Abstract
Myoepitheliomas are rare, benign neoplasms affecting predominantly parotid gland and to a lesser extent minor salivary glands. In this article we have reported three cases with different histomorphological patterns. Two cases are from oral cavity and one from sinonasal tract, a very rare location. We have discussed different histomorphological patterns of myoepitheliomas, which at times possesses a real diagnostic dilemma to a pathologist. Along with its morphology, immunohistochemical profile and clinical behavior are discussed in detail with relevant review of literature.
Keywords: Myoepithelioma, minor salivary glands, sinonasal
INTRODUCTION
Myoepithelioma of salivary glands are very rare, accounting for only 1%-1.5% of all salivary gland neoplasms. The most common site is parotid gland with a few arising in minor salivary glands of the oral cavity and palate.[1] Since 1943 when Sheldon identified them and characterized them as myoepithelioma, less than 100 cases have been reported in the literature.[2] Reports of the tumor from sinonasal tract are scarce in most reviews.[3]
These tumors exhibit complex and variable histomorphological pattern, immune antigenic expression, and clinical behavior leaving many gray areas needing further exploration and study. This has prompted us to report three cases we came across in the recent past having different histomorphology and rare sites of origin. Two arose in the oral cavity and one in the sinonasal tract, an extremely rare site. The clinicopathological profile and histopathological diagnosis are highlighted with review of relevant literature.
CASE REPORTS
A detailed clinical profile of all the three cases is shown in Table 1.
Table 1.
Detailed clinical profile of three cases
Case 1
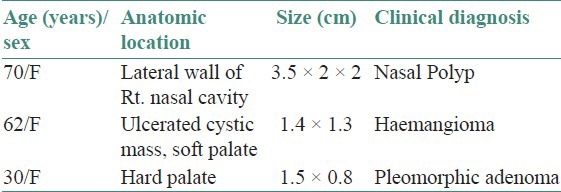
Gross: Received two grayish white masses measuring 2.5 cm × 2 cm × 2 cm and 1 cm × 0.2 cm. each. The histopathological examination showed a tumor tissue containing loose sheets of fairly uniform, plump, oval to spindle cells. These cells were having hyaline eosinophilic cytoplasm and eccentric nuclei giving them plasmacytoid appearance [Figure 1a and b].
Figure 1.
(a) A fairly uniform plump spindle shaped tumor cells arranged in sheets, without pleomorphism and mitosis [H&E stain, ×100]. (b) Polygonal cells showing distinct border, eccentric nuclei, and hyaline eosinophilic cytoplasm (H&E stain, ×400). (c) Photomicrograph showing uniform strong positivity for S100 (IHC stain, ×400). (d) Photomicrograph showing focal strong positivity for smooth muscle actin (IHC stain, ×400)
Occasional ductal elements were seen entrapped within the tumor. There was no evidence of necrosis or abnormal mitoses.
The differential diagnoses considered were plasmacytoma and myoepithelioma. Immunohistochemistry: S100-Uniform strong positivity [Figure 1c] and smooth muscle actin (SMA)-Focal strong positivity [Figure 1d].
Final diagnosis: Plasmacytoid (hyaline) myoepithelioma of sinonasal tract.
Case 2
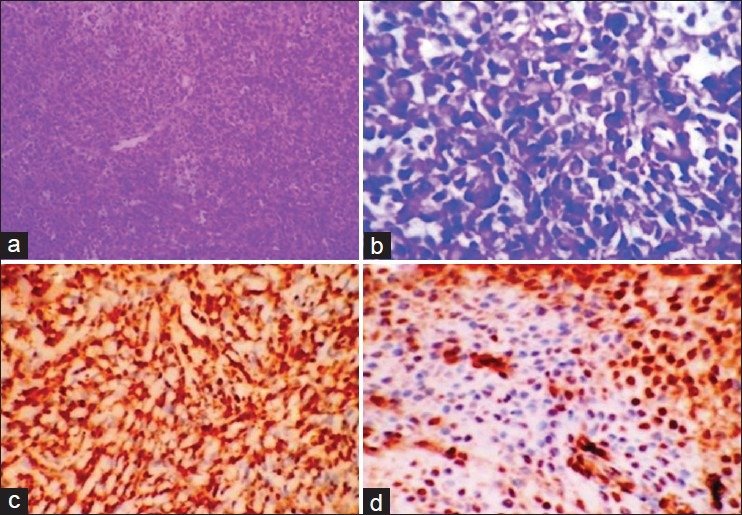
Gross: Received grayish brown ulcerated oval cystic mass, 1.5 cm in diameter. Cut surface was grayish white soft, mucoid, partially cystic. The histopathological examination showed tumor tissue separated from normal salivary gland by fibrocollagenous tissue. The tumor was made up of solid sheets of round to oval cells having moderate amount of clear cytoplasm and regular round to vesicular nuclei [Figure 2a and b]. The diagnosis made was clear cell myoepithelioma.
Figure 2.
(a) Normal salivary gland (arrow), separated from tumor by fibrocollagenous tissue. (H&E stain, ×40). (b) Tumor shows round to oval cells with clear cytoplasm and regular vesicular nuclei (H&E stain, ×400). (c) Photomicrograph showing diffuse strong positivity for S100 (IHC stain, ×100) (d) Photomicrograph showing diffuse positivity for smooth muscle actin (IHC stain, ×400)
Immunohistochemistry: S100 and SMA-positive [Figure 2c and d].
Final diagnosis: Clear cell myoepithelioma of soft palate.
Case 3
Gross: The specimen consisted of two grayish white soft tissue pieces, together measuring 1.5 cm × 1 cm.
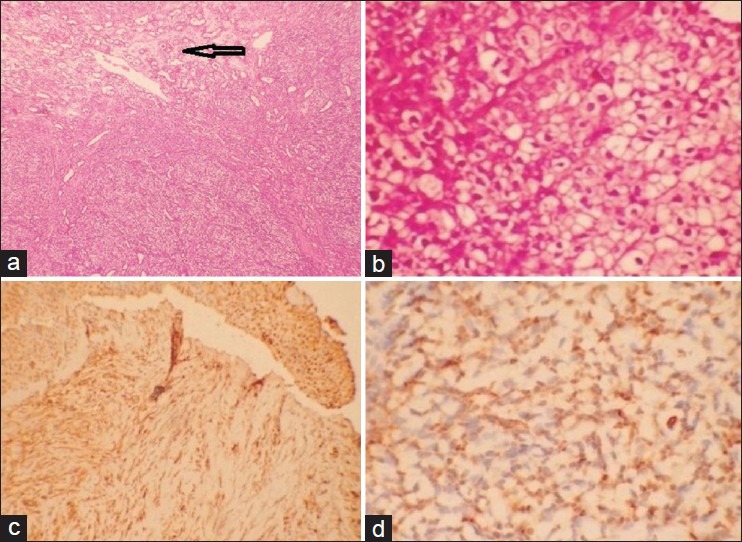
The histopathological examination showed a well-circumscribed tumor made up of anastomosing cords of epithelial cells separated by abundant intercellular hyaline material. These cells were having moderate amount of eosinophilic cytoplasm and round to oval monomorphic nuclei [Figure 3a and b]. The differential diagnoses considered were monomorphic adenoma and reticular (epithelioid)-type myoepithelioma.
Figure 3.
(a) Well-circumscribed tumor tissue consisting of anastomosing cords of epithelial-type tumor cells separated by abundant intercellular hyaline material (H&E stain, ×100. (b) The cells have moderate eosinophilic cytoplasm and round–oval monomorphic vesicular nuclei (H&E stain, ×400). (c) S100 stain showing diffuse strong positivity (IHC stain, ×100). (d) Smooth muscle actin showing diffuse strong positivity. (IHC stain, ×100)
Immunohistochemistry: S100 and SMA: strong positivity [Figure 3c and d].
Final diagnosis: Reticular (epithelioid) myoepithelioma of hard palate.
DISCUSSION
Parotid gland and to a lesser extent glands in the palate are the preferred locations for myoepitheliomas.[1,2] There are no specific clinical features that distinguish them and they usually present as slow-growing painless masses. None of our cases were in parotid gland and one was in a very rare site, namely, nasal cavity. Only three to four tumors reported in the literature earlier were at this site.[3] Although no predilection with regard to age and gender was noted in the literature, which is similar to that of pleomorphic adenoma,[2] all our cases were females in the age group of 30-70 years.
Myoepitheliomas of the parotid are usually encapsulated lesions with no ulceration. Palatal lesions may be unencapsulated as in our case 3 and ulcerated as was in our case 2.[2,4] Histomorphologically, standard text books describe four types mainly spindle cell, hyaline/plasmacytoid, clear cell, and oncocytic (a variant of spindle cell). Others identify the four types as fusiform, epithelioid, plasmacytoid, and clear cell types.[5] There may be mixtures. Pilch et al., recognize four types as spindle cell, hyaline/plasmacytoid, epithelioid, and clear cell type.[6] The epithelioid variant shows interconnected cords of cells with abundant mucoid stroma, also labeled as reticular type by Dardick.[5] Morphology of one of our cases (Case 3) was consistent with that of this type [Figure 3a and b].
All of the few sinonasal myoepitheliomas reported in the literature were spindle cell type,[3,7] whereas the one case we encountered (Case 1) was plasmacytoid/hyaline type [Figure 1a and b].
Cystic change seen in our case 2 is peculiar to the clear cell type of myoepithelioma as suggested by previous reports of cystic changes in two such tumors from the parotid[8,9] and one from the hard palate.[4]
To consider a histomorphological diagnosis of pure myoepithelioma, the epithelial component should be less than 5%-10%, and fibromyxoid stroma should be absent. The presence of fibromyxoid stroma will indicate pleomorphic adenoma. However, some consider even focal epithelial differentiation will be sufficient to label the tumor as pleomorphic adenoma.[9] Many workers believe that salivary adenomas are a part of spectrum of benign salivary gland tumors with monomorphic adenoma and myoepithelioma at the extremes with a wide range of pleomorphic adenomas in between.[2,10]
The differential diagnosis of plasmacytoid hyaline myoepithelioma includes plasmacytoma, rhabdomyoma, and oncocytoma.[2] However, the hyaline cytoplasm of myoepithelioma is distinct from the granular cytoplasm of oncocytoma. The clear cell myoepithelioma should be distinguished from deposit of renal cell carcinoma, the rare clear cell carcinoma of salivary gland, and other salivary gland tumors showing clear cell change.[4]
The spindle cell myoepithelioma may mimic nerve sheath tumors, fibrohistiocytic tumors, nodular fasciitis, or even synovial sarcoma.
Myoepithelial carcinoma can arise in pleomorphic adenoma[8] or benign myoepithelioma.[5] To exclude malignancy one may have to search for infiltrative or destructive growth pattern, cellular pleomorphism, high mitotic rate, multifocal growth pattern, necrosis, and lack of encapsulation. However, encapsulation is not a reliable criterion for palatal myoepitheliomas.[4]
WHO classification attributes a more aggressive behavior to myoepithelioma as compared with pleomorphic adenoma. Others find no difference in the behavior of the two tumors.[3] Conflicting results have been obtained on immunostains of myoepitheliomas. The most consistent were the positive staining for cytokeratin, S100, and SMA, whereas vimentin and GFAP expressions vary.[2,5] All our cases showed strong positivity for S100 and SMA, except that the stain for SMA in the plasmacytoid variant was positive only focally. Ogawa et al.[11] argue that the plasmacytoid variant lacks evidence of myoepithelial differentiation and hence should be classified as adenoma or plasmacytoid adenocarcinoma. However, others do not agree with this view and suggest that SMA expression may vary and may be negative at certain stage of differentiation of myoepithelial cells due to certain inhibitory effects of the extracellular matrix.[5] The focal strong expression of SMA in our case of plasmacytoid myoepithelioma vindicate this stand.
The recommended management of myoepithelioma is surgical excision with a margin of uninvolved tissue around. A recurrence rate of 15%-18% is observed. Recurrence is possible with incomplete resection.
We conclude that rare type of salivary gland tumors, especially those arising in unusual locations with unfamiliar morphological patterns can cause difficulty in diagnosis. Awareness about these with their varied morphological patterns and diligent use of immunohistochemistry will help in their identification. This is important as most of them can be cured by adequate excision.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Piattelli A, Fioroni M, Rubini C. Myoepithelioma of the gingiva. Report of a case. J Periodontol. 1999;70:683–7. doi: 10.1902/jop.1999.70.6.683. [DOI] [PubMed] [Google Scholar]
- 2.Politi M, Toro C, Zerman N, Mariuzzi L, Robiony M. Myoepithelioma of the parotid gland: Case report and review of literature. Oral Oncol Extra. 2005;41:104–8. [Google Scholar]
- 3.Boscolo-Rizzo P, Da Mosto MC, Morchiori C. Sinonasal spindle cell myoepithelioma. The Internet J Otorhinolanyngol. 2004;3 DOI: 10.5580/1c82. [Google Scholar]
- 4.Agarwal AK, Sethi A, Chopra S, Sareen D. Clear cell myoepithelioma of the hard palate. Internet J Head Neck Surg. 2007:1. DOI: 10.5580/9bc. [Google Scholar]
- 5.da Silveira EJ, Pereiva AL, Fontora MC, de Souza LB, de Almeida Freitas R. Myoepithelioma of minor salivary glands - An immunohistochemical analysis of four cases. Braz J Otorhinolaringol. 2006;72:528–32. doi: 10.1016/S1808-8694(15)31000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunn MA. Salivary glands. In: Pilch BZ, editor. Head and Neck Surgical Pathology. 1st ed. New York: Lippincott Williams and Wilkins; 2001. pp. 302–5. [Google Scholar]
- 7.Sayed SI, Kazi RA, Jagade MV, Palav RS, Shinde VV, Pawar PV. A rare myoepithelioma of sinonasal cavity: Case report. Cases J. 2008;1:29. doi: 10.1186/1757-1626-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astarci HM, Celik A, Sungy N, Ustun H. Cystic clear cell myoepithelioma of the parotid gland. A case report. Oral Maxillofac Surg. 2009;13:45–8. doi: 10.1007/s10006-009-0145-9. [DOI] [PubMed] [Google Scholar]
- 9.Zeynep K, Hatice E, Kursat C, Hokan G, Muzeyyen A. Cystic clear cell myoepithelioma of the parotid gland: Case report. Turkiye Kliniktevi J Med Sci. 2009;29:254–7. [Google Scholar]
- 10.Cheuk W, Chan JK. Salivary gland tumors. In: Fletcher CD, editor. Diagnostic histopathology of tumours. 3rd ed. Philadelphia: Elsevier Saunder's Company; 2007. pp. 258–60. [Google Scholar]
- 11.Ogawa Y, Kishino M, Atsumi Y, Kimoto M, Fukuda Y, Ishida T, et al. Plasmacytoid cell in salivary gland pleomorphic adenomas: Evidence of luminal cell differentiation. Virchows Arch. 2003;443:625–34. doi: 10.1007/s00428-003-0890-3. [DOI] [PubMed] [Google Scholar]


