Abstract
Heavy metals contaminate numerous freshwater streams and rivers worldwide. Previous work by this group demonstrated a relationship between the structure of hyporheic microbial communities and the fluvial deposition of heavy metals along a contamination gradient during the fall season. Seasonal variation has been documented in microbial communities in numerous terrestrial and aquatic environments, including the hyporheic zone. The current study was designed to assess whether relationships between hyporheic microbial community structure and heavy-metal contamination vary seasonally by monitoring community structure along a heavy-metal contamination gradient for more than a year. No relationship between total bacterial abundance and heavy metals was observed (R2 = 0.02, P = 0.83). However, denaturing gradient gel electrophoresis pattern analysis indicated a strong and consistent linear relationship between the difference in microbial community composition (populations present) and the difference in the heavy metal content of hyporheic sediments throughout the year (R2 = 0.58, P < 0.001). Correlations between heavy-metal contamination and the abundance of four specific phylogenetic groups (most closely related to the α, β, and γ-proteobacteria and cyanobacteria) were apparent only during the fall and early winter, when the majority of organic matter is deposited into regional streams. These seasonal data suggest that the abundance of susceptible populations responds to heavy metals primarily during seasons when the potential for growth is highest.
Large-scale mining and other activities have resulted in contamination of many aquatic environments around the world (50). Changes in the geochemical characteristics of heavy-metal-contaminated environments are well documented (for a review, see Moore and Luoma [50]). Heavy-metal contamination can reduce water quality and has been shown to harm numerous organisms (12, 45, 50, 69). Several studies have examined the effects of this type of anthropogenic contamination on aquatic macrobiota (1, 12-15, 33, 47). While heavy-metal effects on the activity and composition of microbial communities in terrestrial ecosystems have been well documented (2, 6, 7, 20, 21, 34, 43, 49, 54, 64, 72), relatively little is known about the effects on aquatic microbial communities. In a prior study by our group, heavy-metal contamination was implicated as a structuring factor for hyporheic microbial communities in streambeds (23).
The hyporheic zone is the region of heterogeneous sediments beneath and adjacent to the stream channel that is saturated with a mixture of surface and ground water (46), providing connectivity between terrestrial, groundwater, and lotic habitats. As such, this zone is an important component of lotic ecosystems (11, 26, 35, 58, 60, 68, 70, 71). The microbial communities in the hyporheic zone play important functional roles in lotic environments (18, 31, 32, 51, 52, 57, 59). For example, transformation of dissolved and particulate nutrients by hyporheic microorganisms can influence the distribution of aquatic flora and fauna and affect the productivity of vegetation in the riparian zone (4, 40, 59). Thus, changes induced in hyporheic microbial communities by anthropogenic heavy-metal contamination may be translated to higher trophic levels.
In our previous study, we described a relationship between fluvially deposited heavy metals and the structure of hyporheic microbial communities in samples taken in September 2000 (23). That study indicated that there is a direct linear relationship between the composition of hyporheic microbial communities and the level of heavy-metal contamination in the stream, that the total abundance of bacteria in the hyporheic zone is unaffected by heavy-metal contamination, and that the abundance of β-proteobacteria was negatively correlated with heavy-metal contamination, while the abundance of γ-proteobacteria was positively correlated (23).
Although that study provided an initial indication of a relationship between hyporheic microbial community structure and fluvially deposited heavy-metal contamination, all of the data were from a single time point, and we were thus unable to assess potential seasonal differences in microbial community response to metal contamination. Seasonal variation in microbial communities has been documented in numerous terrestrial (25, 38, 63, 65) and aquatic (5, 8, 20, 36, 44, 55, 66) environments, and we have previously shown that hyporheic microbial communities inhabiting streams of the western Rocky Mountains not impacted by mining exhibit seasonal patterns in diversity, abundance, and activity, which peaks during the fall (24, 33).
The purpose of the current investigation was to test the hypothesis that the influence of fluvially deposited heavy metals on hyporheic microbial community structure and population abundance should vary seasonally, such that populations susceptible to metal effects would exhibit responses primarily when the potential for microbial growth is high. The rationale is that such responses should be most apparent when there is either outgrowth of certain populations or turnover of susceptible populations in the absence of growth, so that the relative contribution of component populations to the total community would be altered. To this end, we employed denaturing gradient gel electrophoresis (DGGE) analysis, real-time quantitative PCR, and direct microscopic enumeration to monitor changes in hyporheic microbial community structure along a heavy-metal contamination gradient over the course of more than a year.
MATERIALS AND METHODS
Study sites.
The current study extends prior work at six sites along a metal contamination gradient. The sampling locations and experimental design have been described in detail elsewhere (23). Briefly, the rivers and sampling locations were chosen based on a comprehensive survey of western Montana streams (16). The six stream sites encompass a range of physical characteristics, such as average discharge, substratum type, drainage area, and sediment metal concentrations (23). Three sites were located within the Clark Fork River watershed: Silverbow Creek, a headwater tributary of the Clark Fork (third-order stream, latitude 46°06′28"N, longitude 112°48′17"W, elevation 4,912 ft); Clark Fork River at Gold Creek (fourth-order stream, latitude 46°35′26"N, longitude 112°55′40"W, elevation 4,172.8 ft); and Clark Fork River at Rock Creek (fourth-order stream, latitude 46°49′34"N, longitude 113°48′48"W, elevation 3,320 ft). The remaining streams included the Little Blackfoot River, sampled near Garrison, Mont. (third-order stream, latitude. 46°31′11"N, longitude 112°47′33"W, elevation 4,344 ft); the Big Hole River, sampled near Glen, Mont. (fourth-order stream, latitude 45°26′26"N, longitude 112°33′20"W, elevation 4,850 ft); and Rock Creek, sampled near Missoula, Mont. (third-order stream, latitude 46°43′21"N, longitude 113°40′56"W, elevation 3,519 ft).
Shallow hyporheic-zone sediments (0 to 20 cm) were gathered by hand sieving as described previously (23). Sediments from each site (with their respective resident microbial communities) were packed into sterile slotted polyvinylchloride columns and then replanted into the hyporheic zone in groups of five columns at three replicate riffles (areas of rough water due to passage over a shallow sand bar or rocks) in the streambed of each site. Three columns (one from each replicate riffle) were sampled from each site at five different time points over the course of more than a year: September 2000 (fall), November 2000 (early winter), April 2001 (spring prerunoff), July 2001 (summer postrunoff), and October 2001 (fall). The sediments were removed from the columns, immediately frozen on dry ice at the time of sampling, and then stored frozen at −70°C until analyzed. Each replicate sample was treated as independent for all statistical analyses.
Geochemical analyses.
Total recoverable metals associated with sediment samples were measured and converted to contamination index (CI) values as in the previous study (23). CI comprises a cumulative measure of the amounts of As, Cd, Cu, Pb, and Zn in sediment and is calculated as CI = Σ[(log Men/log Men at RC)/number of metals included in index), where n = As, Cd, Cu, Pb, and Zn (53). Sediment-associated heavy-metal concentrations from these six sites spanned a linear range over two orders of magnitude (23). Dissolved anions (NO2−, NO3−, and PO43−) in the pore water of each sampled riffle were measured as an estimate of the nutrients available at each site over time with the techniques described previously (23). The general physical characteristics of each stream throughout the year were obtained from the U.S. Geological Survey Montana stream-flow website (http://waterdata.usgs.gov/mt/nwis/current? type = flow).
DNA extraction.
Total bacterial community DNA was purified from 1 g of each sample for DGGE and real-time quantitative PCR analyses as described previously (23, 24).
DGGE and gel pattern analysis.
DGGE analysis of partial 16S rRNA gene sequences was performed to determine and compare the composition of the hyporheic microbial communities along the contamination gradient at each time point. PCR amplification for DGGE analysis was performed with the conserved general 16S rRNA gene primers 536fC (5′-CGC CCG CCG CGC CCC GCG CCC GGC CCG CCG CCC CCG CCC CCA GCM GCC GCG GTA ATW C-3′) and 907r (5′-CCG TCA ATT CMT TTR AGT TT-3′) (23). PCR amplicons generated from each sample were separated by DGGE with the Bio-Rad D-GENE System (Bio-Rad Laboratories, Hercules, Calif.). Detailed descriptions of PCR conditions, DGGE gel reagents, denaturant range, and running conditions are provided elsewhere (24). Briefly, a linear gradient ranging from 25% to 60% denaturant (7 M urea, 40% [wt/vol] formamide) in a 6% acrylamide gel matrix was used. Each gel was run at 60°C and 30 V for 30 min, after which the voltage was increased to 130 V for 5 h.
Following electrophoresis, gels were stained for 2 h at 37°C with a 5× concentration of SYBERGreen I (BioWhittaker Molecular Applications, Rockland, Maine). To eliminate variation between individual gels, digital images of DGGE gels were normalized based on standards included in each gel with GelCompar version 4.0 software (Applied Maths, Kortrijk, Belgium). Gel patterns were analyzed with GelCompar, and a similarity index based on the dice coefficient was calculated as described previously (24). Similarity index values were converted to dissimilarity values by subtracting them from 100 and then related to the CI by using linear modeling to determine whether the previously observed relationship between hyporheic microbial community structure and heavy-metal contamination (23) varied seasonally.
Real-time quantitative PCR.
The development of a suite of group-specific primers derived from sequences obtained from these six sites that detect organisms most closely related to the α-, β-, and γ-proteobacteria and cyanobacteria (groups I, II, III, and IV, respectively) has been described previously (23). These primers were used to quantify the abundance and distribution of each group via real-time quantitative PCR with a Bio-Rad iCycler (Bio-Rad) and the SYBERGreen I detection method. Briefly, each 25-μl PCR mixture contained a 1× concentration of a modified 10× PCR buffer (Roche Diagnostics, Mannheim, Germany) (10 mM Tris-HCl, 0.3 mM MgCl2, 50 mM KCl, pH 8.3, 1:10,000 dilution of SYBERGgreen I), 6.25 mM deoxynucleoside triphosphate mix, 1 pmol of each primer, 7% dimethyl sulfoxide, and 1.25 U of Taq polymerase (Roche Diagnostics). Details of the PCR protocols, construction of quantitative PCR standards for each phylogenetic group, and determination of PCR target copy numbers per gram of sediment have been described previously (23).
Microscopic enumeration of bacteria.
Total bacterial cells associated with 1-g samples of lyophilized sediment were enumerated as described before (33). For each sample, 30 fields of view or 400 bacterial cells were counted on each slide.
Statistical analysis of data.
Linear modeling and multivariate statistics were employed to explore the relationship between microbial community structure and fluvially deposited heavy metals and to determine whether this relationship varied seasonally. Linear modeling was used to determine if there was a direct relationship between the level of heavy-metal contamination (CI) and (i) the composition of hyporheic microbial communities, (ii) the total abundance of bacteria in the hyporheic zone, and (iii) the abundance of the monitored phylogenetic groups.
Multivariate statistics (multivariate analysis of variance and analysis of variance) were used to determine whether the abundance of the four phylogenetic groups differed among sites and between sampling dates. Specifically, multivariate analysis of variance was used to analyze phylogenetic group abundance data to determine whether the abundance of all four monitored groups changed significantly over time and if there were differences between sites. By contrast, univariate analysis of variance was used to determine if any of the individual phylogenetic groups varied significantly with respect to site or sampling date.
Differences in phylogenetic group-level abundance, with respect to site, detected by either statistical test should be due to the heavy-metal contaminants. Furthermore, both statistical procedures can test for interactions of the two factors, site and sampling date, to indicate if the abundance of all of the monitored phylogenetic groups (multivariate analysis of variance) or individual phylogenetic groups (analysis of variance) changed within each site over time. In these analyses, a significant interaction of site and sampling date (site × sampling date) indicates seasonal variation in the relationship between group-level abundance and the level of contamination present in each site.
All statistical tests were performed with the Number Cruncher Statistical System (NCSS) 2001 software (NCSS, Kaysville, Utah). A P value of 0.05 was set as the significance threshold for all Tukey-Kramer multiple-comparison tests. Values for bacterial cell abundance and abundance of individual phylogenetic groups were log transformed prior to statistical analyses.
RESULTS
Contamination index.
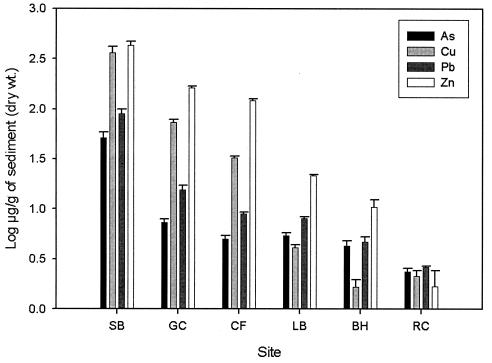
The concentrations of each metal were relatively constant over the course of the study (2001 to 2002) (Fig. 1). There was no significant variation in sediment-associated metal levels over the course of this study (FAs = 0.21, P = 0.95; FCd = 0.05, P = 0.95; FCu = 0.59, P = 0.56; FPb = 0.32, P = 0.72; FZn = 0.07, P = 0.93). Therefore, the means and standard errors of the CI values for all time points at each site were used for the linear modeling and statistical analyses described below (SB, 1.90 ± 0.06; GC, 1.22 ± 0.07; CF, 1.02 ± 0.04; LB, 0.72 ± 0.06; BH, 0.28 ± 0.03; and RC, 0.03 ± 0.02).
FIG. 1.
Means and standard errors of heavy-metal concentrations in sediment for all time points in 2000 and 2001. Log Cd concentrations were not plotted because these values were negative. Log Cd concentrations measured at each site were: Silverbow Creek (SB), 0.23 ± 0.3 μg/g; Gold Creek (GC), −0.24 ± 0.16 μg/g; Clark Fork River at Rock Creek (CF), −0.34 ± 0.01 μg/g; Little Blackfoot River (LB), −0.58 ± 0.02 μg/g; Big Hole River (BH), −0.71 ± 0.13μg/g; and Rock Creek (RC), −0.62 ± 0.08 μg/g).
Microscopic enumeration.
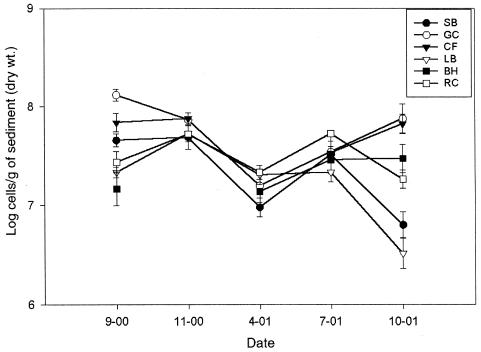
Direct microscopic enumeration was used to estimate total bacterial abundance at each site over the course of this study (Fig. 2). There were significant differences in cells/g values among the sampling locations (Fsite = 15.29, P < 0.001), among sampling dates (Fdate = 10.04, P < 0.001), and these values were significantly affected by the interaction between site and time (Fsite × time = 4.66, P < 0.001). However, a posthoc multiple-comparison test revealed that just two of the GC samples from two time points (September 2000 and October 2001) were the cause of the significant difference between sites. No significant relationship between total bacterial abundance and CI was detected during the course of the study (Table 1), nor were these values significantly correlated with any of the dissolved-anion values (P = 0.07 to 0.99) (not shown).
FIG. 2.
Means and standard errors of bacterial cell densities associated with sediments gathered at each site and time point as determined by direct microscopic enumeration. No values are reported for the Big Hole River (BH) site during the November 2000 sampling due to adverse conditions at the site. Data for September 2000 samples were reported previously (23). See the legend to Fig. 1 for abbreviations.
TABLE 1.
Correlation coefficients and significance values for linear regressions of microbial community response variables versus CI for 2000 to 2001a
| Date sampled | Log no. of bacterial cells/g
|
DGGE dissimilarity scores
|
Group I
|
Group II
|
Group III
|
Group IV
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | R2 | P | R2 | P | R2 | P | |
| September 2000 | 0.34 | 0.23 | 0.67 | <0.001 | 0.22 | 0.34 | 0.67 | 0.04 | 0.70 | 0.04 | 0.01 | 0.95 |
| November 2000 | 0.01 | 0.93 | 0.63 | <0.001 | 0.96 | <0.01 | 0.36 | 0.28 | 0.01 | 0.91 | 0.15 | 0.51 |
| April 2001 | 0.60 | 0.12 | 0.77 | <0.001 | 0.80 | 0.04 | 0.62 | 0.10 | 0.74 | 0.06 | 0.37 | 0.26 |
| July 2001 | 0.08 | 0.59 | 0.66 | <0.001 | 0.04 | 0.69 | 0.20 | 0.37 | 0.27 | 0.29 | 0.04 | 0.68 |
| October 2001 | 0.01 | 0.86 | 0.62 | <0.001 | 0.32 | 0.24 | 0.16 | 0.43 | 0.05 | 0.66 | 0.04 | 0.87 |
| 2000-2001 | 0.01 | 0.83 | 0.58 | <0.001 | 0.22 | 0.01 | 0.04 | 0.28 | 0.11 | 0.08 | 0.01 | 0.77 |
Data for September 2000 are from reference 23.
DGGE and pattern-matching analysis.
DGGE analysis was used as a means of describing and comparing the composition of microbial populations at each site during 2000 to 2001. Visual examination of DGGE patterns can be subjective, making it tenuous to relate perceived DGGE pattern differences to other environmental factors. To lessen potential subjective bias, we used the Pearson coefficient function of the GelCompar software to generate a similarity matrix comparing the composition of microbial communities within and between each site along the metal contamination gradient over the course of this seasonal study. In this analysis, patterns of bands representing populations are compared, independent of their abundance, to determine similarity (or dissimilarity) in the composition of communities independent of the abundance of the component populations.
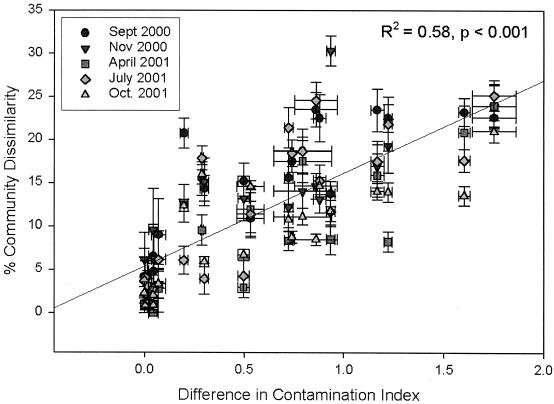
To determine whether community composition could be correlated with the measured environmental factors, the means of dissimilarity scores (100 − similarity scores) for each between-site comparison were plotted against a range of predictor variables, including the difference in dissolved NO2−, NO3−, and PO43− (data not shown) and the difference in the metal content of the sediments (Fig. 3). Correlation coefficients relating microbial community composition with dissolved-anion measurements were consistently low (R2 range = 0.36 to 0.05), with a trend towards nonsignificance (P value range = 0.01 to 0.25) (Table 2). None of the individual anions demonstrated consistent, strong, and significant relationships with microbial community composition. However, if all data points from the entire sampling time course were included in the regression, a weakly significant relationship was detected between each dissolved anion and community composition between sites (mean R2 = 0.13, mean P < 0.001) (Table 2). By contrast, a consistently significant (R2 = 0.58, P < 0.001) and strong relationship between microbial community composition and heavy metal content of the sediments was detected (Fig. 3 and Table 1). The strength of this relationship varied little throughout the course of the study (R2 range = 0.62 to 0.77) (Table 1).
FIG. 3.
Means and standard errors of differences in community composition between sites versus differences in the CI. Comparisons are separated by sampling date, as indicated in the legend. Line represents a regression of all points on the graph. Data for September 2000 samples were reported previously (23).
TABLE 2.
Correlation coefficients (R2) and significance levels of DGGE similarity scores versus each dissolved inorganic nutrienta
| Environmental factor | September 2000
|
November 2000
|
April 2001
|
July 2001
|
October 2001
|
2000-2001
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | R2 | P | R2 | P | R2 | P | |
| NO2− | 0.09 | 0.17 | 0.37 | 0.01 | 0.12 | 0.21 | 0.07 | 0.25 | 0.11 | 0.14 | 0.12 | <0.01 |
| NO3− | 0.24 | 0.03 | 0.05 | 0.36 | 0.31 | 0.03 | 0.07 | 0.23 | 0.14 | 0.06 | 0.13 | <0.01 |
| PO43− | 0.14 | 0.01 | 0.36 | 0.02 | 0.17 | 0.14 | 0.07 | 0.25 | 0.13 | 0.11 | 0.14 | <0.01 |
See Table 1, footnote a.
Quantitative PCR analyses.
Real-time quantitative PCR was used to monitor changes in the relative abundance of four specific microbial phylogenetic groups inhabiting the hyporheic zone of each site over the course of the study. The phylogenetic groups that were monitored, groups I, II, III, and IV, were most closely related to the α-, β-, and γ-proteobacteria and cyanobacteria, respectively.
Multivariate analysis of the data indicated that the quantitative PCR response variables were significantly affected by both the site and sampling date (Wilks Lamda Fsite = 1.73, P = 0.054, and Fdate = 3.69, P < 0.001). However, the interaction of the two factors, site and date, was not significant (Wilks Lambda Fsite × date = 0.94, P = 0.623). To determine which response variables (i.e., which phylogenetic groups in the hyporheic community) were responsible for these significant interactions, individual analysis of variance analyses were performed on each quantitative PCR response variable. The abundance of group I was significantly affected by the sampling site (Fsite = 3.10, P = 0.03) but not by the sampling date (Fdate = 2.44, P = 0.08). The opposite was true for group II, which was significantly affected by the sampling date (Fdate = 3.85, P = 0.017) but not by the sampling site (Fsite = 1.28, P = 0.31). Similarly, group III was significantly affected by the sampling date (Fdate = 14.13, P < 0.001) but not the site (Fsite = 1.85, P = 0.15), as was group IV (Fdate = 4.47, P = 0.009; Fsite = 1.87, P = 0.14).
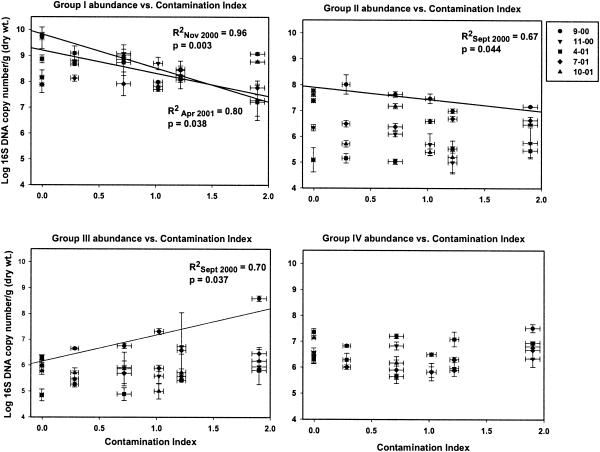
Linear modeling was used to test for a relationship between sediment metal content and the abundance of each phylogenetic group by plotting the values against the CI (Fig. 4). Of the four phylogenetic groups monitored, three exhibited a significant relationship with CI (P < 0.05) (Table 1 and Fig. 4). However, the strength and significance of these relationships were not constant over the course of the study (Table 1). By separately analyzing data for each time point, we were able to reveal for which phylogenetic groups the abundance was correlated with the CI and when that relationship was significant (Fig. 4). Group I was significantly negatively correlated with CI only during winter (November 2000) and early spring (April 2001) (R2 = 0.96, P = 0.003 and R2 = 0.80, P = 0.038, respectively) (Fig. 4). Group II had a negative correlation with CI for most of the year, but it was only significant during the fall of 2000 (September 2000) (R2 = 0.67, P = 0.044). Group III tended to be positively correlated with CI; this relationship was only significant during the fall of 2000 (R2 = 0.70, P = 0.037) but demonstrated a positively correlated trend (P < 0.10) during the early spring of 2001) (R2 = 0.74, P = 0.059). Group IV appeared to be unaffected by CI, with correlation coefficient values (R2) ranging between 0.001 and 0.37. At no time was the abundance of group IV significantly correlated with CI (P > 0.05). There were no significant linear correlations between dissolved-anion concentrations and the abundance of the monitored phylogenetic groups (data not shown).
FIG. 4.
Means and standard errors of group-level abundances as measured by quantitative PCR versus the CI. Symbols for the sampling time points: •, September 2000; ▪, November 2000; ▴, April 2001; ▾, July 2001; ♦, October 2001. Regression lines are provided only for time points that indicated significant linear relationships between the group-level abundance and the CI. Data for samples taken in September 2000 were reported previously (23).
DISCUSSION
The objective of this study was to assess the impact of seasonal changes on the previously established relationship between heavy-metal contamination and microbial community structure in the hyporheic zone of rivers in western Montana (23). To this end, we examined and compared hyporheic microbial community structure at six river sites where there was a heavy-metal contamination gradient at five time points over the course of more than a year. Such gradients can be useful for studying the effects of contaminants on natural populations (3, 19, 33, 34, 54, 64). All of the enriched metals in the contaminated sites, including the five used to calculate the CI, covary, and thus it is difficult to determine the effects of an individual heavy metal on hyporheic-zone microbial community structure (56). This potential limitation was overcome via the development of the CI, which provides a means for relating measured microbial response variables to an ecologically relevant estimate of the total metal contamination to which the hyporheic community is exposed (23).
Sediment-associated metal concentrations rather than pore water values were employed in developing the contamination index because the predominant microbial biomass in the hyporheic zone is sediment surface associated (24) and soluble metal concentrations were very low. Indeed, only the two most highly contaminated sites (SB and GC) had detectable soluble concentrations of As, Cd, Cu, and Zn, and none of the sites had detectable levels of soluble Pb in the pore water. Sediment-associated metals appear to be bioavailable (17), and previous work has described diurnal variations in dissolved-metal levels in the upper Clark Fork River (9), indicating an equilibrium between sorbed and aqueous-phase metals in this system. Thus, the use of a contamination index based on total metals associated with sediments should be a valid estimate of the magnitude of contamination to which hyporheic microbial communities are exposed. By monitoring hyporheic microbial communities along the metal contamination gradient over the course of a year and relating these changes to the contamination index, it was possible to detect seasonally dependent responses in microbial community structure.
DGGE pattern-matching analysis indicated that the fluvially deposited heavy metals impose a consistent selective pressure on the total hyporheic microbial community throughout the year (Fig. 3). The strength of this consistently significant relationship (P < 0.001) is relatively constant throughout the year, with correlation coefficient values ranging between 0.62 and 0.77. Conversely, the correlations between community composition and dissolved-nutrient levels were low and demonstrated a nonsignificant trend (Table 2). This implies that, while dissolved nutrients may have some effect on hyporheic microbial community composition, the effects of heavy metals far outweigh this influence.
The lack of correlation between total bacterial biomass and the sediment metal content was not unexpected, as similar results have been noted in soils (21, 34, 43). Although there is a potential for a relationship to exist between CI and sediment-associated bacterial biomass, it may be undetectable at the total community level due to the low resolution of direct microscopic enumeration (41). Consequently, seasonal fluctuations in relationships between CI and group-level abundances, discussed below, may not be detected in total bacterial biomass measurements. Furthermore, bacterial biomass tends to be correlated with the quantity of available organic matter in soils (42, 43, 54) and aquatic systems (22, 27-31, 66). Since the sites in the current study show little variation in sediment-associated bacterial biomass, it appears that the quantity of dissolved organic carbon available to the hyporheic zone within each site is similar (23), while the composition of the total microbial community at each site along the metal contamination gradient differs. These data indicate that increased sediment metal loads select for metal-tolerant hyporheic communities that maintain bacterial cell densities similar to those in uncontaminated streams throughout the year and demonstrate that metal-related differences in total community structure are a year-round phenomenon.
Although the relationship between CI and total community composition (presence of populations independent of their abundance) determined by DGGE was relatively constant throughout the year, population abundance measurements at the group level (with quantitative PCR) indicated that the relationships between CI and the abundance of groups I, II, and III varied seasonally. This variation may be due to seasonal changes in nutrient availability or other environmental factors affecting this predominantly heterotrophic hyporheic microbial community.
Determining precisely which environmental factors were controlling these group-level responses was beyond the scope of this project. However, previous studies in other streams have identified exogenous organic carbon as an important growth substrate for bacteria in the hyporheic zone (10, 29, 59). The majority of exogenous organic carbon that enters lotic ecosystems in this montane region arrives during the fall and early winter, primarily through deposition of leaf litter (18, 59). This coarse organic matter is processed by aquatic insects, fungi, and bacteria and is eventually entrained in the hyporheic zone as dissolved organic carbon (48, 67). Entrainment of dissolved organic carbon normally promotes increased respiration and growth in hyporheic microbial communities (10, 29, 59). Thus, elevated heavy-metal levels in the hyporheic zone appear to inhibit the growth of group I and II organisms during the fall and early winter, when conditions may otherwise favor their growth. Conversely, metals may promote the growth of group III organisms at these times, perhaps because they can tolerate metals and thus exhibit growth on available nutrient inputs not being consumed by inhibited populations. In other words, group III may be released from competition with groups I and II by the heavy-metal effects on those groups.
The phylogenetic groups monitored were defined based on analysis of partial 16S rRNA sequences from the study sites and appear to represent specific bacterial phyla and subdivisions. Group I consists of members of the α-proteobacteria, group II is made up of representatives of the β-proteobacteria, group III comprises members of the γ-proteobacteria, and the members of group IV align with known cyanobacteria. The members of groups I, II, and III, which exhibited significant correlations with sediment metal content, are predominantly heterotrophic, can form biofilms, and are commonly found in aquatic environments (39). It seems likely that these three groups represent established and active members of the hyporheic community and thus are affected by heavy-metal contaminants in this environment. Groups I and II (representing α-proteobacteria and β-proteobacteria, respectively) have not, to our knowledge, previously been monitored in metal-contaminated sediments or soils. However, there are γ-proteobacteria that are tolerant to Cd, Cu, and Zn (37, 61, 62), three metals included in the CI of the current study.
The lack of any correlation between CI and group IV (representing cyanobacteria) abundance may be due to the transient nature of this group in the hyporheic zone. Since the hyporheic zone is dark, it would not readily support active growth of photosynthetic organisms. Since group IV organisms are most likely entrained from surface water and not permanent and active members of the hyporheos, they may not exhibit toxic effects from heavy metals in the hyporheic zone.
To our knowledge, this is the first indication that the effects of fluvially deposited heavy metals on hyporheic microbial community structure vary seasonally. While the effects of sediment metal content on total microbial community composition are detectable throughout the year, the correlation with the abundances of certain specific populations or groups is most prevalent during fall and early winter. Thus, it is important to consider seasonality when designing sampling regimens to monitor the effects of fluvially deposited heavy metals and likely other perturbations on phylogenetic group abundance.
While these results are based on studying just six river sites within a heavy-metal contamination gradient, it is anticipated that similar responses would be detected in other streams experiencing similar types of heavy-metal contamination. By describing and understanding such seasonal patterns, it may be possible to design better monitoring strategies for detecting immediate as well as long-term impacts of heavy-metal contamination on the microbiota of lotic ecosystems.
Acknowledgments
We thank Nathan Stevens for assistance in gathering the total recoverable metal data.
This research was funded by EPA EPSCoR grant R-82940001-0.
REFERENCES
- 1.Axtmann, E., D. Cain, and S. Luoma. 1997. Effect of tributary inflows on the distribution of trace metals in fine-grained bed sediments and benthic insects of the Clark Fork River, Montana. Environ. Sci. Technol. 31:750-758. [Google Scholar]
- 2.Bååth, E., M. Diaz-Ravina, A. Frostegard, and C. D. Campbell. 1998. Effect of metal-rich sludge amendments on the soil microbial community. Appl. Environ. Microbiol. 64:238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bååth, E., A. Frostegard, M. Diaz-Ravina, and A. Tunlid. 1998. Microbial community-based measurements to estimate heavy metal effects in soil: the use of phospholipid fatty acid patterns and bacterial community tolerance. Ambio 27:58-62. [Google Scholar]
- 4.Barlocher, F., and J. H. Murdoch. 1989. Hyporheic biofilms: a potential food source for interstitial animals. Hydrobiologia 184:61-67. [Google Scholar]
- 5.Böckelmann, D. B. U. 2001. Description and characterization of bacteria attached to lotic organic aggregates (river snow) in the Elbe River of Germany and the South Saskatchewan River of Canada. Ph.D. dissertation. Technischen Universität Berlin, Berlin, Germany.
- 6.Bond, P. L., G. K. Druschel, and J. F. Banfield. 2000. Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl. Environ. Microbiol. 66:4962-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond, P. L., S. P. Smriga, and J. F. Banfield. 2000. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 66:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bott, T. L., and L. A. Kaplan. 1985. Bacterial biomass, metabolic state, and activity in stream sediments: relation to environmental variables and multiple assay comparisons. Appl. Environ. Microbiol. 50:508-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brick, C. M., and J. N. Moore. 1996. Diel variation of trace metals in the upper Clark Fork River, Montana. Environ. Sci. Technol. 30:1953-1960. [Google Scholar]
- 10.Brugger, A., B. Wett, I. Kolar, B. Reitner, and G. Herndl. 2001. Immobilization and bacterial utilization of dissolved organic carbon entering the riparian zone of the alpine Enns River, Austria. Aquat. Microb. Ecol. 24:129-142. [Google Scholar]
- 11.Brunke, M., and T. Gonser. 1997. The ecological significance of exchange processes between rivers and ground water. Freshwater Biol. 37:1-33. [Google Scholar]
- 12.Burton, G. J., A. Drotar, J. Lazorchak, and L. Bahls. 1987. Relationship of microbial activity and Ceriodaphnia responses to mining impacts on the Clark Fork River, Montana. Arch. Environ. Contam. Toxicol. 16:523-530. [DOI] [PubMed] [Google Scholar]
- 13.Cain, D., and S. Luoma. 1998. Metal exposures to native populations of the caddisfly Hydropsyche (Trichoptera: Hydropsychidae) determined from cytosolic and whole body metal concentrations. Hydrobiologia 386:103-117. [Google Scholar]
- 14.Cain, D. J., S. N. Luoma, and E. V. Axtmann. 1995. Influence of gut content in immature aquatic insects on assessments of environmental metal contamination. Can. J. Fish. Aquat. Sci. 52:2736-2746. [Google Scholar]
- 15.Cain, D. J., S. N. Luoma, J. L. Carter, and S. Fend. 1992. Aquatic insects as bioindicators of trace element contamination in cobble-bottom rivers and streams. Can. J. Fish. Aquat. Sci. 49:2142-2154. [Google Scholar]
- 16.Chapman, D. C. I. 1992. Assessment of injury to fish populations: Clark Fork River NPL sites, MT. Montana Department of Natural Resources report. Montana Department of Natural Resources, Missoula, Mont.
- 17.Courtney, L., and W. Clements. 2002. Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshwater Biol. 47:1766-1778. [Google Scholar]
- 18.Craft, J., J. Stanford, and M. Pusch. 2002. Microbial respiration within a floodplain aquifer of a large gravel-bed river. Freshwater Biol. 47:251-261. [Google Scholar]
- 19.Diaz-Ravina, M., and E. Bååth. 1996. Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl. Environ. Microbiol. 62:2970-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards, K. J., T. M. Gihring, and J. F. Banfield. 1999. Seasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environment. Appl. Environ. Microbiol. 65:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis, R. J., P. Morgan, A. J. Weightman, and J. C. Fry. 2003. Cultivation-dependent and -independent approaches for determining bacterial diversity in heavy-metal-contaminated soil. Appl. Environ. Microbiol. 69:3223-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espeland, E. M., S. N. Francoeur, and R. G. Wetzel. 2001. Influence of algal photosynthesis on biofilm bacterial production and associated glucosidase and xylosidase activities. Microb. Ecol. 42:524-530. [DOI] [PubMed] [Google Scholar]
- 23.Feris, K., P. Ramsey, C. Frazar, J. N. Moore, J. E. Gannon, and W. E. Holben. 2003. Differences in hyporheic-zone microbial community structure along a heavy-metal contamination gradient. Appl. Environ. Microbiol. 69:5563-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feris, K. P., P. W. Ramsey, C. F. Frazar, M. C. Rillig, J. E. Gannon, and W. E. Holben. 2003. Structure and seasonal dynamics of hyporheic zone microbial communities in free-stone rivers of the western United States. Microb. Ecol. 46:200-215. [DOI] [PubMed] [Google Scholar]
- 25.Ferris, M., and D. Ward. 1997. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 63:1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Findlay, S. 1995. Importance of surface-subsurface exchange in stream ecosystems: the hyporheic zone. Limnol. Oceanogr. 40:159-164. [Google Scholar]
- 27.Findlay, S., L. Carlough, M. Crocker, H. Gill, J. Meyer, and P. Smith. 1986. Bacterial growth on macrophyte leachate and fate of bacterial production. Limnol. Oceanogr. 31:1335-1341. [Google Scholar]
- 28.Findlay, S., P. Smith, and J. Meyer. 1986. Effect of detritus addition on metabolism of river sediment. Hydrobiologia 137:257-263. [Google Scholar]
- 29.Findlay, S., D. Strayer, C. Goumbala, and K. Gould. 1993. Metabolism of streamwater dissolved organic carbon in the shallow hyporheic zone. Limnol. Oceanogr. 38:1493-1499. [Google Scholar]
- 30.Findlay, S., J. Tank, S. Dye, H. M. Valett, P. J. Mulholland, W. H. McDowell, S. L. Johnson, S. K. Hamilton, J. Edmonds, W. K. Dodds, and W. B. Bowden. 2002. A cross-system comparison of bacterial and fungal biomass in detritus pools of headwater streams. Microb. Ecol. 43:55-66. [DOI] [PubMed] [Google Scholar]
- 31.Fischer, H., and M. Pusch. 2001. Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river. Freshwater Biol. 46:1335-1348. [Google Scholar]
- 32.Fischer, H., M. Pusch, and J. Schwoerbel. 1996. Spatial distribution and respiration of bacteria in stream-bed sediments. Arch. Hydrobiol. 137:281-300. [Google Scholar]
- 33.Frazar, C. F. 2002. Microbial community production and tolerance in heavy metals polluted sediment. Masters thesis. University of Montana, Missoula, Mont.
- 34.Frostegard, A., A. Tunlid, and E. Bååth. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm, N. B., and S. G. Fisher. 1984. Exchange between interstitial and surface water: implications for stream metabolism and nutrient cycling. Hydrobiologia 111:219-228. [Google Scholar]
- 36.Halda-Alija, L., S. P. Hendricks, and T. C. Johnston. 2001. Spatial and temporal variation of Enterobacter genotypes in sediments and the underlying hyporheic zone of an agricultural stream. Microb. Ecol. 42:286-294. [DOI] [PubMed] [Google Scholar]
- 37.Hassen, A., N. Saidi, M. Cherif, and A. Boudabous. 1998. Resistance of environmental bacteria to heavy metals. Bioresource Technol. 64:7-15. [Google Scholar]
- 38.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem, with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. WIlliams. 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams and Wilkins, Baltimore, Md.
- 40.Jones, J. B., S. G. Fisher, and N. B. Grimm. 1995. Nitrification in the hyporheic zone of a desert stream ecosystem. J. N. Am. Benthol. Soc. 14:249-258. [Google Scholar]
- 41.Kepner, R., Jr., and J. Pratt. 1994. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol. Rev. 58:603-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kieft, T. L., J. K. Fredrickson, J. P. McKinley, B. N. Bjornstad, S. A. Rawson, T. J. Phelps, F. J. Brockman, and S. M. Pfiffner. 1995. Microbiological comparisons within and across contiguous lacustrine, peleosol, and fluvial subsurface sediments. Appl. Environ. Microbiol. 61:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight, B. P., S. P. McGrath, and A. M. Chaudri. 1997. Biomass carbon measurements and substrate utilization patterns of microbial populations from soils amended with cadmium, copper, or zinc. Appl. Environ. Microbiol. 63:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leff, L. G., A. A. Leff, and M. J. Lemke. 1998. Seasonal changes in planktonic bacterial assemblages of two Ohio streams. Freshwater Biol. 39:129-134. [Google Scholar]
- 45.Lepowski, W. 1998. Arsenic crisis in Bangladesh. Chem. Eng. News 76:27-29. [Google Scholar]
- 46.Malard, F., J. V. Ward, and C. T. Robinson. 1999. An expanded perpspective of the hyporheic zone. Verh. Int. Verein. Limnol. 27:1-7. [Google Scholar]
- 47.McCormick, F., B. Hill, L. Parrish, and W. Willingham. 1994. Mining impacts on fish assemblages in the Eagle and Arkansas Rivers, Colorado. J. Freshwater Ecol. 9:175-180. [Google Scholar]
- 48.Mermillod-Blondin, F., M. Creuze des Chatelliers, P. Marmonier, and M. J. Dole-Olivier. 2000. Distribution of solutes, microbes and invertebrates in river sediments along a riffle-pool-riffle sequence. Freshwater Biol. 44:255-269. [Google Scholar]
- 49.Mills, A. L., and L. M. Mallory. 1987. The community structure of sessile heterotrophic bacteria stressed by acid mine drainage. Microb. Ecol. 14:219-232. [DOI] [PubMed] [Google Scholar]
- 50.Moore, J. N., and S. N. Luoma. 1990. Hazardous wastes from large-scale metal extraction: a case study. Environ. Sci. Technol. 24:1278-1285. [Google Scholar]
- 51.Mulholland, P. J., E. R. Marzolf, J. R. Webster, D. R. Hart, and S. P. Hendricks. 1997. Evidence that hyporheic zones increase heterotrophic metabolism and phosphorous uptake in forest streams. Limnol. Oceanogr. 42:443-451. [Google Scholar]
- 52.Naegeli, M. W., and U. Uehlinger. 1997. Contribution of the hyporheic zone to ecosystem metabolism in a prealpine gravel-bed river. J. N. Am. Benthol. Soc. 16:794-804. [Google Scholar]
- 53.Nimick, D. A., and J. N. Moore. 1991. Prediction of water-soluble metal concentrations in fluvially deposited tailings sediments, Upper Clark Fork Valley, Montana, USA. Appl. Geochem. 6:635-646. [Google Scholar]
- 54.Palmborg, C., A. Nordgren, and E. Bååth. 1998. Multivariate modelling of soil microbial variables in forest soil contaminated by heavy metals using wet chemical analyses and pyrolysis GC/MS. Soil Biol. Biochem. 30:345-357. [Google Scholar]
- 55.Pernthaler, J., F.-O. Glockner, S. Unterholzner, A. Alfreider, R. Psenner, and R. Amann. 1998. Seasonal community and population dynamics of pelagic Bacteria and Archaea in a high mountain lake. Appl. Environ. Microbiol. 64:4299-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philippi, T. E. 1993. Multiple regression: herbivory, p. 183-210. In S. M. Scheiner and J. Gurevitch (ed.), Design and analysis of ecological experiments. Chapman and Hall, New York, N.Y.
- 57.Pusch, M. 1997. Community respiration in the hyporheic zone of a riffle-pool sequence, p. 51-56. In J. Gibert, J. Mathieu, and F. Fournier (ed.), Groundwater/surface water ecotones: biological and hydrological interactions and management options. Cambridge University Press, Cambridge, United Kingdom.
- 58.Pusch, M. 1996. The metabolism of organic matter in the hyporheic zone of a mountain stream, and its spatial distribution. Hydrobiologia 323:107-118. [Google Scholar]
- 59.Pusch, M., D. Fiebig, I. Brettar, H. Eisenmann, B. K. Ellis, L. A. Kaplan, M. A. Lock, M. W. Naegeli, and W. Traunspurger. 1998. The role of microorganisms in the ecological connectivity of running waters. Freshwater Biol. 40:453-495. [Google Scholar]
- 60.Pusch, M., and J. Schwoerbel. 1994. Community respiration in hyporheic sediments of a mountain stream (Steina, Black Forest). Arch. Hydrobiol. 130:35-52. [Google Scholar]
- 61.Roane, T. M., K. L. Josephson, and I. L. Pepper. 2001. Dual-bioaugmentation strategy to enhance remediation of cocontaminated soil. Appl. Environ. Microbiol. 67:3208-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roane, T. M., and I. L. Pepper. 1999. Microbial responses to environmentally toxic cadmium. Microb. Ecol. 38:358-364. [DOI] [PubMed] [Google Scholar]
- 63.Rooney-Varga, J., R. Devereux, R. Evans, and M. Hines. 1997. Seasonal changes in the relative abundance of uncultivated sulfate-reducing bacteria in a salt marsh sediment and in the rhizosphere of Spartina alterniflora. Appl. Environ. Microbiol. 63:3895-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandaa, R. A., O. Enger, and V. L. Torsvik. 1999. Abundance and diversity of Archaea in heavy-metal-contaminated soils. Appl. Environ. Microbiol. 65:3293-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smalla, K., G. Wieland, A. Buchner, A. Zock, J. Parzy, S. Kaiser, N. Roskot, H. Heuer, and G. Berg. 2001. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smoot, J. C., and R. H. Findlay. 2001. Spatial and seasonal variation in a reservoir sedimentary microbial community as determined by phospholipid analysis. Microb. Ecol. 42:350-358. [DOI] [PubMed] [Google Scholar]
- 67.Sridhar, K. R., and F. Barlocher. 2000. Initial colonization, nutrient supply, and fungal activity on leaves decaying in streams. Appl. Environ. Microbiol. 66:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stanford, J. A., and J. V. Ward. 1993. An ecosystem perspective of alluvial rivers: connectivity and the hyporheic corridor. J. N. Am. Benthol. Soc. 12:48-60. [Google Scholar]
- 69.Traina, S. J., and V. Laperche. 1999. Contaminant bioavailability in soils, sediments, and aquatic environments. Proc. Natl. Acad. Sci. USA 96:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valett, H. M., S. G. Fisher, and E. H. Stanley. 1990. Physical and chemical characteristics of the hyporheic zone of a Sonoran Desert stream. J. N. Am. Benthol. Soc. 16:239-247. [Google Scholar]
- 71.Vervier, P., and R. J. Naiman. 1992. Spatial and temporal fluctuations of dissolved organic carbon in subsurface flow of the Stillaguamish (Washington, USA). Arch. Hydrobiol. 123:401-412. [Google Scholar]
- 72.Wenderoth, D., and H. Reber. 1999. Correlation between structural diversity and catabolic versatility of metal affected prototrophic bacteria in soil. Soil Biol. Biochem. 31:345-352. [Google Scholar]