Abstract
Inflammatory myofibroblastic tumors (IMTs) are extremely rare neoplasms with a variable natural history and biologic behavior, ranging from completely benign to malignant tumors with fatal outcome. They have no common identifiable cause, although some authors have assumed that any inflammatory stimulus may cause these pseudotumors. They are most commonly found in the lungs. Extrapulmonary sites include abdomen, retroperitoneum and extremities. IMTs rarely affect the head and neck, but the most common subsites in this region include the orbit, larynx, mouth, tonsils, parapharyngeal space, thyroid, parotid and lacrimal glands. There are few reports of inflammatory pseudotumors in the paranasal sinuses. In the maxillary sinus, the initial presenting sign is usually a nonspecific sinonasal mass, which has been growing over a period of weeks or months. On rare occasions, IMT may exhibit malignant transformation. Herein we present a rare case of pathologically proved IMT with malignant transformation which originated in the maxillary sinus of a 29-year-old male.
Keywords: Inflammatory myofibroblastic tumor, malignant transformation, maxillary sinus
INTRODUCTION
Inflammatory myofibroblastic tumors (IMTs) are clinicopathologically distinctive but biologically controversial entities. IMT is an uncommon benign neoplasm with locally aggressive behavior, but malignant change is rare.[1] The lesion is known by different synonyms, including inflammatory pseudotumor (IPT), plasma cell granuloma, plasma cell pseudotumor, myofibroblastoma, myofibroblastic proliferation and inflammatory fibrosarcoma; reflecting its uncertain histogenesis.[1,2]
The etiology and pathogenesis of IMT still remain unknown, with various reports indicating infection or an abnormal immunological reaction as possibilities.[1,3] Whether IMT is a neoplastic or a reactive process is controversial, but finding of specific genetic alteration suggests more of a neoplastic etiology than a reactive inflammatory process.[4]
The World Health Organization (WHO) defined IMT as an intermediate soft tissue tumor that is composed of spindle cells with myofibroblastic differentiation accompanied by numerous inflammatory cells, plasma cells and/or lymphocytes.[1]
IMTs are most commonly found in the lung, abdomen, retroperitoneum, and extremities; but their occurrence in the head and neck region is less common.[5] There is no age preference identified, with equal incidence in male and female patients.[3] In the head and neck, IMTs most commonly occur in the orbit. However, they have been reported in the larynx, tonsils, parapharyngeal space, nasal cavity and maxillary sinus.[3,5,6]
IMT of the maxillary sinus is very uncommon and behaves as an aggressive lesion with extensive destruction. It is easily misinterpreted as malignant epithelial or mesenchymal spindle cell neoplasm according to clinical, radiological and histopathological evidences.[3,5] The diagnosis of IMT cannot be based on clinical findings alone and supplemental histopathological and immunohistochemical studies are usually required.[4] However, it is difficult to diagnose because of its complicated pathologic characteristics.[2]
Herein, we report an extremely unusual case of IMT with malignant transformation originating from maxillary sinus in a 29-year-old male.
CASE REPORT
In January 2012, a 29-year-old apparently healthy man was admitted to the Department of Oral and Maxillofacial Pathology, with 1 month history of left facial numbness, swelling and pain radiating to the left maxillary sinus, upper teeth, and zygomatic region. Pain was severe, aggravated during the night and relieved on medication. There was no nausea, vomiting or hypopsia. Clinical examination revealed a firm, diffuse swelling involving the left midfacial area, extending into the oral cavity, accompanied by pain in the left upper teeth.
The patient's medical history was otherwise unremarkable. No enlarged cervical lymph node was palpable and laboratory tests suggested no significant abnormality.
Intraorally, both right permanent maxillary premolars and the first molar were luxated. The covering mucosa was normal. There were no signs of infection such as, erythema, purulent drainage, fluctuance or indurated swelling of the surrounding soft tissues.
An urgent computerized tomography (CT) scan revealed a homogenous soft tissue mass involving the left maxillary sinus. The mass destructed the anterior and inferior walls and extended further downward, until the alveolar bone was destroyed. Furthermore, cortex was destroyed from both buccal and palatal sides. No obvious calcification or ossification was noted [Figure 1]. The clinical and radiological impression was one of a malignant maxillary sinus tumor.
Figure 1.
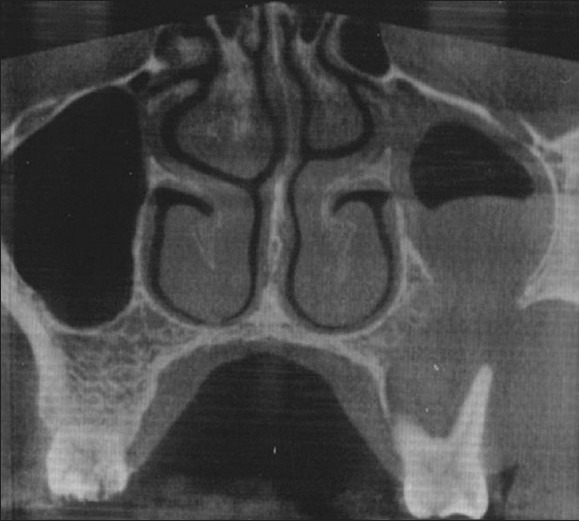
Computerized tomography scan of the left maxillary sinus shows homogenous mass with destruction of anterior and inferior walls
The patient therefore underwent an excisional biopsy via an extraoral approach under general anesthesia. The intraoperative findings revealed a highly vascular, friable lesion occupying the left maxillary sinus. The whole mass was excised.
Histopathologically, this tumor was composed of diffuse storiform proliferation of spindle-shaped fibroblasts and myofibroblasts in a predominantly fibromyxoid stroma interspersed with dense bands of collagen. This was accompanied by inflammatory cells which included lymphocytes, plasma cells, and foamy histiocytes [Figures 2 and 3]. Mitotic figures were rare, but the cellularity of the tumor together with hyperchromatism, variations in the size of nuclei and the invasive quality of the tumor, indicated malignancy.
Figure 2.
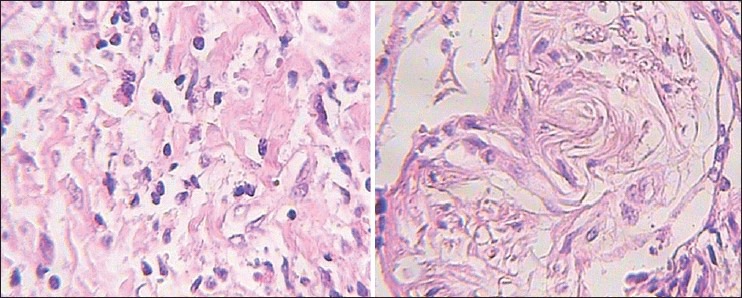
Photomicrograph reveals spindled myofibroblasts and ganglion-like cells dispersed in a myxoid background with inflammatory reaction (H&E stain, ×400)
Figure 3.
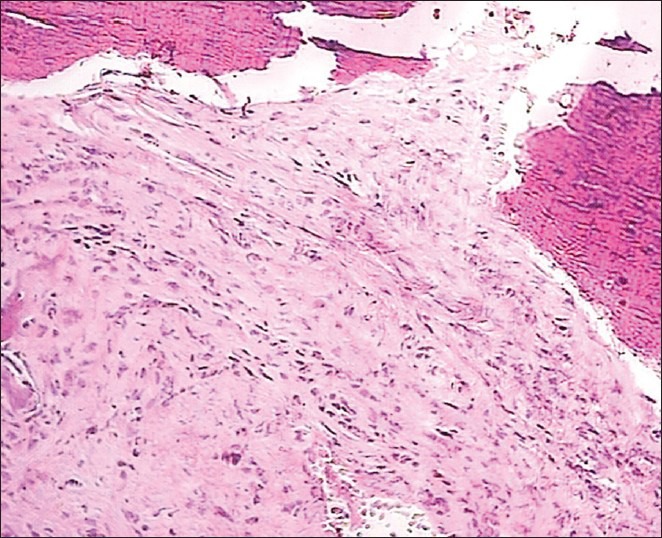
Low-power view of the inflammatory myofibroblastic tumor showing proliferation of spindle cells admixed with inflammatory cells such as lymphocytes, plasma cells, and histiocytes. The background is vascular, varying from myxoid to collagenous stroma (H&E stain, ×100)
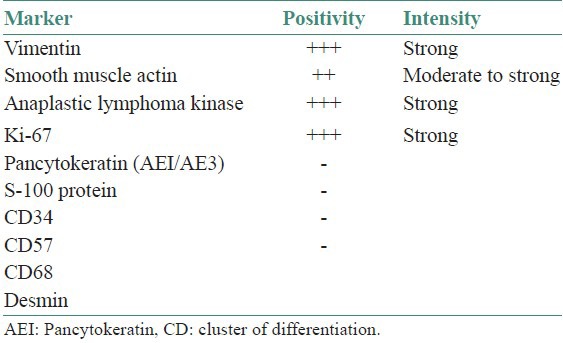
Immunohistochemical analysis revealed that the spindle-shaped cells were strongly and diffusely positive for vimentin, smooth muscle actin, anaplastic lymphoma kinase (ALK), and revealed a high proliferative index (Ki-67 = 60%) [Figure 4]. None were positive for desmin, pancytokeratin, S-100 protein, CD57, CD34, and CD68. All immunohistochemical studies that have been performed are summarized in Table 1. Special stains revealed no acid fast bacilli, other microorganisms or foreign materials.
Figure 4.

Immunohistochemical stained section showing reactivity for smooth muscle actin (IHC stain, ×200)
Table 1.
Immunohistochemical results in the present case
On the basis of the above histopathologic and immunohistochemical findings, a diagnosis of IMT with malignant transformation was established. There was no clinical evidence of local or distant metastatic spread.
The patient was referred to an oncologist for further treatment. As the patient did not keep his appointment with the dental school, no additional follow-up information is available.
DISCUSSION
IPT is a heterogeneous group of lesions, which is histologically characterized by fibroblastic and myofibroblastic proliferation with inflammatory infiltrate. IMT is a neoplastic counterpart of IPT, which shows aberrant expression of ALK and its gene translocation.[7,8] However, there is still controversy over the exact classification of IMT as a benign or malignant neoplasm.[9] In most cases, IMTs behave as benign lesions; but examples of recurrence, distant metastasis, and even malignant transformation have occurred and been reported in the literature.[9,10] Therefore, to avoid any further confusion, World Health Organization (WHO) classified IMTs as tumors of intermediate biological potential due to a tendency of local recurrence and mild risk of distant metastasis.[10]
The etiology and pathogenesis of IMT still remains unknown.[1,2] However, the opinion of this disease has changed from IPT to a true neoplasm, based on the cytogenic studies demonstrating clonal genetic alteration and chromosomal abnormality.[9] Moreover, various stimuli may act as triggers for its development; such as microorganisms, trauma and irritation. Interestingly, evidence for a role of Epstein-Barr and human herpes virus-8 (HHV8) viruses has been noted in some cases of IMTs.[8] Other organisms found in association with tumor include Mycobacteria, Actinomycetes, Nocardia, Mycoplasma, Escherichia coli, Klebsiella, Bacillus sphaericus, Pseudomonas, human immunodeficiency virus (HIV), and Helicobacter pylori.[1] In this particular case, there was no definite evidence of related infection or any traumatic episode.
IMTs are more frequent among children and young adults.[11] They may occur throughout the body, but the lung is the most common affected organ.[4] Less common extrapulmonary sites include soft tissues, mediastinum, gastrointestinal tract, pancreas, liver, spleen, lymph nodes, kidney, skin, breasts, bone, and central nervous system.[3,8] Such lesions are rarely encountered in the head and neck region and when they do, they are commonly found in the orbit; but have been reported in locations such as the oral cavity, nasal cavity, parapharyngeal space, infratemporal fossa, nasopharynx, major salivary glands, larynx, trachea, and paranasal sinuses.[5,6,7,12,13]
IMT of the maxillary sinus is very rare.[5] It usually appears as a nonspecific mass that has been growing over a period of months or years. The most frequent symptom is a local swelling and pain usually associated with at least one sinus wall destruction.[10] Unlike IMTs at other locations, systemic manifestations such as malaise, weight loss, and fever have not been observed in cases of the maxillary sinus IMTs.[3]
CT scan imaging of IMTs in the maxillary sinus might be nonspecific and often suggests either infiltrative growth with aggressive malignant potential or a granulomatous disease.[3,5] They usually appear as homogeneous soft tissue masses filling the maxillary sinuses. There is usually no evidence of calcification or central necrosis. Bone destruction has been described especially in symptomatic aggressive cases. Although root resorption associated with rapid onset malignant IMT is unexpected, root resorption was observed in our case, as the invasion was too rapid. Thus an association between the tumor extension and the root resorption was investigated in our malignant IMT.[3,5,10]
Histologically, IMT is composed of variable admixture of fascicles of myofibroblastic spindle cells with a prominent polyclonal infiltrate of numerous plasma cells, lymphocytes, and acute inflammatory cells; in a loose myxoid or edematous stroma.[1,14] Three basic histological variants of IMT, none of which appears to have a distinct association with the clinical behavior, have been recognized, namely: (1) myxoid/vascular pattern, resembling inflammatory granulation tissue; (2) nodular fasciitis-like compact spindle cell pattern with haphazard fascicular and/or storiform areas and variation of cellular density; and (3) a fibrous scar-like hypocellular pattern, of densely collagenized stroma, reminiscent of a fibrous scar.[1,2,5,8] IMTs may display more than one cellular pattern within the same tumor, often blending into one another, with one or two patterns predominating.[8] IMT in this case was subcategorized as a compact spindle cell pattern.
Immunohistochemistry is usually utilized to confirm the myofibroblastic phenotype of the tumor spindle cells, which are typically reactive to vimentin (99%), smooth muscle actin (92%), and muscle specific actin (89%). Other myogenic markers positivity is less consistent including desmin (69%), cytokeratin (36%), and CD68 (25%). IMTs are typically negative to myoglobin and S-100 protein.[7,12,13,15]
The differential diagnosis of the head and neck IMTs should include various benign and malignant spindle cell proliferations which may pose considerable histological overlap with IMTs. The main benign entities include nodular fasciitis, fibromatosis, myofibroma, myofibromatosis, solitary fibrous tumor, benign fibrous histiocytoma, Wegener's granulomatosis, neural and smooth muscle lesions; the malignant differential diagnostic entities include fibrosarcoma, myofibrosarcoma, low-grade myofibroblastic sarcoma, malignant fibrous histiocytoma, spindle cell carcinoma, sarcomatoid carcinoma, leiomyosarcoma, rhabdomyosarcoma, and malignant peripheral nerve sheath tumor (MPNST). If there was a predominant lymphocytic and/or plasmacytic component, lymphoma and plasma cell neoplasms (plasmacytoma and multiple myeloma) also should be excluded. In general, knowledge of the different histologic patterns of IMT, identification of a predominant inflammatory component and contributory immunohistochemical investigation allow distinct and definitive diagnosis.
Cytologic atypia with nuclear pleomorphism, prominent nucleoli, and increased mitotic activity, including atypical forms; are uncommon and rare features and may be associated with malignant transformation.[9,16] With regard to the present case, the supporting histology was more consistent with a diagnosis of malignant IMT, as evidenced by the presence of atypical mitotic figures and apparent cellular atypia in the spindle cell populations.
Most of the IMTs reported in head and neck region showed a benign course.[8] However, cases related to paranasal sinuses seem to be showing highly aggressive behavior, with poor response to surgery, radiotherapy, and chemotherapy showing multiple recurrences and fatal outcome.[10] Since the head and neck IMTs usually erode critical structures, surgical resection with negative margins remained the gold standard treatment for head and neck IMTs.[8] Thus, early primary radical resection is greatly recommended. Radiotherapy was used as adjunct modality of treatment for both high-risk and moderate-risk groups.[17] Radiation therapy has also been reported as being applied for unresectable and recurrent cases. Reports in which chemotherapy has been used to treat IPT are scarce.[4,5] To date, no standardized chemotherapeutic regimens had been introduced. The benefit of chemotherapy and radiation therapy may yet be unproven, but high-dose steroid therapy was effective in some cases. Early lesions that include lymphoid follicles tend to be more responsive to corticosteroids, while mature lesions with a more fibrous content respond less.[10,16]
Clinical behavior and prognosis are highly diverse, ranging from being completely benign to malignant with fatal outcome.[16] The potential for aggressive growth, recurrence, and malignant transformation is often correlative with a high degree of atypia, increased mitotic figures, multinodularity, deoxyribonucleic acid (DNA) aneuploidy, the presence of ganglion-like cells, elevated Ki-67 proliferative index, and oncogenic protein overexpression such as ALK, p53, and bcl-2.[1,9] Malignant transformation poses a serious concern, ranging from 8 to 18% in some investigations.[1,16] The local recurrence predisposition rates to the originating site of the IMT. The frequency of local recurrence is approximately 25% in extrapulmonary IMTs, including head and neck lesions.[8] IMT recurrence is common in the nasal sinus where it is always aggressively progressive.[5] Very large lesions, or those arising in areas difficult to excise completely tend to recur, with a potential for metastatic spread in rare instances.[10] According to the World Health Organization, rare cases (<5%) also metastasize. Pulmonary and brain metastasis were reported.[17] Strict and careful follow-up of these cases are mandatory as its behavior is unpredictable. Patients must be reviewed once in 1-2 months for the first 2 years; once in 2-3 months for the following year, and once in 6 months for the 4th and 5th year.[3,8,17]
ACKNOWLEDGEMENT
This study was supported by fund received from research deputy of Mashhad University of Medical Sciences.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: Comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–20. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 2.Fisher C. Myofibroblastic malignancies. Adv Anat Pathol. 2004;11:190–201. doi: 10.1097/01.pap.0000131773.16130.aa. [DOI] [PubMed] [Google Scholar]
- 3.Ushio M, Takeuchi N, Kikuchi S, Kaga K. Inflammatory pseudotumor of the paranasal sinuses: A case report. Auris Nasus Larynx. 2007;34:533–6. doi: 10.1016/j.anl.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro AC, Joshi VM, Funkhouser WK, Mukherji SK. Inflammatory myofibroblastic tumor involving the pterygopalatine fossa. Am J Neuroradiol. 2001;22:518–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Sindi K, Al-Shehabi MH, Al-Khalifa SA. Inflammatory myofibroblastic tumor of paranasal sinuses. Saudi Med J. 2007;28:623–7. [PubMed] [Google Scholar]
- 6.Cho KJ, Lee DH, Jung SH, Kim JH. A case of an inflammatory myofibroblastic tumor of the mastoid presenting with chronic suppurative otitis media. Auris Nasus Larynx. 2007;34:523–6. doi: 10.1016/j.anl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Van Weert S, Manni JJ, Driessen A. Inflammatory myofibroblastic tumor of the parotid gland: Case report and review of the literature. Acta Otolaryngol. 2005;125:433–7. doi: 10.1080/00016480410025225. [DOI] [PubMed] [Google Scholar]
- 8.Saiji E, Guillou L. Fibroblastic and myofibroblastic tumors of the head and neck. Ann Pathol. 2009;29:335–46. doi: 10.1016/j.annpat.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Chun YS, Wang L, Nascimento AG, Moir CR, Rodeberg DA. Pediatric inflammatory myofibroblastic tumor: Anaplastic lymphoma kinase (ALK) expression and prognosis. Pediatr Blood Cancer. 2005;45:796–801. doi: 10.1002/pbc.20294. [DOI] [PubMed] [Google Scholar]
- 10.Gale N, Zidar N, Podboj J, Volavsek M, Luzar B. Inflammatory myofibroblastic tumour of paranasal sinuses with fatal outcome: Reactive lesion or tumour? J Clin Pathol. 2003;56:715–7. doi: 10.1136/jcp.56.9.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein AM, Schoem SR, Altman A, Eisenfeld L. Inflammatory myofibroblastic tumor in the neonate: A case report. Otolaryngol Head Neck Surg. 2003;128:145–7. doi: 10.1067/mhn.2003.45. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues M, Taylor RJ, Sun CC, Wolf JS. Inflammatory myofibroblastic tumor of the larynx in a 2-year-old male. ORL J Otorhinolaryngol Relat Spec. 2005;67:101–5. doi: 10.1159/000084997. [DOI] [PubMed] [Google Scholar]
- 13.Gleizal A, Ranchere-Vince D, Abou-Chebel N, Nimeskern N, Beziat JL. Inflammatory myofibroblastic pseudotumor of the tongue. Rev Stomatol Chir Maxillofac. 2005;106:304–7. doi: 10.1016/s0035-1768(05)86046-2. [DOI] [PubMed] [Google Scholar]
- 14.Browne M, Abramson LP, Chou PM, Acton R, Holinger LD, Reynolds M. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the neck infiltrating the trachea. J Pediatr Surg. 2004;39:e1–4. doi: 10.1016/j.jpedsurg.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Marioni G, Bottin R, Staffieri A, Altavilla G. Spindle-cell tumours of the larynx: Diagnostic pitfalls. A case report and review of the literature. Acta Otolaryngol. 2003;123:86–90. doi: 10.1080/0036554021000028070. [DOI] [PubMed] [Google Scholar]
- 16.Oda Y, Tamiya S, Oshiro Y, Hachitanda Y, Kinukawa N, Iwamoto Y, et al. Reassessment and clinicopathological prognostic factors of malignant fibrous histiocytoma of soft parts. Pathol Int. 2002;52:595–606. doi: 10.1046/j.1440-1827.2002.01399.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Wang K, Liu WK, Zhang YK. Is radical surgery necessary to head and neck inflammatory myofibroblastic tumor (IMT) in children? Childs Nerv Syst. 2009;25:285–91. doi: 10.1007/s00381-008-0718-1. [DOI] [PubMed] [Google Scholar]


