Abstract
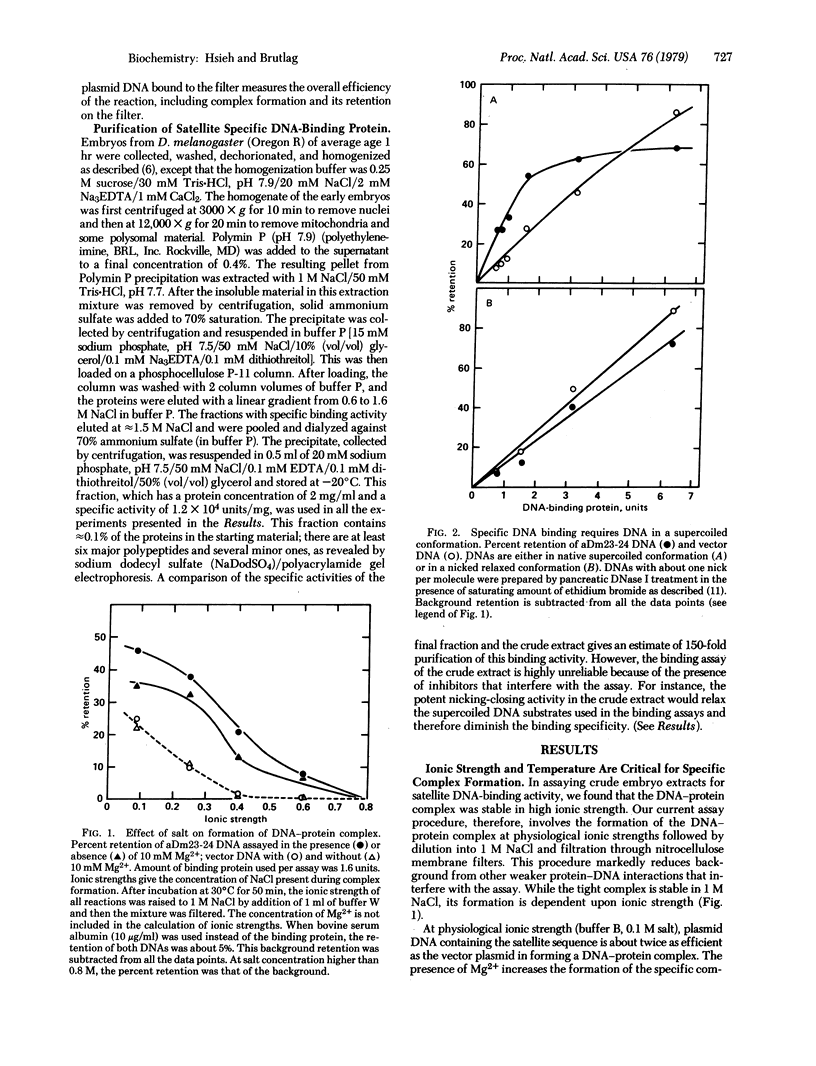
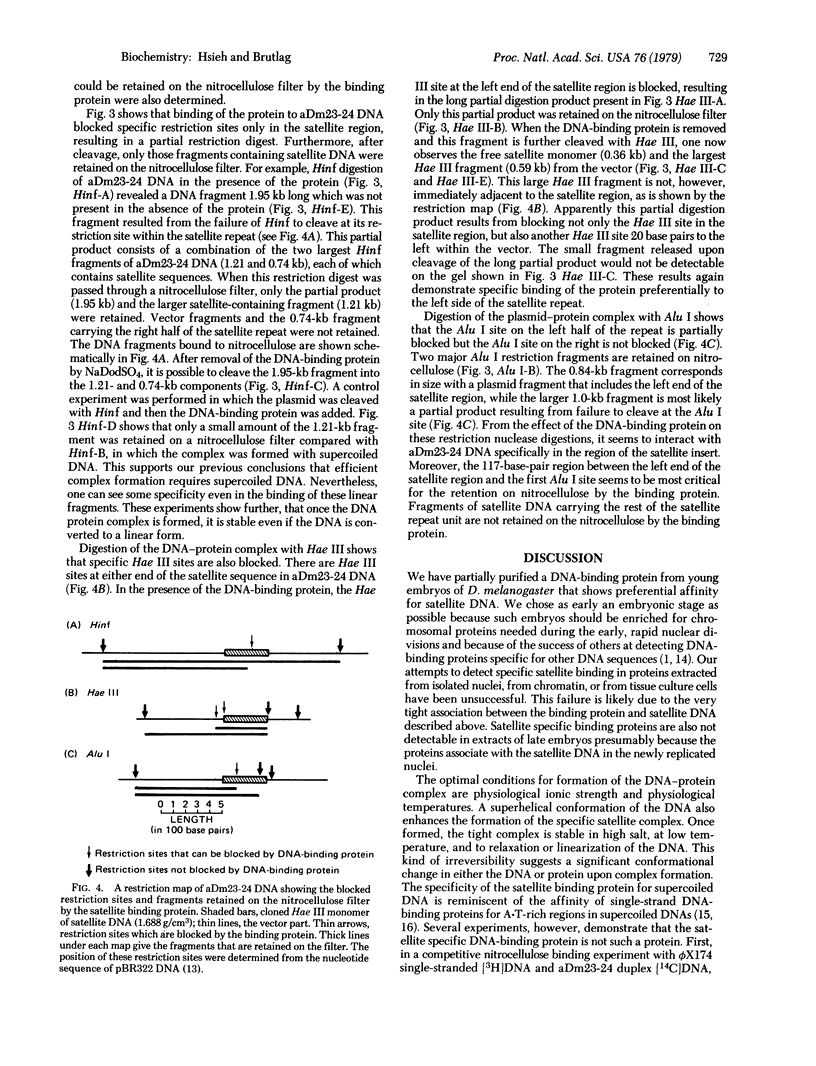
Using a nitrocellulose filter binding assay, we have detected and partially purified a protein from embryos of Drosophila melanogaster that preferentially binds to a highly repeated satellite DNA of the same species. Formation of the satellite DNA-protein complex requires physiological conditions of salt and temperature, but once formed, the complex is stable in high salt (1 M NaCl) or at low temperature. Optimal formation of the specific complex also requires the satellite DNA to be in a supertwisted conformation. The protein interacts with a limited region within the 359-base-pair repeated sequence of the satellite DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker W. K. Position-effect variegation. Adv Genet. 1968;14:133–169. [PubMed] [Google Scholar]
- Bennett G. N., Yanofsky C. Sequence analysis of operator constitutive mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):179–192. doi: 10.1016/s0022-2836(78)80004-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brack C., Bickle T. A., Yuan R. The relation of single-stranded regions in bacteriophage PM2 supercoiled DNA to the early melting sequences. J Mol Biol. 1975 Aug 25;96(4):693–702. doi: 10.1016/0022-2836(75)90146-1. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Appels R., Dennis E. S., Peacock W. J. Highly repeated DNA in Drosophila melanogaster. J Mol Biol. 1977 May 5;112(1):31–47. doi: 10.1016/s0022-2836(77)80154-x. [DOI] [PubMed] [Google Scholar]
- COOPER K. W. MEIOTIC CONJUNCTIVE ELEMENTS NOT INVOLVING CHIASMATA. Proc Natl Acad Sci U S A. 1964 Nov;52:1248–1255. doi: 10.1073/pnas.52.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Brutlag D. Cloning and characterization of a complex satellite DNA from Drosophila melanogaster. Cell. 1977 Jun;11(2):371–381. doi: 10.1016/0092-8674(77)90054-x. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Developmental genetics of Drosophila. Annu Rev Genet. 1976;10:209–252. doi: 10.1146/annurev.ge.10.120176.001233. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS E. B. The phenomenon of position effect. Adv Genet. 1950;3:73–115. doi: 10.1016/s0065-2660(08)60083-8. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi J. C. Dosage compensation in Drosophila. Annu Rev Genet. 1973;7:225–237. doi: 10.1146/annurev.ge.07.120173.001301. [DOI] [PubMed] [Google Scholar]
- Manteuil S., Hamer D. H., Thomas C. A., Jr Regular arrangement of restriction sites in Drosophila DNA. Cell. 1975 Aug;5(4):413–422. doi: 10.1016/0092-8674(75)90060-4. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Location of the T4 gene 32 protein binding site on simian virus 40 DNA. J Virol. 1973 Dec;12(6):1631–1632. doi: 10.1128/jvi.12.6.1631-1632.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler L. Evidence for a set of closely linked autosomal genes that interact with sex-chromosome heterochromatin in Drosophila melanogaster. Genetics. 1977 Jul;86(3):567–582. doi: 10.1093/genetics/86.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. J., Wiesehahn G., Hearst J. E. Cleavage patterns of Drosophila melanogaster satellite DNA by restriction enzymes. Nucleic Acids Res. 1976 Apr;3(4):931–951. doi: 10.1093/nar/3.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weideli H., Schedl P., Artavanis-Tsakonas S., Steward R., Yuan R., Gehring W. J. Purification of a protein from unfertilized eggs of Drosophila with specific affinity for a defined DNA sequence and the cloning of this DNA sequence in bacterial plasmids. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):693–700. doi: 10.1101/sqb.1978.042.01.071. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Revzin A., Gross C. A., Wang A. C. Non-specific DNA binding of genome regulating proteins as a biological control mechanism: I. The lac operon: equilibrium aspects. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4808–4812. doi: 10.1073/pnas.71.12.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]



