Abstract
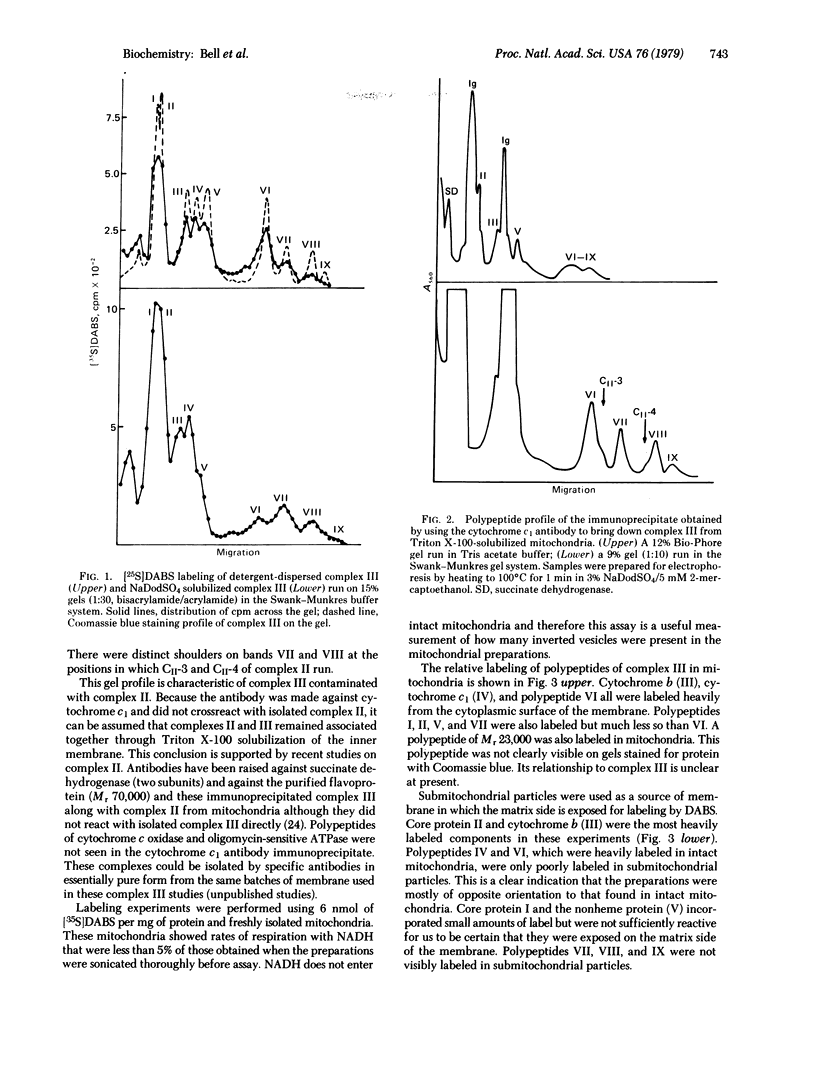
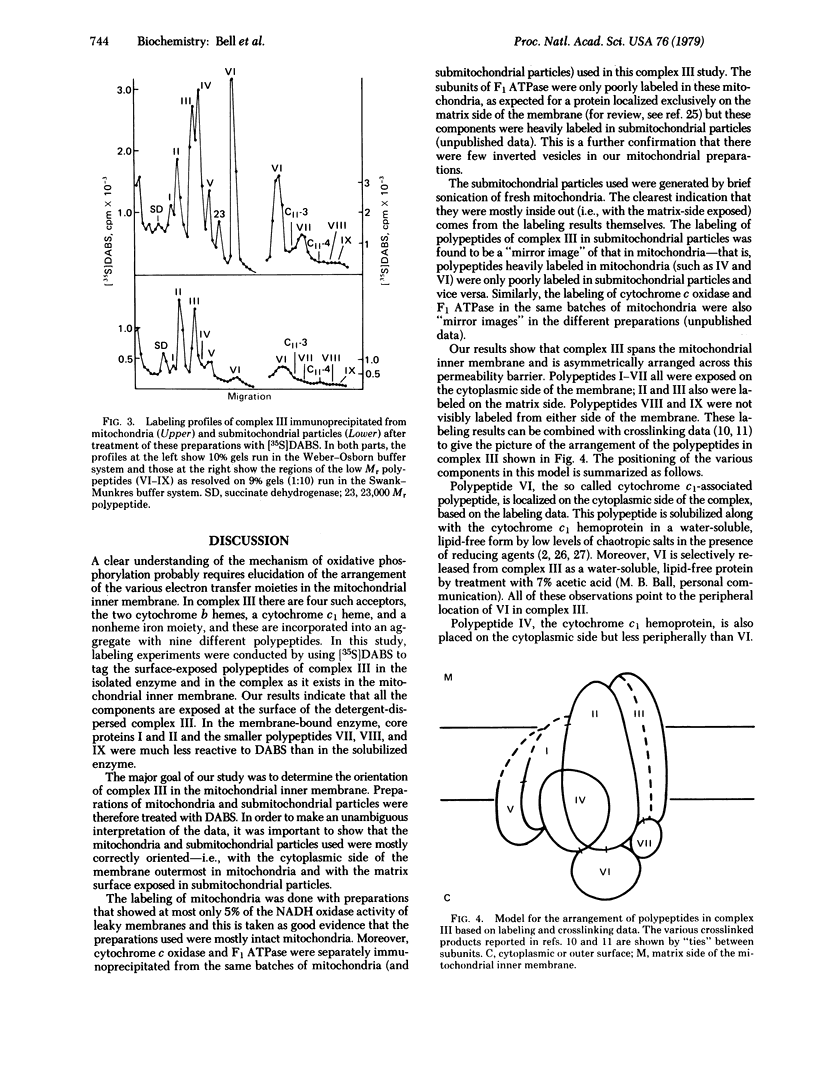
[34S]Diazobenzenesulfonate has been used to tag the surface-exposed polypeptides of isolated complex III. All nine different component polypeptides were labeled, indicating that each is at least partially exposed on the surface of the isolated, detergent-dispersed complex. Labeling studies were also conducted on the membrane-bound complex. Preparations of intact mitochondria and submitochondrial particles were separately labeled with [35S]diazobenzenesulfonate in order to determine the distribution of the polypeptides of complex III between the outer (cytoplasmic) and inner (matrix) surfaces of the mitochondrial inner membrane, respectively. Polypeptides II and III were the only components labeled in a significant amount in submitochondrial particles (i.e., from the matrix side). Polypeptides III, IV, and VI were heavily labeled in mitochondria (i.e., from the cytoplasmic side). Polypeptides I,II, V, and VII were also labeled in mitochondria but to a much lesser extent. Polypeptides VIII and IX were not significantly labeled from either side of the membrane. The labeling data and information obtained from previous crosslinking studies [Smith, R.J. & Capaldi, R.A. (1977) Biochemistry 16, 2629-2633] are used to derive a picture of the arrangement of complex III in the mitochondrial inner membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball M. B., Bell R. L., Capaldi R. A. Trypsin cleavage of ubiquinone-cytochrome c reductase (complex III). FEBS Lett. 1977 Nov 1;83(1):99–102. doi: 10.1016/0014-5793(77)80650-9. [DOI] [PubMed] [Google Scholar]
- Baum H., Silman H. I., Rieske H. S., Lipton S. H. On the composition and structural organization of complex 3 of the mitochondrial electron transfer chain. J Biol Chem. 1967 Nov 10;242(21):4876–4887. [PubMed] [Google Scholar]
- Bell R. L., Capaldi R. A. The polypeptide composition of ubiquinone-cytochrome c reductase (complex III) from beef heart mitochondria. Biochemistry. 1976 Mar 9;15(5):996–1001. doi: 10.1021/bi00650a008. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Case G. D., Leigh J. S., Jr Intramitochondrial positions of cytochrome haem groups determined by dipolar interactions with paramagnetic cations. Biochem J. 1976 Dec 15;160(3):769–783. doi: 10.1042/bj1600769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case G. D., Ohnishi T., Leigh J. S., Jr Intramitochondrial positions of ubiquinone and iron-sulphur centres determined by dipolar interactions with paramagnetic ions. Biochem J. 1976 Dec 15;160(3):785–795. doi: 10.1042/bj1600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Gellerfors P., Nelson B. D. Topology of the peptides in free and membrane-bound complex III (ubiquinol--cytochrome c reductase) as revealed by lactoperoxidase and p-diazoniumbenzene [35S] sulfonate labelling. Eur J Biochem. 1977 Oct 17;80(1):275–282. doi: 10.1111/j.1432-1033.1977.tb11880.x. [DOI] [PubMed] [Google Scholar]
- Katan M. B., Pool L., Groot G. S. The cytochrome bc1 complex of yeast mitochondria. Isolation and partial characterization of the cytochrome bc1 complex and cytochrome b. Eur J Biochem. 1976 May 17;65(1):95–105. doi: 10.1111/j.1432-1033.1976.tb10393.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung K. H., Hinkle P. C. Reconstitution of ion transport and respiratory control in vesicles formed from reduced coenzyme Q-cytochrome c reductase and phospholipids. J Biol Chem. 1975 Nov 10;250(21):8467–8471. [PubMed] [Google Scholar]
- Marres C. M., Slater E. C. Polypeptide composition of purified QH2:cytochrome c oxidoreductase from beef-heart mitochondria. Biochim Biophys Acta. 1977 Dec 23;462(3):531–548. doi: 10.1016/0005-2728(77)90099-8. [DOI] [PubMed] [Google Scholar]
- Riccio P., Schägger H., Engel W. D., Von Jagow G. bc1-Complex from beef heart. One-step purification by hydroxyapatite chromatography in Triton X-100, polypeptide pattern and respiratory chain characteristics. Biochim Biophys Acta. 1977 Feb 7;459(2):250–262. doi: 10.1016/0005-2728(77)90026-3. [DOI] [PubMed] [Google Scholar]
- Rieske J. S. Composition, structure, and function of complex III of the respiratory chain. Biochim Biophys Acta. 1976 Sep 27;456(2):195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Capaldi R. A., Muchmore D., Dahlquist F. Cross-linking of ubiquinone cytochrome c reductase (complex III) with periodate-cleavable bifunctional reagents. Biochemistry. 1978 Sep 5;17(18):3719–3723. doi: 10.1021/bi00611a007. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Capaldi R. A. Nearest neighbor relationships of the polypeptides in ubiquinone cytochrome c reductase (complex III). Biochemistry. 1977 Jun 14;16(12):2629–2633. doi: 10.1021/bi00631a008. [DOI] [PubMed] [Google Scholar]
- Staros J. V., Richards F. M. Photochemical labeling of the cytoplasmic surface of the membranes of intact human erythrocytes. J Biol Chem. 1975 Oct 25;250(20):8174–8178. [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Melnick R. L., Maguire J., Packer L. Studies on mitochondrial proteins. II. Localization of components in the inner membrane: labeling with diazobenzenesulfonate, a non-penetrating probe. Biochim Biophys Acta. 1974 Apr 12;345(1):118–128. doi: 10.1016/0005-2736(74)90251-x. [DOI] [PubMed] [Google Scholar]
- Trumpower B. L., Katki A. Controlled digestion with trypsin as a structural probe for the N-terminal peptide of soluble and membranous cytochrome c. Biochemistry. 1975 Aug 12;14(16):3635–3642. doi: 10.1021/bi00687a019. [DOI] [PubMed] [Google Scholar]
- Von Jagow G., Schägger H., Engel W. D., Machleidt W., Machleidt I., Kolb H. J. Beef heart complex III: isolation and characterization of cytochrome b. FEBS Lett. 1978 Jul 1;91(1):121–125. doi: 10.1016/0014-5793(78)80031-3. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. N., Jr A METHOD FOR THE SIMULTANEOUS QUANTITATIVE ESTIMATION OF CYTOCHROMES A, B, C1, AND C IN MITOCHONDRIA. Arch Biochem Biophys. 1964 Sep;107:537–543. doi: 10.1016/0003-9861(64)90313-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss H. Subunit composition and biogenesis of mitochondrial cytochrome b. Biochim Biophys Acta. 1976 Nov 30;456(3-4):291–313. doi: 10.1016/0304-4173(76)90002-1. [DOI] [PubMed] [Google Scholar]


