Abstract
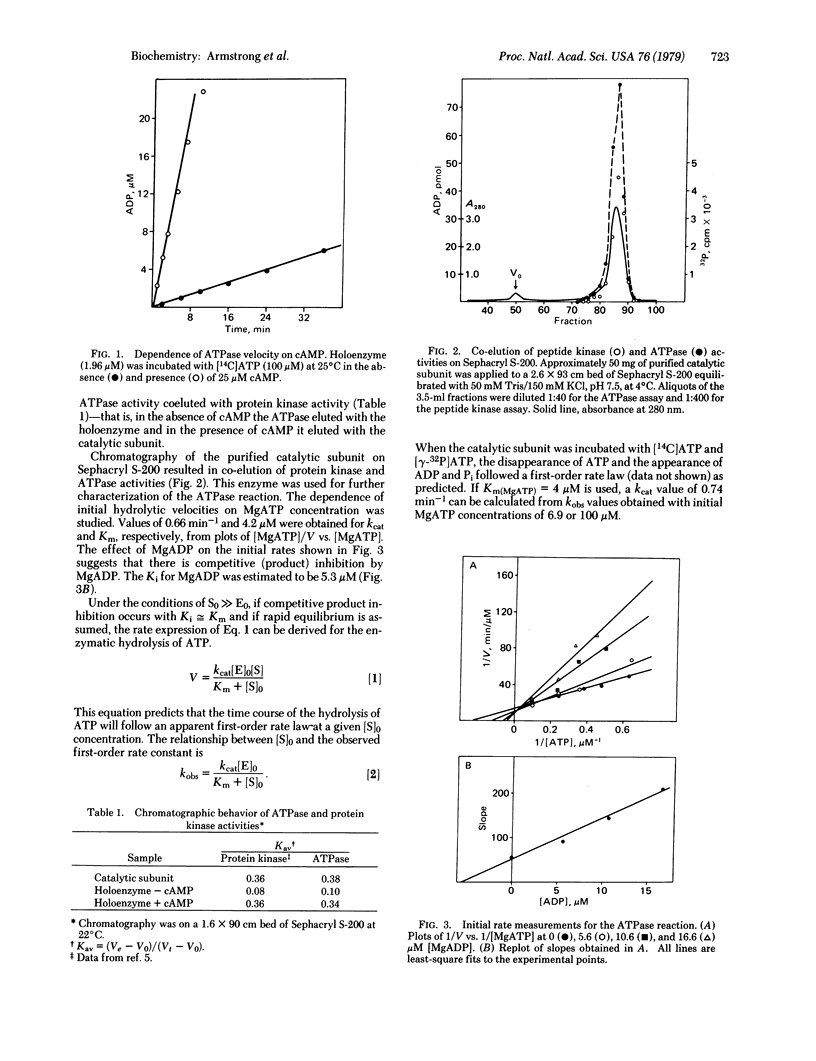
The adenosine 3",5"-monophosphate (cAMP)-dependent ATPase (ATP phosphohydrolase, EC 3.6.1.3) activity of cAMP-dependent protein kinase (ATP:protein phosphotransferase, EC 2.7.1.37) from bovine heart is characterized. That the ATPase activity is intimately associated with the catalytic subunit of the enzyme is suggested by the following: (i) the similar dependences of ATPase and protein kinase activities on cAMP; (ii) the dissociation of ATPase activity from the holoenzyme on addition of cAMP and its co-elution with the catalytic subunit on gel filtration chromatography; (iii) the similarity of the relative effectiveness of divalent metal ions in ATPase and protein kinase catalysis; and (iv) the correspondence of kinetically determined Km(MgATP) and Ki(MgADP) values with thermodynamic dissociation constants determined by equilibrium dialysis. The hydrolysis of ATP is stimulated 10- to 20-fold by cAMP in the holoenzyme. The molar specific activity of the catalytic subunit ATPase is approximately 0.7 min-1 with Km(MgATP) = 5 muM. MgADP is a competitive inhibitor of the reaction with a Ki value of approximately muM. The order of the relative effectiveness of metal ions for both ATPase and peptide kinase activities is Mg2+ greater than Mn2+ greater than Ca2+. A possible interpretation of these observations is that the role that the metal ion plays is more directly manifested in bond-breaking than in bond-forming.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. N., Kaiser E. T. Sulfhydryl group reactivity of adenosine 3',5'-monophosphate dependent protein kinase from bovine heart: a probe of holoenzyme structure. Biochemistry. 1978 Jul 11;17(14):2840–2845. doi: 10.1021/bi00607a022. [DOI] [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Rosenfeld R., Rosen O. M. Phosphorylation of a cyclic adenosine 3':5'-monophosphate-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1974 Aug 10;249(15):5000–5003. [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Moll G. W., Jr, Kaiser E. T. Phosphorylation of histone catalyzed by a bovine brain protein kinase. J Biol Chem. 1976 Jul 10;251(13):3993–4000. [PubMed] [Google Scholar]
- Pomerantz A. H., Allfrey V. G., Merrifield R. B., Johnson E. M. Studies on the mechanism of phosphorylation of synthetic polypeptides by a calf thymus cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4261–4265. doi: 10.1073/pnas.74.10.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Erlichman J. Reversible autophosphorylation of a cyclic 3':5'-AMP-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1975 Oct 10;250(19):7788–7794. [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic AMP-dependent protein kinase from bovine heart muscle. Methods Enzymol. 1974;38:308–315. doi: 10.1016/0076-6879(74)38047-0. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todhunter J. A., Purich D. L. Autophosphorylation of cardiac 3',5'-cyclic AMP-stimulated protein kinase. Kinetic evidence for the regulatory subunit directly acting at the active site in the R2C2 complex. Biochim Biophys Acta. 1977 Nov 23;485(1):87–94. doi: 10.1016/0005-2744(77)90195-4. [DOI] [PubMed] [Google Scholar]



