Abstract
The peripartum control of diabetes is very important for the well-being of the newborn as higher incidence of neonatal hypoglycemia is seen if maternal hyperglycemia happens during this period. Type of diabetes (type 1, type 2 or gestational diabetes) also has an effect on the glucose concentration during intrapartum period. During the latent phase of labor, the metabolic demands are stable but during active labor there is increased metabolic demand and decreased insulin requirement. After delivery once the placenta is extracted, insulin resistance rapidly comes down and in patients with pre-gestational diabetes there will be a sudden drop in insulin requirement and the insulin may not be required in women with gestational diabetes, but they just need close monitoring. During breast-feeding blood glucose levels fall because of high metabolic demand and women need to take extra calories to maintain the levels and more vigilance especially in type 1 and type 2 diabetic mothers is required. The protocols used for the management of peripartum management of diabetes mostly rely on glucose and insulin infusion to maintain maternal blood sugars between 70 and 110 mg/dl. The data is mostly from retrospective studies and few randomized control trials done mainly in type 1 diabetes patients. The review summarizes guidelines, which are used for peripartum management of blood glucose.
Keywords: Diabetes, insulin, peripartum
INTRODUCTION
The most important aspect of peripartum management of diabetes is to avoid maternal hyperglycemia as it increases chances of neonatal hypoglycemia and fetal acidemia. The management in the postpartum period is less critical, but maternal hypoglycemia is more of a concern because of the rapid changes in the placental hormones in the postpartum period. It is universally accepted that tight glycemic control is necessary for women with pre-gestational or gestational diabetes mellitus (GDM) throughout pregnancy and it is more crucial during labor.[1] The monitoring of blood sugars along with continuous glucose infusion has been used for the peripartum management of diabetes in many centers as it causes less hypoglycemia and fewer incidence of side effects.[2]
The requirement of insulin in the intrapartum period is largely dependent on the type of diabetes in the mother (type 1, type 2 or gestational diabetes) and also on the phase of labor. The women with type 2 diabetes and gestational diabetes have adequate endogenous insulin production while women with type 1 diabetes have almost no endogenous insulin production. During the latent phase of labor, the requirement of insulin is stable, but during the active phase insulin requirement comes down markedly especially in patients with type 2 diabetes mellitus (type 2DM) and GDM. The control of the sugars during pregnancyalso has a bearing on the requirement of insulin during peripartum period. Women with previously uncontrolled sugars may require a high dose of insulin during peripartum period while requirement will be lower in women with good control of sugars during pregnancy.[3,4] Newborns born to poorly controlled diabetic mothers are prone for severe neonatal hypoglycemia because of secondary hyperplasia of pancreas and hyperinsulinemia and even with tight glycemia during the peripartum period it is difficult to prevent hypoglycemia in these infants as there will be excess of insulin levels in response to prolonged hyperglycemia.[5]
MANAGEMENT OF DIABETES DURING LABOR
During labor and delivery the goal is to maintain the sugars in the normal range as safely as possible as increased blood sugars 4-6 h prior to delivery leads to increased rates of hypoglycemia in the neonate.[6] Studies have shown that continuous monitoring of blood glucose during labor may improve the outcome of the mother and the baby. During labor normoglycemia is mostly achieved with short or rapid acting insulin when indicated so as to prevent hypoglycemia in the neonate.[7]
INTRAPARTUM TARGETS FOR GLUCOSE
The American College of Obstetrics and Gynecology and the American College of Endocrinology recommends maintenance of blood glucose between 70 and 110 mg/dl during labor (3.9-6.1 mmol/L),[8,9] this goal is the same irrespective of whether the women has type 1 diabetes, type 2DM or GDM. Some studies recommend an upper target of 100 mg/dl[10] to minimize the risk of neonatal hypoglycemia while some studies have relaxed the target to 126 mg/dl as this level does not further increase the risk of hypoglycemia in the neonate. A maternal blood glucose value of more than 180 mg/dl has been conclusively proven to be associated with high risk of neonatal hypoglycemia.[11]
In a retrospective analysis of 137 singleton cases, managed with a conservative approach during delivery of singleton pregnancy, 87% of neonatal hypoglycemia (n = 26) occurred in infants born to mothers with blood sugar levels between 72 and 144 mg/dl (4-8 mmol/L). Thirteen of these neonates who required neonatal intensive care unit admission were born to mothers with blood sugar <144 mg/dl (8 mmol/L). Thus, blood glucose needs to be monitored very closely to keep it within the targets.[12]
INSULIN AND GLUCOSE THERAPY DURING INTRAPARTUM PERIOD
The hepatic glucose supply is sufficient during the latent phase of labor, but during the active phase of labor the hepatic glucose supply is depleted so calorie supplementation is required. During the active phase of labor, the supplementation is mostly in the form of intravenous glucose as the oral supplementation is restricted.[13]
The guidelines for insulin therapy during pregnancy mostly suggest infusion of insulin and glucose. The protocols for use of insulin during pregnancy are mostly based on studies in type 1 diabetes mellitus patients.
An audit of 40 pregnancies over a 4 year period was conducted to find out the blood sugar control during labor using the insulin glucose infusion and it demonstrated the practical use of a simple regimen for control of blood sugar during pregnancy. Mean blood glucose of 94 ± 40 mg/dl (5.2 ± 2.2 mmol) before delivery and 85 ± 33 mg/dl (4.7 ± 1.8 mmol) just before labor prevented neonatal hypoglycemia.[14]
In women with type 1 diabetes mellitus, a glucose infusion with insulin is mostly required during the latent period of spontaneous labor, but when the patients go into active labor the requirement of insulin drops to almost zero and the glucose requirement is equivalent to that required during rigorous exercise There is an eight-fold increase in the glucose substrate requirement during this time.
In women with type 1 diabetes mellitus, a protocol with a normal saline infusion can also be used and when the blood sugar falls below 70 mg/dl then an intravenous glucose drip can be started[15] while some protocols favor the use of glucose infusion at the rate of 125 mg/h with a simultaneous use of insulin infusion at the rate of 0.5-1 unit/h.[16,17] Both protocols are associated with low rates of complications. One less studied approach is rotating fluid, where patients can be on insulin drip or rotating fluids with continuous glucose and non-glucose drip, it has been observed that there is no difference in mean intrapartum maternal capillary blood glucose (CBG) levels between the two groups. In a study on 15 patients comparing intrapartum insulin drip or rotating fluids protocol, both regimes were found to have the same effect on the control of maternal blood glucose.[18]
In women with type 1, type 2 DM or gestational diabetes during labor either a dextrose 5% solution can be used with a fixed dose of insulin to be added to the drip or a simultaneous infusion is started with glucose infusion and the dose is titrated as per the patients’ blood sugar levels and the blood sugar is mostly maintained between 72 and 144 mg/dl (4-8 mmol/l).
The insulin requirement during the peripartum period also depends on the diabetes control during the pregnancy period. Women having uncontrolled sugars during pregnancy may require a higher dose of insulin during labor depending on whether it is active or latent labor. The women who have good glycemic control during pregnancy do not require very high dose of insulin during labor. The rapidly acting insulin analogues are comparable option in pregnant women as they help in avoiding the postprandial excursions and cause less incidence of hypoglycemia wherever it is a concern.[19,20]
In clinical practice, 6-8 units of conventional regular insulin or analogue like lispro or aspart in the same dose can be added to 500 ml of 5% dextrose normal saline (DNS) according to the requirement of the patient or if the patient's requirements are high it is advisable to start an insulin infusion with monitoring of the blood glucose every 1-2 h and to titrate the insulin infusion rate accordingly and to start simultaneous glucose infusion when the blood sugars. Patients with GDM well-controlled on life-style modification need to be monitored for blood sugars during labor. A similar protocol is followed in our center.
MONITORING OF BLOOD SUGARS DURING INTRAPARTUM PERIOD
The rapid changes in glucose and the insulin requirement in labor mandate frequent monitoring of capillary sugars in these patients which should be done every 2-4 h during the latent phase, every 1-2 h during the active phase and hourly in patients on glucose infusion. Measurement of CBG is an accurate method to check the blood sugars bedside and promptly except over high ranges and low ranges where they may give less accurate value so a laboratory confirmation for venous blood may need to be done in such cases.
During labor in a case with GDM controlled only on life-style modification it is not compulsory to monitor blood sugars periodically but monitoring once in every 4-6 h is sufficient during labor but in patients on insulin it is mandatory to monitor the blood sugar every 1-2 hourly and in all cases the blood sugars should be maintained between 80 and 110 mg/dl.
Figure 1.
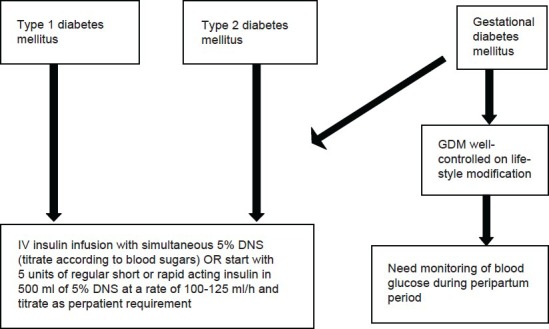
Flow chart for management of diabetes during labour (type 1DM, type 2DM, GDM)[8,16,17]
MANAGEMENT DURING CESAREAN SECTION AND INDUCTION
Any medical procedure induces physiological stress, caesarian section and general anesthesia cause release of counter regulatory hormones such as glucagon, cortisol, growth hormone, epinephrine, all the counter regulatory hormones cause insulin resistance and hyperglycemia and few cases develop ketosis. In one study, which included 21 patients in whom serial blood samples were taken before, during and after the operation and assessed for the measurement of plasma glucagon, plasma insulin and blood glucose concentrations. It was noted that there was a significant rise in plasma glucagon level during the operation and fall in insulin levels in the post-operative period despite hyperglycemia during and after the operation.[21]
In patients for whom cesarean is planned, it always preferred to do the procedure early morning. Patient needs to take her usual night dose of intermediate-acting insulin and the morning dose of insulin has to be withheld and patient needs to be kept nil by mouth. If surgery is delayed it is needed to start basal and corrective regimen (DNS with short acting insulin) with one-third of the morning intermediate insulin dose with a 5% dextrose infusion to avoid ketosis. Blood glucose has to be monitored second hourly and if required subcutaneous dose of corrective dose of short acting insulin to be given. Hyperglycemia should be avoided during the surgery to reduce the risk of neonatal hypoglycemia and wound infections in the mother. During the induction of labor which again should be planned early morning and similarly the woman should take her usual night dose of intermediate acting insulin and if labor is prolonged they have to be supplemented with 5% dextrose when blood glucose falls below100 mg/dl.
MANAGEMENT IMMEDIATELY AFTER DELIVERY
After delivery, the requirement of insulin shows a sharp decline and in GDM it is advisable to continue the monitoring to see if the sugars have become normal in the postpartum period but in cases with type 1 and type 2 DM it is not prudent to stop the insulin rather the dose of insulin needs to be decreased by 20-40% of the pregnancy dose as the requirement of insulin during lactation is less. During the breast-feeding, sometimes the requirement of insulin can fall drastically and these women may develop hypoglycemia, so the dose of insulin needs to be adjusted accordingly.[22,23] The monitoring of GDM women with a fasting and postprandial sugar is a good method to diagnose T2DM, about 15% of GDM remain glucose intolerant or develop type 2DM in the postpartum period and 10-50% of the women develop diabetes within 5 years of delivery.[24] Provision of proper obstetric and neonatal care is of prime concern after delivery. Immediate postpartum type 2 diabetes is uncommon in women with GDM. The glucose targets during the postpartum period in women who have undergone cesarean section are not very clear, but strict control is needed to prevent any infections. Patients with uncontrolled sugars have improper control have high chances of post-operative complications.
AFTER DISCHARGE
If the patient has diabetes post-pregnancy then she needs to be counseled about medical nutrition therapy and self-monitoring of blood glucose and physical activity is advised.[25] The diet is advised such that it is good for the mother's nutrition, lactation and also for the infant's health. If the patient continues to have type 1 diabetes, which was diagnosed for the first time in pregnancy as GDM then insulin therapy is immediately instituted. If type 2 diabetes is suspected after delivery then insulin therapy is continued until the mother is breast-feeding but among the oral hypoglycemic agents like glibenclamide and glipizide can be used as they don’t pass in large amounts in breast milk.[26] As for the use of metformin small studies have shown that it is excreted in small amounts in breast milk.[27,28] The women should be counseled about the potential problems with the use of oral hypoglycemic agents during lactation and should preferably be put on insulin till they breastfeed the baby.
More thrust should be given to the life-style modification in women with GDM as they have a high chance of developing diabetes if they are not cautious with their life-style modification. For women with GDM whose sugars are normal after delivery an OGTT with 75 g of glucose can be planned after 6 weeks of delivery to see if the sugars have normalized.[29] Some studies have even used short message service (text) system for these women to remind them for testing for type 2DM.[30]
CONTROVERSIES - ROLE OF ORAL ANTI-DIABETIC AGENTS VERSUS INSULIN IN PREGNANCY
Only few studies have assessed the risk of oral anti diabetic agents versus insulin in patients with gestational diabetes. In the largest trial (n = 404) comparing glyburide and insulin, 49% of the women on insulin underwent cesarean delivery as compared with 46% of those on glyburide.[31] A second trial reported no difference in cesarean delivery rates for 51 women on glyburide, insulin or acarbose. Three trials found no statistically significant differences in glucose control between women treated with insulin and those receiving glyburide.[31,32] One of these studies considered pre-eclampsia, two studies included maternal weight and two other studies included information on maternal hypoglycemia. In one large trial which compared glyburide versus insulin and which included 404 patients 46% of the women underwent cesarean section in the glyburide group versus 49% in the insulin group.[32,33] Because of the limited number of randomized control trials and the lack of consistency in the maternal outcomes measured across studies; the overall strength of evidence is very low. The oral anti diabetic agents are not used during peripartum period as most of these patients are not orally allowed and are on insulin and glucose infusions during that period and also there is no Food and Drug Administration approval for their use during pregnancy.
CONCLUSION
The women with diabetes in pregnancy need a good control irrespective of the type of diabetes and antepartum control of sugars is necessary to achieve a good peripartum control which reduces the risk of neonatal complications. The judicious use of insulin is the key to have a normal delivery of a healthy child and to prevent neonatal hypoglycemia. Postpartum counseling for life-style modification in the future is very important for future prevention of type 2DM in women with GDM.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mimouni F, Miodovnik M, Siddiqi TA, Khoury J, Tsang RC. Perinatal asphyxia in infants of insulin-dependent diabetic mothers. J Pediatr. 1988;113:345–53. doi: 10.1016/s0022-3476(88)80282-8. [DOI] [PubMed] [Google Scholar]
- 2.Yeast JD, Porreco RP, Ginsberg HN. The use of continuous insulin infusion for the peripartum management of pregnant diabetic women. Am J Obstet Gynecol. 1978;131:861–4. doi: 10.1016/s0002-9378(16)33132-5. [DOI] [PubMed] [Google Scholar]
- 3.Garabedian C, Deruelle P. Delivery (timing, route, peripartum glycemic control) in women with gestational diabetes mellitus. Diabetes Metab. 2010;36:515–21. doi: 10.1016/j.diabet.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Castorino K, Jovanovič L. Pregnancy and diabetes management: Advances and controversies. Clin Chem. 2011;57:221–30. doi: 10.1373/clinchem.2010.155382. [DOI] [PubMed] [Google Scholar]
- 5.Kline GA, Edwards A. Antepartum and intra-partum insulin management of type 1 and type 2 diabetic women: Impact on clinically significant neonatal hypoglycemia. Diabetes Res Clin Pract. 2007;77:223–30. doi: 10.1016/j.diabres.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Iafusco D, Stoppoloni F, Salvia G, Vernetti G, Passaro P, Petrovski G, et al. Use of real time continuous glucose monitoring and intravenous insulin in type 1 diabetic mothers to prevent respiratory distress and hypoglycaemia in infants. BMC Pregnancy Childbirth. 2008;8:23. doi: 10.1186/1471-2393-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol. 2011;25:65–76. doi: 10.1016/j.bpobgyn.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675–85. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 9.Garber AJ, Moghissi ES, Bransome ED, Jr, Clark NG, Clement S, Cobin RH, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(Suppl 2):4–9. doi: 10.4158/EP.10.S2.4. [DOI] [PubMed] [Google Scholar]
- 10.Curet LB, Izquierdo LA, Gilson GJ, Schneider JM, Perelman R, Converse J. Relative effects of antepartum and intrapartum maternal blood glucose levels on incidence of neonatal hypoglycemia. J Perinatol. 1997;17:113–5. [PubMed] [Google Scholar]
- 11.Carron Brown S, Kyne-Grzebalski D, Mwangi B, Taylor R. Effect of management policy upon 120 Type 1 diabetic pregnancies: Policy decisions in practice. Diabet Med. 1999;16:573–8. doi: 10.1046/j.1464-5491.1999.00124.x. [DOI] [PubMed] [Google Scholar]
- 12.Barrett HL, Morris J, McElduff A. Watchful waiting: A management protocol for maternal glycaemia in the peripartum period. Aust N Z J Obstet Gynaecol. 2009;49:162–7. doi: 10.1111/j.1479-828X.2009.00969.x. [DOI] [PubMed] [Google Scholar]
- 13.Maheux PC, Bonin B, Dizazo A, Guimond P, Monier D, Bourque J, et al. Glucose homeostasis during spontaneous labor in normal human pregnancy. J Clin Endocrinol Metab. 1996;81:209–15. doi: 10.1210/jcem.81.1.8550753. [DOI] [PubMed] [Google Scholar]
- 14.Njenga E, Lind T, Taylor R. Five year audit of peripartum blood glucose control in type 1 diabetic patients. Diabet Med. 1992;9:567–70. doi: 10.1111/j.1464-5491.1992.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 15.Kitzmiller JL, Gavin L. Preexisting diabetes and pregnancy. In: Lavin N, editor. Manual of Endocrinology and Metabolism. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2002. Copyright © 2002 Lippincott Williams and Wilkins. [Google Scholar]
- 16.Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep. 2012;12:33–42. doi: 10.1007/s11892-011-0249-0. [DOI] [PubMed] [Google Scholar]
- 17.Caplan RH, Pagliara AS, Beguin EA, Smiley CA, Bina-Frymark M, Goettl KA, et al. Constant intravenous insulin infusion during labor and delivery in diabetes mellitus. Diabetes Care. 1982;5:6–10. doi: 10.2337/diacare.5.1.6. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg VA, Eglinton GS, Rauch ER, Skupski DW. Intrapartum maternal glycemic control in women with insulin requiring diabetes: A randomized clinical trial of rotating fluids versus insulin drip. Am J Obstet Gynecol. 2006;195:1095–9. doi: 10.1016/j.ajog.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic L, Pettitt DJ. Treatment with insulin and its analogs in pregnancies complicated by diabetes. Diabetes Care. 2007;30(Suppl 2):S220–4. doi: 10.2337/dc07-s220. Erratum in: Diabetes Care 2007;30:3154. [DOI] [PubMed] [Google Scholar]
- 20.Lapolla A, Dalfrà MG, Fedele D. Insulin therapy in pregnancy complicated by diabetes: Are insulin analogs a new tool? Diabetes Metab Res Rev. 2005;21:241–52. doi: 10.1002/dmrr.551. [DOI] [PubMed] [Google Scholar]
- 21.Russell RC, Walker CJ, Bloom SR. Hyperglucagonaemia in the surgical patient. Br Med J. 1975;1:10–2. doi: 10.1136/bmj.1.5948.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: The SWIFT cohort. Diabetes Care. 2012;35:50–6. doi: 10.2337/dc11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringholm L, Mathiesen ER, Kelstrup L, Damm P. Managing type 1 diabetes mellitus in pregnancy – From planning to breastfeeding. Nat Rev Endocrinol. 2012;8:659–67. doi: 10.1038/nrendo.2012.154. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25:1862–8. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 25.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 26.Feig DS, Briggs GG, Kraemer JM, Ambrose PJ, Moskovitz DN, Nageotte M, et al. Transfer of glyburide and glipizide into breast milk. Diabetes Care. 2005;28:1851–5. doi: 10.2337/diacare.28.8.1851. [DOI] [PubMed] [Google Scholar]
- 27.Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia. 2002;45:1509–14. doi: 10.1007/s00125-002-0939-x. [DOI] [PubMed] [Google Scholar]
- 28.Gardiner SJ, Kirkpatrick CM, Begg EJ, Zhang M, Moore MP, Saville DJ. Transfer of metformin into human milk. Clin Pharmacol Ther. 2003;73:71–7. doi: 10.1067/mcp.2003.9. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heatley E, Middleton P, Hague W, Crowther C. The DIAMIND study: Postpartum SMS reminders to women who have had gestational diabetes mellitus to test for type 2 diabetes: A randomised controlled trial-Study protocol. BMC Pregnancy Childbirth. 2013;13:92. doi: 10.1186/1471-2393-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–8. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 32.Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract. 2007;76:474–5. doi: 10.1016/j.diabres.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Bertini AM, Silva JC, Taborda W, Becker F, Lemos Bebber FR, Zucco Viesi JM, et al. Perinatal outcomes and the use of oral hypoglycemic agents. J Perinat Med. 2005;33:519–23. doi: 10.1515/JPM.2005.092. [DOI] [PubMed] [Google Scholar]


