancy in human OSCC.[73] The invasive tumour front of an OSCC is composed of tumour subpopulations with high proliferative activity.[74] This amplified and uninhibited cell proliferation at the invasive front may be one feature contributory to the invasion of OSCCs. The density of proliferating cells at the ITF has a positive relationship with prognostic and risk factors in OSCC.[73] Various antigens, such as proliferating cell nuclear antigen (PCNA),[75] Ki-67,[74,76] and argyrophilic nucleolar organizer regions (AgNORs)[9] have been used in the immunohistochemical analysis of cell proliferation.
PCNA is a 36-kDa nonhistonic nuclear polypeptide associated with the cell cycle, and is considered necessary for DNA replication and cell proliferation.[75] Kobayashi et al. and Wang et al., reported it to be elevated in both oral dysplastic lesions and OSCCs.[77,78] However, the correlation between the expression of PCNA at ITF of OSCC and the prognostic outcome is yet to be elucidated.[79]
Ki-67 is a nonhistonic nuclear protein that is present throughout all the active phases of the cell cycle (G1, S, G2, and M), but is absent from resting cells (G0), and reaches its peak concentration in phases G2 and M. Various studies have demonstrated that elevated expression of Ki-67 antigen at the ITF of OSCC indicates proliferative activity of a cell population at the ITF.[13,75,76] Immunohistochemical levels of Ki-67 antigen at the ITF have a positive relationship with clinical staging, tumour thickness, smoking status of the patient, and alcohol consumption. Importantly, this study shows that tumours that have metastasised have a significantly higher Ki-67 labeling index than tumours where distant metastasis was not detected.[73]
The nucleolar organizer regions (NORs) are loops of DNA, which transcribe ribosomal RNA. They are associated with proteins, which are considered to be required for RNA transcription. NORs can be identified indirectly by means of argyrophilia of their associated proteins (AgNORs) as nuclear dark dots. Bankfalvi and Piffko found that the number of AgNORs per nucleus is related to cellular proliferation and differentiation.[9] This finding could be useful in differentiating between normal, benign, and malignant lesions.[47] The AgNOR quantity is strictly related to the rapidity of cell proliferation: The higher the AgNOR quantity, the shorter the doubling time.[80] Standardized AgNOR analysis shows a strong independent prognostic value of cell proliferation at ITF of OSCCs.[9,81]
CONCLUSION
It is now well documented that the deepest and presumably most aggressive cells reside at the invasive front of tumours. The invasive front of the tumour frequently shows a lower degree of differentiation and a higher grade of cellular dissociation than the residual parts. Furthermore, several molecular events of importance for tumour spread, such as gain and loss of adhesion molecules, secretion of proteolytic enzymes, increased cell proliferation, and initiation of angiogenesis occur at the tumour–host interface or invasive front. The tumour budding in the invasive tumour front is the most important predictor of poor prognosis particularly in patients who receive neoadjuvant chemotherapy followed by surgery. It is well established that tumour cells at the most invasive part of the tumour differ significantly from central and superficial parts. These events which are linked to the risk of metastasis in OSCC have been elucidated by molecular events that are associated with tumour dissemination and spread. Thus, the ITF may reflect tumour prognosis better than other parts. A comprehensive understanding of the ITF of OSCCs may lead to sound prognostic assessment and appropriate treatment planning thus reducing the possibility recurrence or relapse.
The review critically analyses the molecular events at the ITF and the experimental validation that could be employed for the demonstration of this has been further emphasized.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Broder AC. Carcinoma of the mouth: Types and degrees of malignancy. Am J Roentgenol Radium Ther Nucl Med. 1927;17:90–3. [Google Scholar]
- 2.Bryne M, Nielsen K, Koppang HS, Dabelsteen E. Reproducibility of two malignancy grading systems with reportedly prognostic value for oral cancer patients. J Oral Pathol Med. 1991;20:369–72. doi: 10.1111/j.1600-0714.1991.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 3.Umeda M, Nishimatsu N, Teranobu O, Shimada K. Criteria for diagnosing lymph node metastasis from squamous cell carcinoma of oral cavity study of relationship between computed tomographic and histologic findings and outcome. J Oral Maxillofac Surg. 1998;56:585–93. doi: 10.1016/s0278-2391(98)90457-8. [DOI] [PubMed] [Google Scholar]
- 4.Giacomarra V, Tirelli G, Papanikolla L, Bussani R. Predictive factors of nodal metastasis in oral cavity and oropharynx carcinomas. Laryngoscope. 1999;109:795–9. doi: 10.1097/00005537-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Willen R, Nathanson A, Moberger G, Anneroth G. Squamous cell carcinoma of the gingiva. Histological classification and grading of malignancy. Acta Otolaryngol. 1975;79:146–54. doi: 10.3109/00016487509124667. [DOI] [PubMed] [Google Scholar]
- 6.Anneroth G, Batsakis J, Luna M. Review of literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scan J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 7.Piffko J, Bánkfalvi A, Ofner D, Rasch D, Joos U, Schmid KW. Standardized demonstration of silver-stained nucleolar organizer regions–associated proteins in archival oral squamous cell carcinomas and adjacent non-neoplastic mucosa. Mod Pathol. 1997;10:98–104. [PubMed] [Google Scholar]
- 8.Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication. Oral Dis. 1998;4:70–7. doi: 10.1111/j.1601-0825.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 9.Bankfalvi A, Piffko J. Prognostic and predictive factors in oral cancer: The role of invasive tumor front. J Oral Pathol Med. 2000;29:291–8. doi: 10.1034/j.1600-0714.2000.290701.x. [DOI] [PubMed] [Google Scholar]
- 10.Welkoborsky HJ, Gluckman JL, Jacob R, Bernauer H, Mann W. Tumor biologic prognostic parameters in T1N0M0 squamous cell carcinoma of the oral cavity. Laryngorhinootologie. 1999;78:131–8. doi: 10.1055/s-2007-996845. [DOI] [PubMed] [Google Scholar]
- 11.Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi M, Kinjyo H, Kohama GI, Nakamori K. Invasive front in oral squamous cell carcinoma: Image and flow cytometric analysis with clinicopathologic correlation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:682–7. doi: 10.1067/moe.2002.122341. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa H, Zhang M, Matsumoto S, Yamashita S, Tanaka T, Tomoyose H, et al. The relationship of the histologic grade at the deep invasive front and the expression of Ki-67 antigen and p53 protein in oral squamous cell carcinoma. J Oral Pathol Med. 2005;34:602–7. doi: 10.1111/j.1600-0714.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 14.Haas M, Berndt A, Hyckel P, Stiller KJ, Kosmehl H. Laminin-5 in diseases of the oral cavity. Mund Kiefer Gesichtschir. 2000;4:25–9. doi: 10.1007/s100060050007. [DOI] [PubMed] [Google Scholar]
- 15.Harada T, Shinohara M, Nakamura S, Oka M. An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Arch. 1994;424:257–66. doi: 10.1007/BF00194609. [DOI] [PubMed] [Google Scholar]
- 16.Kosmehl H, Berndt A, Strassburger S, Borsi L, Rousselle P, Mandel U, et al. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br J Cancer. 1999;81:1071–9. doi: 10.1038/sj.bjc.6690809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berndt A, Borsi L, Hyckel P, Kosmehl H. Fibrillary co-deposition of laminin-5 and large unspliced tenascin-C in the invasive front of oral squamous cell carcinoma in vivo and in vitro. J Cancer Res Clin Oncol. 2001;127:286–92. doi: 10.1007/s004320000205. [DOI] [PubMed] [Google Scholar]
- 18.Patel V, Aldridge K, Ensley JF, Odell E, Boyd A, Jones J, et al. Laminin-gamma 2 overexpression in head-and-neck squamous cell carcinoma. Int J Cancer. 2002;99:583–8. doi: 10.1002/ijc.10403. [DOI] [PubMed] [Google Scholar]
- 19.Kulasekara KK, Lukandu OM, Neppelberg E, Vintermyr OK, Johannessen AC, Costea DE. Cancer progression is associated with increased expression of basement membrane proteins in three-dimensional in vitro models of human oral cancer. Arch Oral Biol. 2009;54:924–31. doi: 10.1016/j.archoralbio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DF, Jiang D, Pierce AM, Wiebkin OW. Oral cancer: Role of the basement membrane in invasion. Aust Dent J. 1999;44:93–7. doi: 10.1111/j.1834-7819.1999.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu MH, Hong TM, Cheng HW, Pan SH, Liang YR, Hong H, et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res. 2009;7:311–8. doi: 10.1158/1541-7786.MCR-08-0297. [DOI] [PubMed] [Google Scholar]
- 22.Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–83. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Marinkovich MP. Laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–80. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 24.Franz M, Richter P, Geyer C, Hansen T, Acuna LD, Hyckel P, et al. Mesenchymal cells contribute to the synthesis and deposition of the laminin-5 gamma2 chain in the invasive front of oral squamous cell carcinoma. J Mol Histol. 2007;38:183–90. doi: 10.1007/s10735-007-9086-5. [DOI] [PubMed] [Google Scholar]
- 25.Lindberg P, Larsson A, Nielsen BS. Expression of plasminogen activator inhibitor-1, urokinase receptor and laminin γ2 chain is an early coordinated event in incipient oral squamous cell carcinoma. Int J Cancer. 2006;118:2948–56. doi: 10.1002/ijc.21568. [DOI] [PubMed] [Google Scholar]
- 26.Franz M, Hansen T, Borsi L, Geier C, Hyckel P, Schleier P, et al. A quantitative co-localization analysis of large unspliced tenascin-C (L) and laminin-5/gamma2-chain in basement membranes of oral squamous cell carcinoma by confocal laser scanning microscopy. J Oral Pathol Med. 2007;36:6–11. doi: 10.1111/j.1600-0714.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 27.Franz M, Hansen T, Richter P, Borsi L, Bohmer FD, Hyckel P, et al. Complex formation of the laminin-5, gamma2 chain and large unspliced tenascin-C in oral squamous cell carcinoma in vitro and in situ: Implications for sequential modulation of extracellular matrix in the invasive tumor front. Histochem Cell Biol. 2006;126:125–31. doi: 10.1007/s00418-005-0126-5. [DOI] [PubMed] [Google Scholar]
- 28.Jones PL, Jones FS. Tenascin-C in development and disease: Gene regulation and cell function. Matrix Biol. 2000;19:581–96. doi: 10.1016/s0945-053x(00)00106-2. [DOI] [PubMed] [Google Scholar]
- 29.Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61:8586–94. [PubMed] [Google Scholar]
- 30.Tiitta O, Happonen RP, Virtanen I, Luomanen M. Distribution of tenascin in oral premalignant lesions and squamous cell carcinoma. J Oral Pathol Med. 1994;23:446–50. doi: 10.1111/j.1600-0714.1994.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 31.Shrestha P, Sakamoto F, Takagi H, Yamada T, Mori M. Enhanced tenascin immunoreactivity in leukoplakia and squamous cell carcinoma of the oral cavity: An immunohistochemical study. Eur J Cancer B Oral Oncol. 1994;30:132–7. doi: 10.1016/0964-1955(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 32.Mori M, Muramatsu Y, Yamada K, Shrestha P, Takai Y. Intracellular localization of tenascin in squamous cell carcinoma of oral cavity: An immunohistochemical study. Anticancer Res. 1996;16:3075–9. [PubMed] [Google Scholar]
- 33.Hindermann W, Berndt A, Borsi L, Luo X, Hyckel P, Katenkamp D, et al. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol. 1999;189:475–80. doi: 10.1002/(SICI)1096-9896(199912)189:4<475::AID-PATH462>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Inki P, Jalkanen M. The role of syndecan-1 in malignancies. Ann Med. 1996;28:63–7. doi: 10.3109/07853899608999076. [DOI] [PubMed] [Google Scholar]
- 35.Ro Y, Muramatsu T, Shima K, Yajima Y, Shibahara T, Noma H, et al. Correlation between reduction of syndecan-1 expression and clinico-pathological parameters in squamous cell carcinoma of tongue. Int J Oral Maxillofac Surg. 2006;35:252–7. doi: 10.1016/j.ijom.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Kurokawa H, Zhang M, Matsumoto S, Yamashita Y, Tanaka T, Takamori K, et al. Reduced syndecan-1 expression is correlated with the histological grade of malignancy at the deep invasive front in oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:301–6. doi: 10.1111/j.1600-0714.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 38.Chiang WF, Liu SY, Fang LY, Lin CN, Wu MH, Chen YC, et al. Overexpression of galectin-1 at the tumor invasion front is associated with poor prognosis in early-stage oral squamous cell carcinoma. Oral Oncol. 2008;44:325–34. doi: 10.1016/j.oraloncology.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Sahai E, Marshall CJ. Rho-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruoslathi E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 42.Clark ES, Weaver AM. A new role for cortactin in invadopodia: Regulation of protease secretion. Eur J Cell Biol. 2008;87:581–90. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 44.Williams ME, Gaffey MJ, Weiss LM. Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1993;119:1238–43. doi: 10.1001/archotol.1993.01880230084013. [DOI] [PubMed] [Google Scholar]
- 45.Yamada S, Yanamoto S, Kawasaki G, Mizuno A, Nemoto TK. Overexpression of cortactin increases invasion potential in oral squamous cell carcinoma. Pathol Oncol Res. 2010;4:523–31. doi: 10.1007/s12253-009-9245-y. [DOI] [PubMed] [Google Scholar]
- 46.Bánkfalvi A, Krassort M, Buchwalow IB, Vegh A, Felszeghy E, Piffko J. Gains and losses of adhesion molecules (CD44, E-cadherin, and beta-catenin) during oral carcinogenesis and tumour progression. J Pathol. 2002;198:343–51. doi: 10.1002/path.1204. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca LM, do Carmo MA. AgNORs in hyperplasia, papilloma and oral squamous cell carcinoma. Braz Dent J. 2000;11:105–10. [PubMed] [Google Scholar]
- 48.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-Beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Zhang J, Fan M, Zhou Q, Deng H, Aisharif MJ. The expression of E-cadherin at the invasive tumor front of oral squamous cell carcinoma: Immunohistochemical and RT-PCR analysis with clinicopathological correlation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:547–54. doi: 10.1016/j.tripleo.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Lo ML, Staibano S, Pannone G, Grieco M, Mignogna MD, Cerrato A, et al. Beta- and gamma-catenin expression in oral squamous cell carcinomas. Anticancer Res. 1999;19:3817–26. [PubMed] [Google Scholar]
- 52.Ohara T, Kawashiri S, Tanaka A, Noguchi N, Kitahara H, Okamune A, et al. Integrin expression levels correlate with invasion, metastasis and prognosis of oral squamous cell carcinoma. Pathol Oncol Res. 2009;15:429–36. doi: 10.1007/s12253-008-9142-9. [DOI] [PubMed] [Google Scholar]
- 53.Thomas GJ, Jones J, Speight PM. Integrins and oral cancer. Oral Oncol. 1997;33:381–8. doi: 10.1016/s0964-1955(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 54.Hamidi S, Salo T, Kainulainen T, Epstein J, Lerner K, Larjava H. Expression of alpha (v) beta 6 integrin in oral leukoplakia. Br J Cancer. 2000;82:1433–40. doi: 10.1054/bjoc.1999.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas GJ, Jones J, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncol. 1999;35:227–33. doi: 10.1016/s1368-8375(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 56.Kurahara S, Shinohara M, Ikebe T, Nakamura S, Beppu M, Hiraki A, et al. Expression of MMPs, MT-MMP, and TIMPs in squamous cell carcinoma of the oral cavity: Correlations with tumor invasion and metastasis. Head Neck. 1999;21:627–38. doi: 10.1002/(sici)1097-0347(199910)21:7<627::aid-hed7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 57.Hadler-Olsen E, Wetting HL, Ravuri C, Omair A, Rikardsen O, Svineng G, et al. Organ specific regulation of tumour invasiveness and gelatinolytic activity at the invasive front. Eur J Cancer. 2011;47:305–15. doi: 10.1016/j.ejca.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Sutinen M, Kainulainen T, Hurskainen T, Vesterlund E, Alexander JP, Overall CM, et al. Expression of matrix metalloproteinases (MMP-1 and -2) and their inhibitors (TIMP-1, -2 and -3) in oral lichen planus, dysplasia, squamous cell carcinoma and lymph node metastasis. Br J Cancer. 1998;77:2239–45. doi: 10.1038/bjc.1998.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O-Charoenrat P, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001;127:813–20. [PubMed] [Google Scholar]
- 60.Katayama A, Bandoh N, Kishibe K, Takahara M, Ogino T, Nonaka S, et al. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Can Res. 2004;10:634–40. doi: 10.1158/1078-0432.ccr-0864-02. [DOI] [PubMed] [Google Scholar]
- 61.de Vicente JC, Fresno MF, Villalain L, Vega JA, Lopez Arranz JS. Immunoexpression and prognostic significance of TIMP-1 and -2 in oral squamous cell carcinoma. Oral Oncol. 2005;41:568–79. doi: 10.1016/j.oraloncology.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Ondruschka C, Buhtz P, Motsch C, Freigang B, Scheneider-Stock R, Roessner A, et al. Prognostic value of MMP-2, -9 and TIMP-1, -2 immunoreactive protein at the invasive front in advanced head and neck squamous cell carcinomas. Pathol Res Pract. 2002;198:509–15. doi: 10.1078/S0344-0338(04)70292-7. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto N, Mizoe Je, Numasawa H, Tsujii H, Shibahara T, Noma H. Allelic loss on chromosomes 2q, 3p and 21q: Possibly a poor prognostic factor in oral squamous cell carcinoma. Oral Oncol. 2003;39:796–805. doi: 10.1016/s1368-8375(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 64.Partridge M, Emilion G, Pateromichelakis S, A’Hern R, Lee G, Phillips E, et al. The prognostic significance of allelic imbalance at key chromosomal loci in oral cancer. Br J Cancer. 1999;79:1821–7. doi: 10.1038/sj.bjc.6990290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang XH, Fan MW, Sun ZJ, Chen XM, Wang L, Li Y. The TP53 and RPS6 alterations at the invasive tumor front, center and stroma of oral squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 2007;42:140–3. [PubMed] [Google Scholar]
- 66.Day GL, Blot WJ, Shore RE, McLaughlin JK, Austin DF, Greenberg RS, et al. Second cancers following oral and pharyngeal cancers: Role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–7. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 67.Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H. Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol. 2004;40:13–20. doi: 10.1016/s1368-8375(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 68.Richter P, Umbreit C, Franz M, Berndt A, Grimm S, Uecker A. EGF/TGFβ1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J Oral Pathol Med. 2011;40:46–54. doi: 10.1111/j.1600-0714.2010.00936.x. [DOI] [PubMed] [Google Scholar]
- 69.Tang S, Xu D, Zhou B. Analysis of P53 mutation and invasion front grading in oral squamous cell carcinomas. J Huazhong Univ Sci Technolog Med Sci. 2010;30:525–9. doi: 10.1007/s11596-010-0462-0. [DOI] [PubMed] [Google Scholar]
- 70.Kato K, Kawashiri S, Tanaka A, Noguchi N, Nakaya H, Hase T, et al. Predictive value of measuring p53 labeling index at the invasive front of oral squamous cell carcinomas. Pathol Oncol Res. 2008;14:57–61. doi: 10.1007/s12253-008-9022-3. [DOI] [PubMed] [Google Scholar]
- 71.Horta MC, de Assis LA, de Souza AF, de Araujo VC, Gomez RS, Aquiar MC. p53 and p21WAF1/CIP1 overexpression at the invasive front of lower lip squamous cell carcinoma. J Oral Pathol Med. 2007;36:88–92. doi: 10.1111/j.1600-0714.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 72.Piffkò J, Bànkfalvi A, Tory K, Fuzesi L, Bryne M, Ofner D, et al. Molecular assessment of p53 abnormalities at the invasive front of oral squamous cell carcinomas. Head Neck. 1998;20:8–15. doi: 10.1002/(sici)1097-0347(199801)20:1<8::aid-hed2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 73.Tumuluri V, Thomas GA, Fraser IS. The relationship of proliferating cell density at the invasive tumor front with prognostic and risk factors in human oral squamous cell carcinomas. J Oral Pathol Med. 2004;33:204–8. doi: 10.1111/j.0904-2512.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 74.Piffkó J, Bánkfalvi A, Ofner D, Kusch F, Bocker W, Joos U, et al. In situ assessment of cell proliferation at the invasive front of oral squamous cell carcinomas. Virchows Arch. 1996;429:229–34. doi: 10.1007/BF00198338. [DOI] [PubMed] [Google Scholar]
- 75.Myoung H, Kim MJ, Lee JH, Ok YJ, Paeng JY, Yun PY. Correlation of proliferative markers (Ki-67 and PCNA) with survival and lymph node metastasis in oral squamous cell carcinoma: A clinical and histopathological analysis of 113 patients. Int J Oral Maxillofac Surg. 2006;35:1005–10. doi: 10.1016/j.ijom.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Dissanayake U, Johnson NW, Warnakulasuriya KA. Comparison of cell proliferation in the centre and advancing fronts of oral squamous cell carcinomas using Ki-67 index. Cell Prolif. 2003;36:255–64. doi: 10.1046/j.1365-2184.2003.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kobayashi I, Matsuo K, Ozeki S, Ohishi M, Ishibashi Y, Sakai H. The proliferative activity in oral epithelial dysplasia analyzed by proliferating cell nuclear antigen immunostaining and argyrophilic nucleolar organizer region staining. Human Pathol. 1995;26:907–13. doi: 10.1016/0046-8177(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 78.Wang XH, Wang SZ, Chen XM, Li Y. The study of proliferation of cells at the invasive tumor front of squamous cell carcinoma of tongue. Zhonghua Kou Qiang Yi Xue Za Zhi. 2004;39:49–52. [PubMed] [Google Scholar]
- 79.Watanabe S, Watanabe R, Oton-Leite AF, Alencar Rde C, Oliveira JC, Leles CR, et al. Analysis of cell proliferation and pattern of invasion in oral squamous cell carcinoma. J Oral Sci. 2010;52:417–24. doi: 10.2334/josnusd.52.417. [DOI] [PubMed] [Google Scholar]
- 80.Dong H, Bertler C, Schneider E, Ritter MA. Assessment of cell proliferation by AgNOR scores and Ki-67 labeling indices and a comparison with potential doubling times. Cytometry. 1997;28:280–8. doi: 10.1002/(sici)1097-0320(19970801)28:4<280::aid-cyto2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 81.Piffko J, Bankfalvi A, Öfner D, Rasch D, Joos U, Schmid KW. Standardized AgNOR analysis of the invasive tumour front in oral squamous cell carcinomas. J Pathol. 1997;182:450–6. doi: 10.1002/(SICI)1096-9896(199708)182:4<450::AID-PATH883>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]


