Abstract
The association of multisystem pathologic conditions and epidermal nevi, known as the epidermal nevus syndrome, includes disorders of bone, central nervous system, eye, kidney, vasculature and skin. Rarely, congenital nevomelanocytic nevus also known as hairy nevus has also been reported in association with hypophosphatemic rickets. Studies suggest that phosphaturia, caused by circulating factors, called “phosphatonins” may be secreted by an epidermal or hairy nevus. We report here, a rare case of hypophosphatemic rickets associated with a giant hairy nevus in a 10-year-old boy.
Keywords: Giant hairy nevus, hypophosphatemia, rickets
INTRODUCTION
Epidermal nevus syndrome (ENS) is characterized by epidermal nevi associated with abnormalities involving the nervous, skeletal and other systems.[1,2] Rarely, hypophosphatemic rickets has also been observed in association with epidermal nevi. Studies suggest that phosphaturia, caused by circulating factor, called “phosphatonins” including fibroblast growth factor-23 (FGF-23), which may be secreted by an epidermal nevus.[3]
The congenital nevomelanocytic nevus (CNN), commonly known as the congenital hairy nevus is a pigmented surface lesion present at birth. Based on diameter, CNN are characterized as small (<1.5 cm), medium (1.5-19.5 cm) and giant (>20 cm in adolescents and adults).[4] Studies report lifetime risk of developing a melanoma for patients with a large CNN ranging from 6.3% to 12.2% while for small CNN, risk rates have been reported between 0.8% and 4.9%.[5] ENS and linear sebaceous nevus have been associated with various abnormalities including hypophosphatemic rickets, but only one case of giant hairy nevus associated with hypophosphatemic rickets has been reported until date.[6] We report here a case of giant CNN associated with severe hypophosphatemic rickets, probably due to secretion of FGF-23.
SUMMARY
A 10-year-old boy presented with a difficulty in walking and progressive deformity of the lower limbs since 2 years of age. History of delayed milestones started walking at 2 years of age, history of poor height gain and history of difficulty in getting up from the squatting position. History of abnormal skin pigmentation present since birth. Patient was born at full term of non-consanguineous marriage. The family history was non-contributory. No history of seizures, carpopedal spasms, periodic muscle weakness, dental abscess, hearing loss, pain abdomen, hematuria, polyuria, diarrhea and pain abdomen. There was no history of anti-tubercular and anti-epileptic drug intake. He had been on treatment with oral vitamin D and phosphate supplementation irregularly. On examination, Pulse: 70/min, BP: 107/70 mmHg. Physical examination revealed asymmetry of the thighs and left knee extension limitation. He was normotensive with widened bilateral wrists, genu varum and mild scoliosis. Height was 107 cm (<3rd percentile). He was prepubertal in his development. His skin was remarkable for the presence of few hairy nevi, largest one covering his entire back and shoulders [Figure 1]. Other small nevi were present over his arms, legs and face. He had been treated with oral vitamin D with no significant clinical benefit. Investigations revealed serum calcium 8.7 mg/dL (normal 8.1-10.4 mg%), serum phosphate 2.1 mg/dL (normal 2.1-4.5 mg%) and serum alkaline phosphatase 2685 U/L (normal 0-18 years: 240-840 IU). 24 h urine collection showed creatinine, calcium and phosphate excretion of 0.12 g, 0.01 g and 0.17 g respectively. Fractional excretion of phosphate was 26.98%. Tubular resorption of phosphate was 73%. Serum intact parathormone levels was 127.7 pg/ml and serum 25(OH) vitamin D levels was 69 ng/ml. Atreial blood pH was 7.394. Urine pH was 5.3. Urine examination for glucose and amino acids was negative and ruling out any possibility of proximal renal tubular acidosis. Magnetic resonance imaging brain performed previously was normal. Fundus and slit lamp examination of eyes were normal. No evidence of KF rings was there. Skeletal survey showed generalized osteopenia, metaphyseal cupping and fraying of long bones. Radiographs of long bones showed bowing of both upper and lower limbs. 99m-Tc methylene-diphosphonate whole body scintigraphy revealed diffusely increased radiotracer uptake in the entire axial and peripheral skeleton, more prominent in calvarium, mandible and bilateral costochondral junctions suggestive of metabolic bone disease. Serum FGF-23 levels were found to be raised (171 U/L) [Figures 1–3]. Patient was diagnosed as a case of CNN with hypophosphatemic rickets and started on phosphate supplement and calcitriol.
Figure 1.
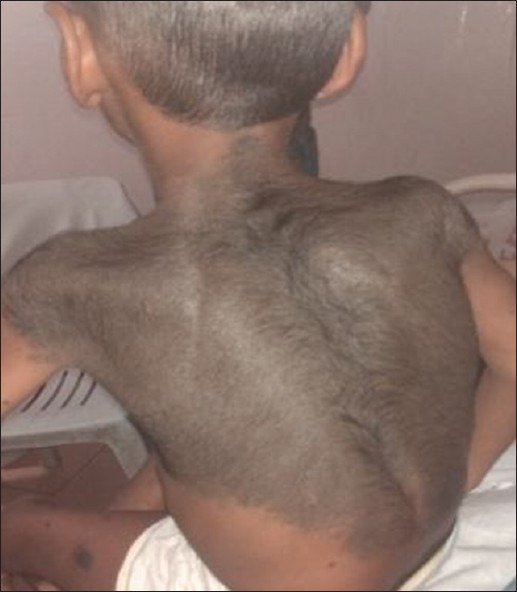
Giant hairy nevus on the back of patient with small nevi over left thigh
Figure 3.
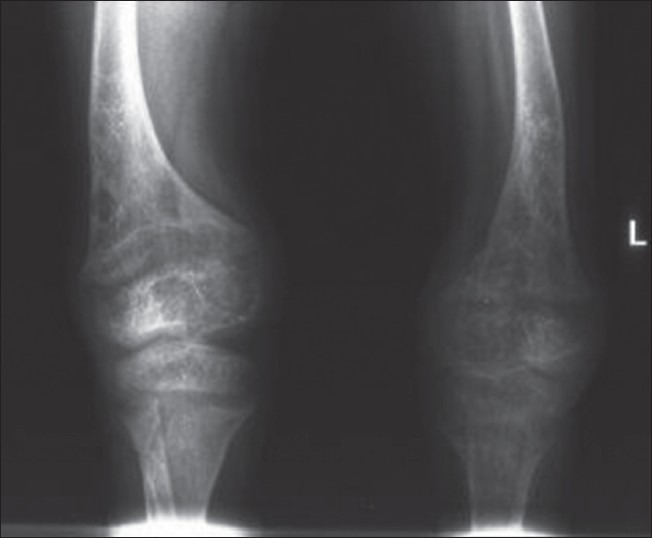
X-ray of both knees showing rachitic changes
Figure 2.
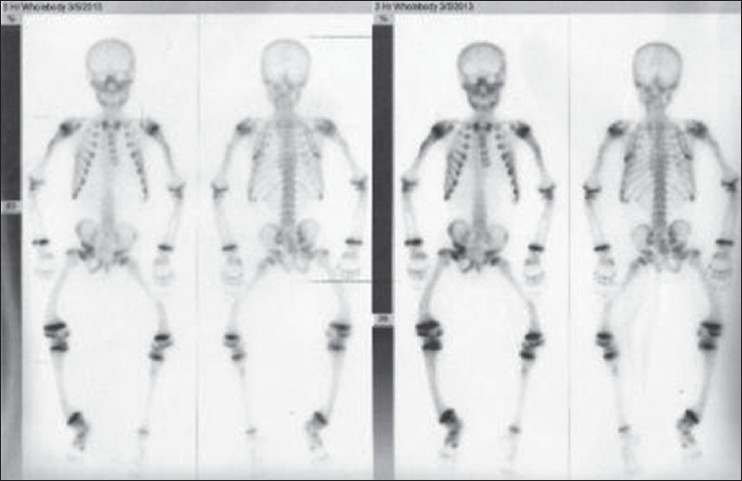
99m-Tc methylene-diphosphonate whole body scintigraphy
Figure 4.
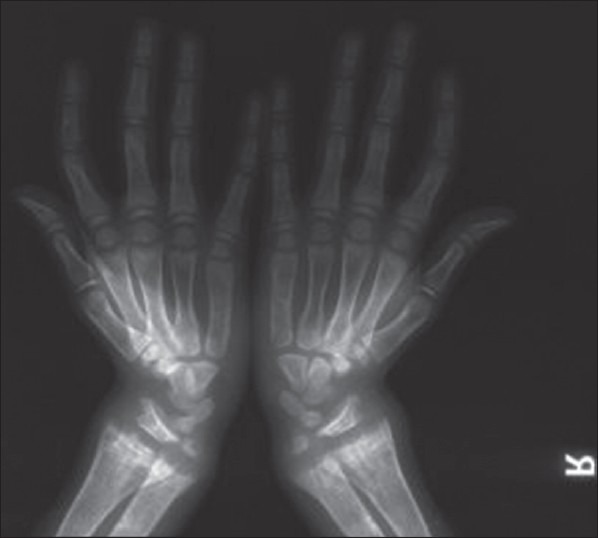
X-ray both hands with wrists showing rachitic changes
DISCUSSION
Hypophosphatemic rickets constitutes a group of disorders in metabolic bone disorders due to the defective renal phosphate resorption. The most common form is X-linked hypophosphatemic rickets. Acquired forms of hypophosphatemic rickets have been reported to be associated with various tumors including mesenchymal tumors, hemangiomas and soft-tissue tumors. However, the combination of hypophosphatemic rickets with ENS or giant hairy nevus is rarely reported. Other skin lesions that can occur in ENS are vascular nevi, hypopigmented macules and café au lait macules. The central nervous system abnormalities in patients with ENS include seizures, hemiparesis, developmental delay, mental retardation, abnormal cerebral gyration, underdeveloped temporal lobe and sensorineural deafness. Precocious puberty is also observed rarely in association with ENS.[2] Surgical excision of the nevus has been reported to improve or cure the metabolic derangement.[7] Injection of the supernate from homogenized portion of excised tissue into experimental animals has been shown to induce excessive phosphaturia suggestive of the causative role of epidermal nevus.[7] The nature of the phosphaturic factor in ENS is not well-understood, but elevated circulating FGF-23 levels has been reported in one patient with hypophosphatemic rickets.[3] This case illustrates the rare association of hypophosphatemic rickets with giant hairy nevus and it also highlights the significance of clinical and biochemical examination of children with epidermal nevi. This association is hence important to recognize so that early surgical correction can be planned and permanent disability can be avoided.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Olivares JL, Ramos FJ, Carapeto FJ, Bueno M. Epidermal naevus syndrome and hypophosphataemic rickets: Description of a patient with central nervous system anomalies and review of the literature. Eur J Pediatr. 1999;158:103–7. doi: 10.1007/s004310051027. [DOI] [PubMed] [Google Scholar]
- 2.Ivker R, Resnick SD, Skidmore RA. Hypophosphatemic vitamin D-resistant rickets, precocious puberty, and the epidermal nevus syndrome. Arch Dermatol. 1997;133:1557–61. [PubMed] [Google Scholar]
- 3.Heike CL, Cunningham ML, Steiner RD, Wenkert D, Hornung RL, Gruss JS, et al. Skeletal changes in epidermal nevus syndrome: Does focal bone disease harbor clues concerning pathogenesis? Am J Med Genet A. 2005;139A:67–7. doi: 10.1002/ajmg.a.30915. [DOI] [PubMed] [Google Scholar]
- 4.Bittencourt FV, Marghoob AA, Kopf AW, Koenig KL, Bart RS. Large congenital melanocytic nevi and the risk for development of malignant melanoma and neurocutaneous melanocytosis. Pediatrics. 2000;106:736–41. doi: 10.1542/peds.106.4.736. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes AR. Melanocytic precursors of cutaneous melanoma. Estimated risks and guidelines for management. Med Clin North Am. 1986;70:3–37. doi: 10.1016/s0025-7125(16)30966-x. [DOI] [PubMed] [Google Scholar]
- 6.Chou YY, Chao SC, Shiue CN, Tsai WH, Lin SJ. Hypophosphatemic rickets associated with epidermal nevus syndrome and giant hairy nevus. J Pediatr Endocrinol Metab. 2005;18:93–5. doi: 10.1515/jpem.2005.18.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Aschinberg LC, Solomon LM, Zeis PM, Justice P, Rosenthal IM. Vitamin D-resistant rickets associated with epidermal nevus syndrome: Demonstration of a phosphaturic substance in the dermal lesions. J Pediatr. 1977;91:56–60. doi: 10.1016/s0022-3476(77)80444-7. [DOI] [PubMed] [Google Scholar]


