Abstract
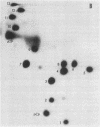
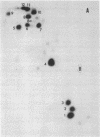
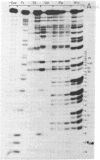
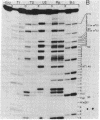
The mitochondrial tyrosine tRNA from Neurospora crassa has been sequenced and found to have several interesting features: (i) It resembles prokaryotic rather than eukaryotic tyrosine tRNAs in that it possesses a large variable loop (loop III); moreover, it can be quantitatively aminoacylated by Escherichia coli tyrosyl-tRNA synthetase but not by yeast tyrosyl-tRNA synthetase. (ii) This tRNA differs from all tRNA's sequenced to date in lacking the A residue at position 14 and the constant purine residue at position 15, two nucleosides that have been found so far in loop I of all tRNA's and that have been implicated in base-base tertiary interactions, respectively, with the universal U residue at position 8 and the constant pyrimidine residue at the end of loop III. (iii) Unlike the N. crassa mitochondrial initiator tRNA, this tRNA contains the usual TpsiC sequence in loop IV and the highly conserved GG sequence in loop I common to other tRNAs.
Full text
PDF
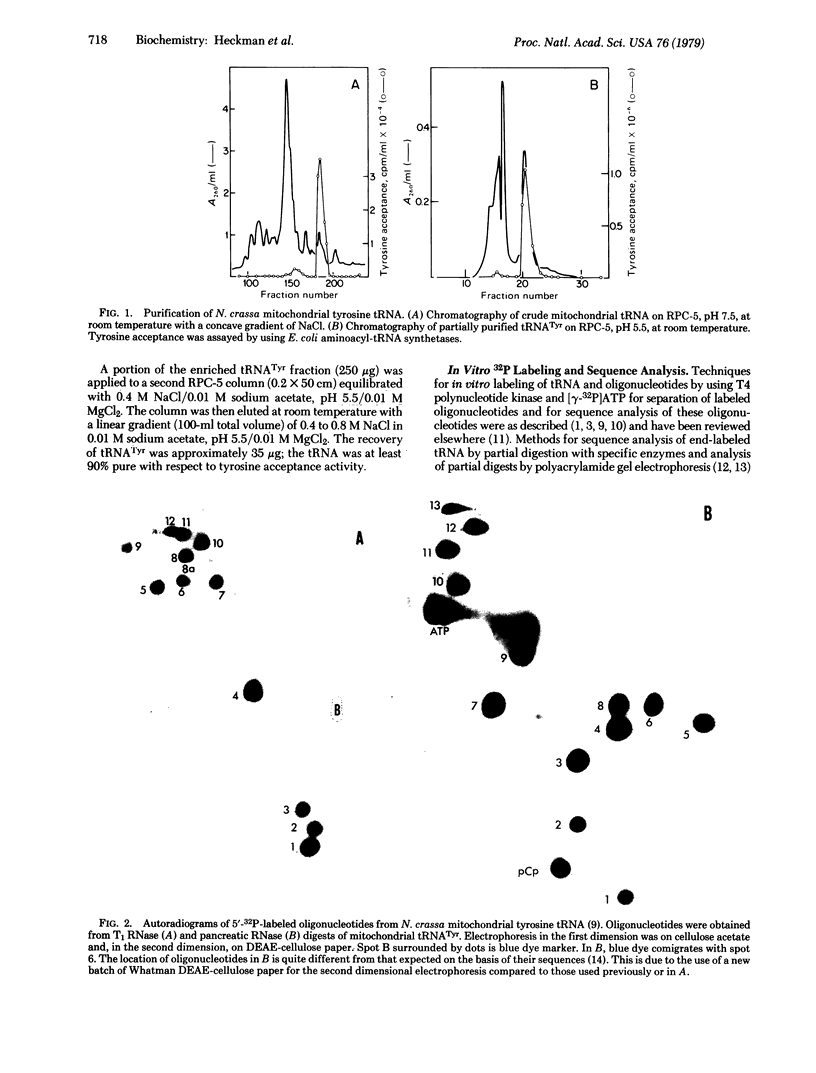
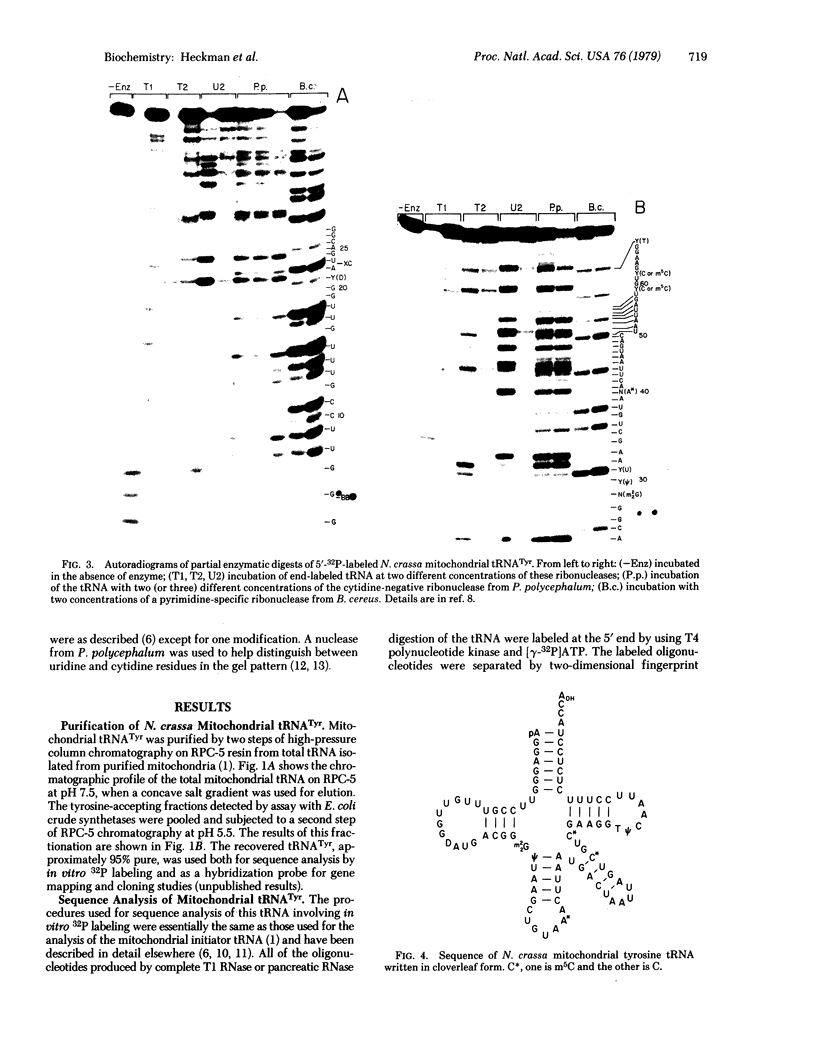


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonen L., Cunningham R. S., Gray M. W., Doolittle W. F. Wheat embryo mitochondrial 18S ribosomal RNA: evidence for its prokaryotic nature. Nucleic Acids Res. 1977 Mar;4(3):663–671. doi: 10.1093/nar/4.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R., Behrens K. A ribonuclease from Physarum. Biochemical properties and synthesis in the mitotic cycle. Biochim Biophys Acta. 1969 Nov 19;195(1):87–98. doi: 10.1016/0005-2787(69)90605-4. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Rubin J. R., Rhodes D., Guilley H., Simoncsits A., Brownlee G. G. The nucleoside sequence of tyrosine tRNA from Bacillus stearothermophilus. Nucleic Acids Res. 1978 Jan;5(1):23–36. doi: 10.1093/nar/5.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Chase J. W. Mitochondrial RNA in Xenopus laevis. II. Molecular weights and other physical properties of mitochondrial ribosomal and 4 s RNA. J Mol Biol. 1972 Jan 28;63(2):217–231. doi: 10.1016/0022-2836(72)90371-3. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin D. T., Friend D. A. Comparison of cytoplasmic and mitochondrial 4 S RNA from cultured hamster cells: physical and metabolic properties. J Mol Biol. 1972 Nov 14;71(2):163–175. doi: 10.1016/0022-2836(72)90344-0. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Takemura S., Miyazaki M. Partial digestion with ribonuclease T 1 and derivation of the complete sequence of tyrosine transfer ribonucleic acid from Torulopsis utilis. J Biochem. 1972 Jul;72(1):123–124. doi: 10.1093/oxfordjournals.jbchem.a129877. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Hecker L. I., Schwartzbach S. D., Barnett W. E., Baumstark B., RajBhandary U. L. Structure and function of initiator methionine tRNA from the mitochondria of Neurospora crassa. Cell. 1978 Jan;13(1):83–95. doi: 10.1016/0092-8674(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison J. T., Everett G. A., Kung H. Nucleotide sequence of a yeast tyrosine transfer RNA. Science. 1966 Jul 29;153(3735):531–534. doi: 10.1126/science.153.3735.531. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Rajbhandary U. L., Chang S. H., Stuart A., Faulkner R. D., Hoskinson R. M., Khorana H. G. Studies on polynucleotides, lxviii the primary structure of yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1967 Mar;57(3):751–758. doi: 10.1073/pnas.57.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Structures of two glycyl-tRNAs from Staphylococcus epidermidis. Nat New Biol. 1972 May 10;237(71):44–45. doi: 10.1038/newbio237044a0. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Grüter F., Gauss D. H. Collection of published tRNA sequences. Nucleic Acids Res. 1978 May;5(5):r15–r27. [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Formylatable methionine transfer RNA from Mycoplasma: purification and comparison of partial nucleotide sequences with those of other prokaryotic initiator tRNAs. Nucleic Acids Res. 1975 Jan;2(1):61–78. doi: 10.1093/nar/2.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]