Abstract
We report a case of recurrent chondrosarcoma of the maxilla in a 29 yr old male patient. The lesion presented as a small diffuse swelling on the left maxillary anterior region which had progressed over a period of one year. On aspiration, a chondromyxoid matrix was noted with cells arranged singly or in groups. Bi and tri-nucleation was noted with a moderate degree of nuclear pleomorphism. A diagnosis of well differentiated chondrosarcoma was made which was confirmed on histopathology. The clinicopathological findings of this case and a review of chondrosarcoma is presented and discussed.
Keywords: Chondrosarcoma, chondromyxoid matrix, maxilla
INTRODUCTION
Chondrosarcomas are uncommon malignant tumors characterized by the formation of cartilage but not bone by the tumor cells. They are rarely found in the head and neck region accounting for 5.76% of all the cases of chondrosarcomas. In contrast to osteosarcoma, chondrosarcomas usually occur in adulthood. In the head and neck region, chondrosarcomas occur more commonly in the maxilla, nasal cavity, nasal septum, and mandible.[1]
The presenting symptoms are usually nonspecific and vary depending on the location of the tumor. Histologic differentiation between this tumor and other relevant bone lesions is fairly difficult. These lesions often attain a large size before diagnosis, thus delaying treatment. Lifelong treatment is essential since this lesion shows a high incidence of local recurrence as well as regional and distant metastasis more than 2 decades later.[2] We document a case of a patient who despite being diagnosed as a well-differentiated chondrosarcoma of the maxilla showed recurrence within 2 years.
CASE REPORT
A 29-year-old male patient was referred to our department for evaluation and treatment of a swelling in the left anterior maxillary region. The patient complained of a slow growing swelling over the left cheek which had increased over a period of 1 year. The patient had noticed an intraoral swelling 25-30 days back for which he had consulted his dentist. He also noted teeth displacement over a period of 1 year. The patient denied any history of trauma and was asymptomatic.
The patient's medical and family history was unremarkable. He had the habit of chewing tobacco and areca nut since 5 years. Clinical examination revealed a diffuse swelling on the left side of the face extending from the ala of the nose up to the zygomatic region measuring approximately 3 × 3 cm. On palpation, the swelling was hard with defined borders. The overlying and adjacent skin was normal; no regional lymphadenopathy was noted.
On intraoral examination, an irregular lobulated swelling was noted involving the entire left side of the palate, which had crossed the midline [Figure 1]. Labially the swelling extended from 21 to 24 with obliteration of the labial vestibule; the teeth in this region were displaced with mobility in relation to 21 and 22. The consistency was firm to hard and the mucosa was intact.
Figure 1.
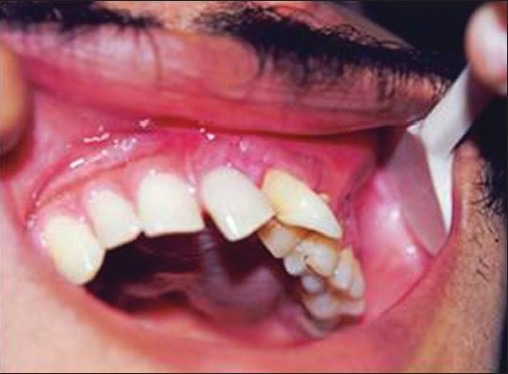
Irregular lobulated swelling on left side of the palate with displaced 21 and 22
Water's view showed a well-corticated radiolucent lesion destroying the inferior border of the maxillary sinus on the left side. A mixed lesion was seen in 22 and 23 region in continuity with the above mentioned lesion. Intraoral periapical radiographs showed displaced teeth i.r.t 21, 22, and 23. Computed tomography (CT) imaging showed an irregular soft tissue mass causing osteolytic destruction of left upper maxillary alveolus, floor and lateral wall of left maxillary sinus, medial wall of right maxillary sinus, and posteromedial part of right orbit [Figure 2].
Figure 2.
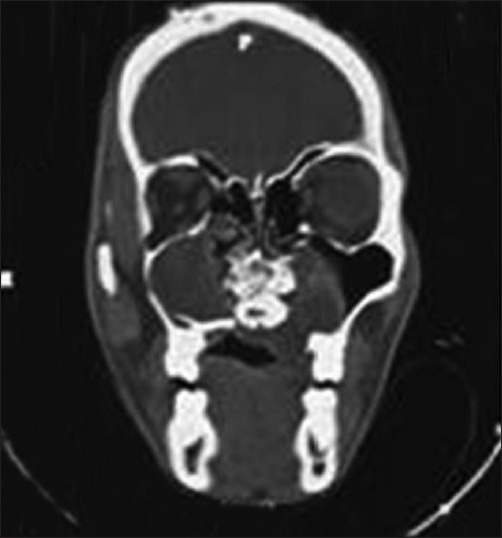
Computed tomography scan. Coronal section at posterior part of maxillary sinus showing a large mass occupying the left maxilla; break in the superior and medial margin of right maxillary sinus with suspicious contralateral contiguous involvement of left maxillary sinus
The differential diagnosis included odontogenic tumors since lesions like adenomatoid odontogenic tumor are common in this location and present a mixed appearance radiographically and therefore was considered. The clinical features such as displacement and mobility of teeth and the radiographic extent of the lesion suggested that it may be a non-odontogenic tumor of the bone.
Fine needle aspiration biopsy smears showed a matrix dominated lesion with moderate cellularity. The matrix was chondromyxoid in nature. Cells either single or in groups were embedded in the matrix. The cells showed abundant to moderate amounts of well-defined cytoplasm. The cytoplasm was eosinophilic and vacuolated. Binucleation and trinucleation was seen. Moderate degree of nuclear pleomorphism was noted. Some cells showed large nuclei with increased nuclear cytoplasmic ratio. Cartilage with cells in lacunae were not seen [Figure 3]. In view of the clinical and radiological data, a diagnosis favoring well-differentiated chondrosarcoma was offered. Following this, an incisional biopsy was carried out.
Figure 3.
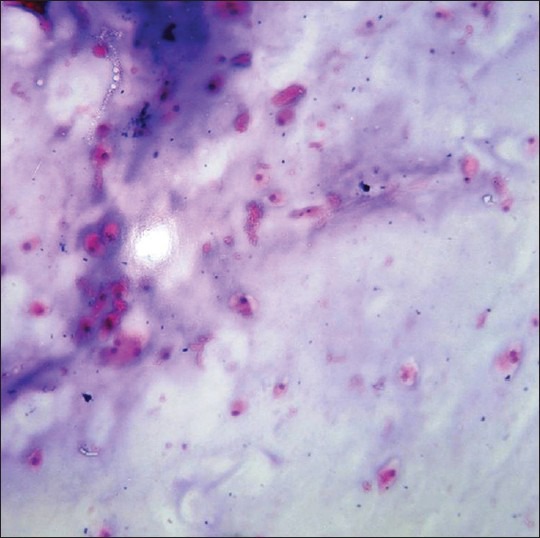
Fine needle aspiration cytology stained smear showing a matrix dominated lesion containing a chondromyxoid background with moderate cellularity (H&E stain, ×40)
MICROSCOPIC EXAMINATION
The histopathological sections showed lobules of hyaline type cartilage which was calcified at many places. The tumor periphery showed an invasive growth of neoplastic lobules into adjacent marrow spaces of ossified cartilage. The cells containing enlarged nuclei were arranged haphazardly. Increased cellularity and cells in lacunae were noted, some of which showed multi-nucleation. Mild cellular and nuclear pleomorphism was noted. The stroma was scanty. Mitotic activity was low to almost absent. Neoplastic cells did not show osteoid formation. [Figures 4–6].
Figure 4.
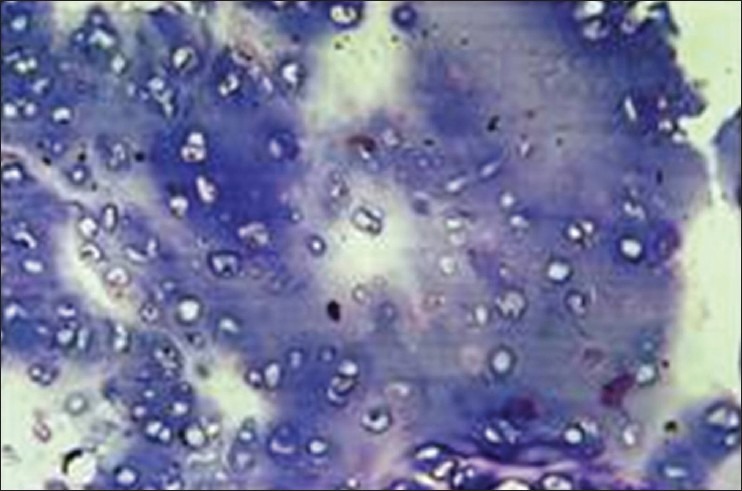
Lobules of cartilage with increased cellularity and cells in lacunae (H&E stain, ×100)
Figure 6.
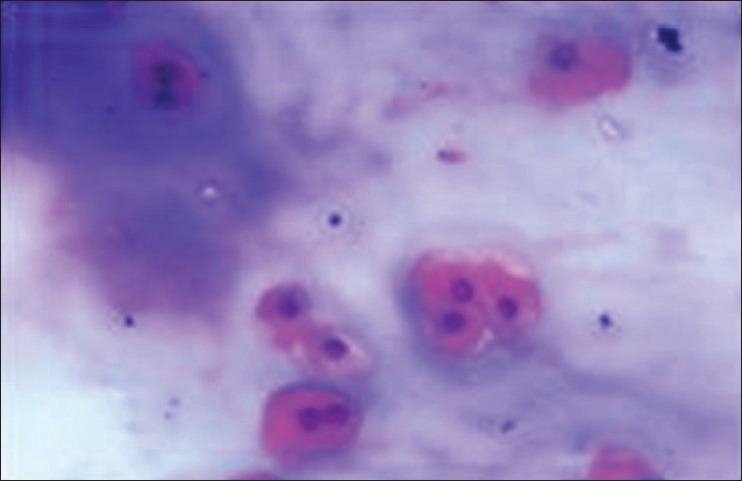
Cells both single and in groups showing binucleation and trinucleation (H&E stain, ×400)
Figure 5.
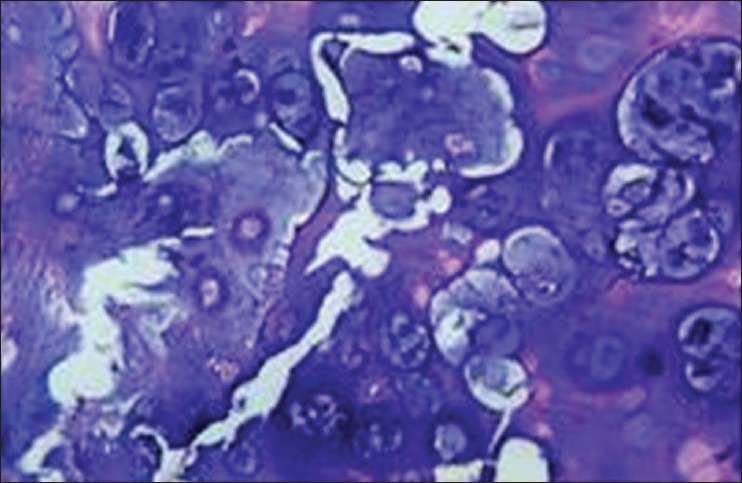
Hypercellular calcified areas with large and irregular cells and nuclei (H&E stain, ×400)
The diagnosis of well-differentiated chondrosarcoma was confirmed.
SURGERY
A midfacial degloving incision was made and a left total and right subtotal maxillectomy was performed. The tumor mass was then removed after the cartilaginous septum was cut along its length. A rectus abdominus flap was used for reconstruction. The postoperative course was uneventful.
On gross examination, the resection specimen was soft to firm in consistency; most areas were soft and gelatinous, whitish grey in color. Examination of the surgical specimen showed that the surgical margins were free of tumor. Histopathology showed similar features as that of the incisional biopsy.
The patient was kept on a regular follow-up. He was disease free for 2 years following surgical resection following which he developed a recurrence after which the patient committed suicide.
DISCUSSION
Chondrosarcoma is a malignant tumor characterized by the formation of cartilage, but not bone by the tumor cells. They comprise about 10% of all primary tumors of skeleton, but are considered to rarely involve the jaws.[1] The incidence of chondrosarcomas in the head and neck region varies from 4.2 to 6.7%.[3]
Clinical features
The Chondrosarcoma is a neoplasm that may arise in any bone but shows a predilection for the pelvic girdle, chest wall, and scapula.[4] In contrast to osteosarcoma, chondrosarcoma is uncommon in the first 2 decades of life, with most patients in the 4th-6th decade.[5] In head and neck cases, the mean patient ages range from 35 to 45 years, although patients younger than 20 years of age are reported.[6] Chondrosarcomas of the jaws do not show a sex predilection. Patients with Maffucci syndrome and Ollier disease have a 25-30% risk of developing chondrosarcoma with these patients being generally younger than those with primary chondrosarcoma.[3] Chondrosarcomas in the head and neck region have occurred in virtually every site. The most common locations include the maxilla, base of the skull, cervical vertebrae, nasal cavity, and the nasal septum. In the mandible, the most common location is the premolar-molar area although the symphysis, coronoid process, and condylar process may be involved.[3] The anterior part of the maxilla and the posterior region of the mandible are more common sites of occurrence and it has been postulated that these lesions arise from remnants of embryonic cartilage precursors from nasal and septal development in the anterior part of the maxilla and from Meckel cartilage precursors in the posterior aspect of the mandible.[7]
This case is of specific interest since there was a recurrence in a well-differentiated chondrosarcoma despite performing a left total and right subtotal maxillectomy. Our case followed the biological trend of the maxillary anterior region being more commonly involved in the jaws.
The symptoms vary from being painless to painful to headache and hearing loss with neurological problems depending on the tumor location. Jaw lesions may be associated with separation or loosening of teeth, expansion of cortical plates, and premature exfoliation of teeth.[1,4]
Radiologic features
Radiographically the tumor presents as an irregular intramedullary radiolucency causing cortical expansion and destruction. Punctate radiopacities may be present because of dystrophic calcifications or focal ossifications of cartilage. In some cases, the tumor may grow in a lobular pattern with minimal or no foci of calcification. In such instances, the lesion can appear as a multilocular radiolucency and mimic a benign process.[1] Calcification is seen in 45-80% of cases.[6] Those chondrosarcomas with an extreme myxoid component frequently lack calcification. These tumors in the base of the skull cannot be radiologically distinguished from chordomas.[3]
In tooth bearing areas, a widening of the periodontal ligament space (Garrington sign) may be seen as an early sign of chondrosarcoma.[7] In general, slow growing tumors cause reactive thickening of the cortex, whereas a more aggressive high grade neoplasm destroys the cortex and forms a soft tissue mass. The more radiolucent the tumor, the greater the likelihood that it is high grade.[8] CT and magnetic resonance imaging are quite valuable in determining the nature and extent of the lesion, but a definitive diagnosis needs to be made histologically. In this presently discussed case, CT imaging showed the exact extent of the lesion which was much more extensive than that what was visualized on conventional radiographs.
Histopathology
The histology of chondrosarcoma was first described by Lichtenstein and Jaffe.[3] It usually contains an abundant amount of hyaline type cartilage, a lobulated growth pattern with round and oval cells in lacunae with enlarged nuclei, and is hypercellular. Tumor cells with large, single, or multiple nuclei may be found. Numerous binucleated cells are seen with increased frequency in chondrosarcoma than chondroma especially in poorly differentiated tumors. Features such as foci of atypical spindle cells, myxoid degeneration of the matrix, and calcification or ossification of the matrix may be seen. Evans and coworkers have further classified chondrosarcomas into grades I, II, and III on the basis of mitotic rate, cellularity, and nuclear size. Most chondrosarcomas in the head and neck region are well-differentiated (grade I). Mitotic activity in a cartilaginous tumor is an excellent indication of malignancy, although some high grade chondrosarcomas may lack this feature. The finding of individual cell necrosis within areas not marred by calcification or degeneration is an important clue to the diagnosis of chondrosarcoma. Chondrosarcomas infiltrate between existing bone trabaculae, cause endosteal erosion and invade the cortex filling the Haversian canals and extend into the soft tissues.[3,8] The absence of osteoid and neoplastic bone in the case presented here ruled out chondroblastic variant of osteosarcoma.
Differential diagnosis
The diagnosis of chondrosarcoma is among the most difficult problems in orthopedic tumor pathology. The diagnosis of a low-grade, well-differentiated chondrosarcoma from an enchondroma based on histology alone is often difficult if not impossible especially in small biopsy specimens. Indicators of malignancy include occurrence of chondrocytes with enlarged atypical nuclei having a distinct chromatin pattern and individual cell necrosis. Islands of cartilage remain separated from the trabecular bone in enchondroma unlike chondrosarcoma where bone is infiltrated. It is imperative to assess clinical features, location, and radiographic appearance to determine whether the lesion is malignant or not. Further, the incidence of chondromas of the jaw is extremely low and therefore any symptomatic cartilage lesion in this area is best considered malignant.[6]
The differential diagnosis of a chondrosarcoma from an osteosarcoma is also a difficult distinction to make, since chondroid differentiation in osteosarcoma of jaw is more common than in other sites. In chondroblastic osteosarcomas, there is spindling of tumor cells towards the periphery of the lobules and the tumor cells surrounding the lobules are cytologically malignant with the presence of lace-like osteoid between the spindle cells. In chondrosarcoma, bone formation takes place on the framework of a preexisting cartilage matrix; whereas in osteosarcoma, it is directly by the malignant stromal cells.[3]
In Ollier's and Maffucci's syndrome the cartilage may be hypercellular with a haphazard distribution of cells and may exhibit nuclear atypia similar to chondrosarcoma. In such cases, a careful review of the clinical features and radiology will aid in a correct diagnosis.
Our case presented in a 29-year-old male with a swelling in the anterior maxilla and palate. The only complaint the patient presented with was teeth displacement, but with no other specific symptoms. Although the patient was relatively asymptomatic, there was extensive involvement which was revealed by CT images. Cytology was useful in making a diagnosis which was further confirmed on histopathology. The lobular growth pattern and the absence of osteoid and neoplastic bone in our case confirmed the diagnosis of chondrosarcoma. Although features of well-differentiated chondrosarcoma were noted and treatment included a complete resection with wide excision, a recurrence occurred within 2 years. This emphasizes the fact that importance of survival time in chondrosarcomas is minimal.
Treatment and prognosis
The prognosis for chondrosarcomas depends on the size, location, grade, and surgical respectability of the tumor. Complete resection is the most effective treatment for conventional chondrosarcomas. Maxillary and antral tumors are more difficult to eradicate and therefore are less amenable to cure. Local recurrence leads to death by direct extension of the tumor into vital structures of the head and neck.[1] Patients presenting with mandibular tumors and with tumors of better differentiated histologic grade enjoy a better survival time.[9] Mesenchymal and dedifferentiated chondrosarcomas are usually treated by chemotherapy because of their aggressive clinical course.
Recently, molecular and genomic studies are thought to be useful tools in rendering a more definite diagnosis, accurate treatment,and quality of life of the patients.[10] The 5 year survival rate for chondrosarcomas of the jaws and facial bones is reported to be 67.6%, although the importance of 5 year survival is minimal because these lesions show a wide variation in time of recurrence and metastasis; and hence lifelong follow-up is essential.[11]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Angela C. Chi. Bone Pathology. In: Neville, Damm, Allen, Bouquot, editors. Text Book of Oral and Maxillofacial Pathology. 3rd ed. Missouri: Elsevier; 2009. pp. 664–9. [Google Scholar]
- 2.Mohammadinezhad C. Chondrosarcoma of the jaw. J Craniofac Surg. 2009;20:2097–100. doi: 10.1097/SCS.0b013e3181bec5d3. [DOI] [PubMed] [Google Scholar]
- 3.Gnepp DR. 2nd ed. Philadelphia: Saunders Elsevier; 2009. Diagnostic surgical pathology of the head and neck. [Google Scholar]
- 4.Hackney FL, Aragon SB, Aufdemorte TB, Holt GR, Van Sickels JE. Chondrosarcoma of the jaws: Clinical findings, histopathology and treatment. Oral Surg Oral Med Oral Pathol. 1991;71:139–43. doi: 10.1016/0030-4220(91)90454-k. [DOI] [PubMed] [Google Scholar]
- 5.Huvos AG, Marcove RC. Chondrosarcoma in the young. A clinicopathologic analysis of 79 patients younger than 21 years of age. Am J Surg Pathol. 1987;11:930–42. doi: 10.1097/00000478-198712000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Saito K, Unni KK, Wollan PC, Lund BA. Chondrosarcoma of the jaw and facial bones. Cancer. 1995;76:1550–8. doi: 10.1002/1097-0142(19951101)76:9<1550::aid-cncr2820760909>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Robert EM, Diane S. Oral and Maxillofacial Pathology. Chicago: Quintessence Publishing Co, Inc; 2003. Rationale for diagnosis and treatment; p. 810. [Google Scholar]
- 8.Robbins, Kumar, Abbas, Fausto . Mitchell-Basic Pathology. 8th ed. Pennsylvania: Elsevier Inc, Saunders; 2007. p. 815. [Google Scholar]
- 9.Inwards CY. Update on cartilage forming tumors of the head and neck. Head Neck Pathol. 2007;1:67–74. doi: 10.1007/s12105-007-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrington GE, Collett WK. Chondrosarcoma II Chondrosarcoma of the jaws: Analysis of 37 cases. J Oral Pathol. 1988;17:12–20. doi: 10.1111/j.1600-0714.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 11.Shirato T, Onizawa K, Yamagata K, Yusa H, IIjima T, Yoshida H. Chondrosarcoma of mandibular symphysis. Oral Oncol Extra. 2006;42:247–50. [Google Scholar]


