Abstract
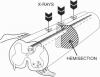
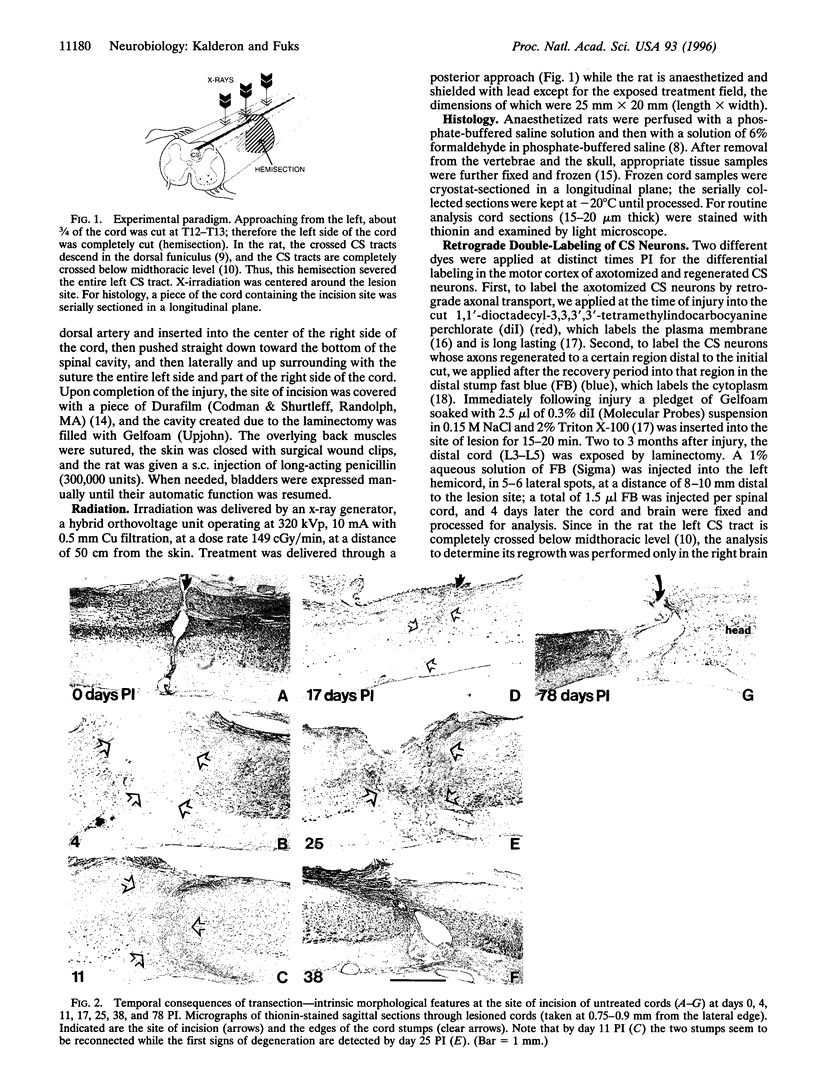
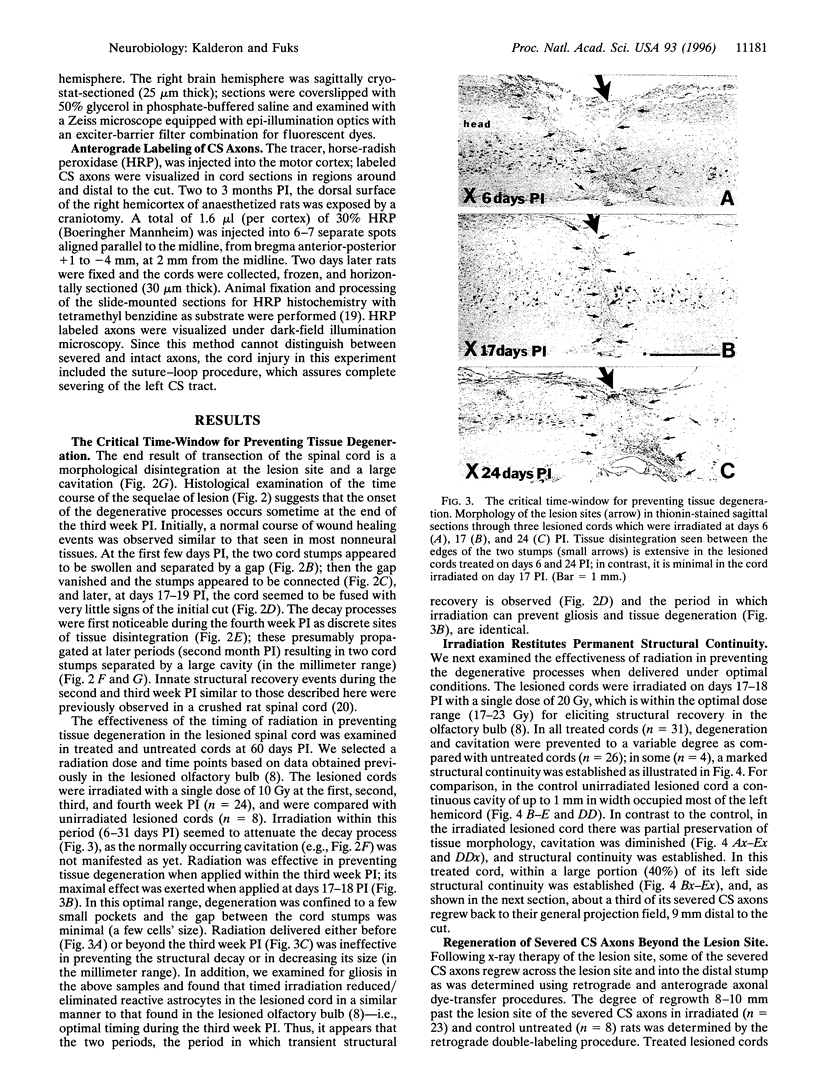
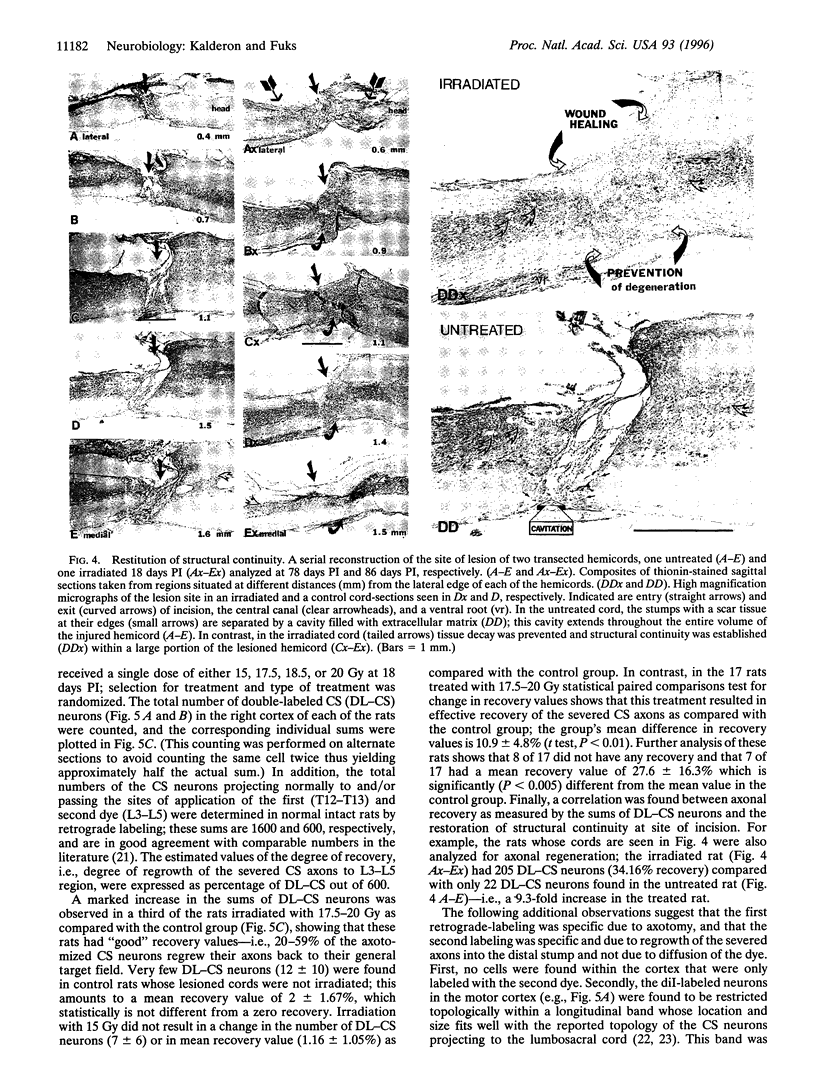
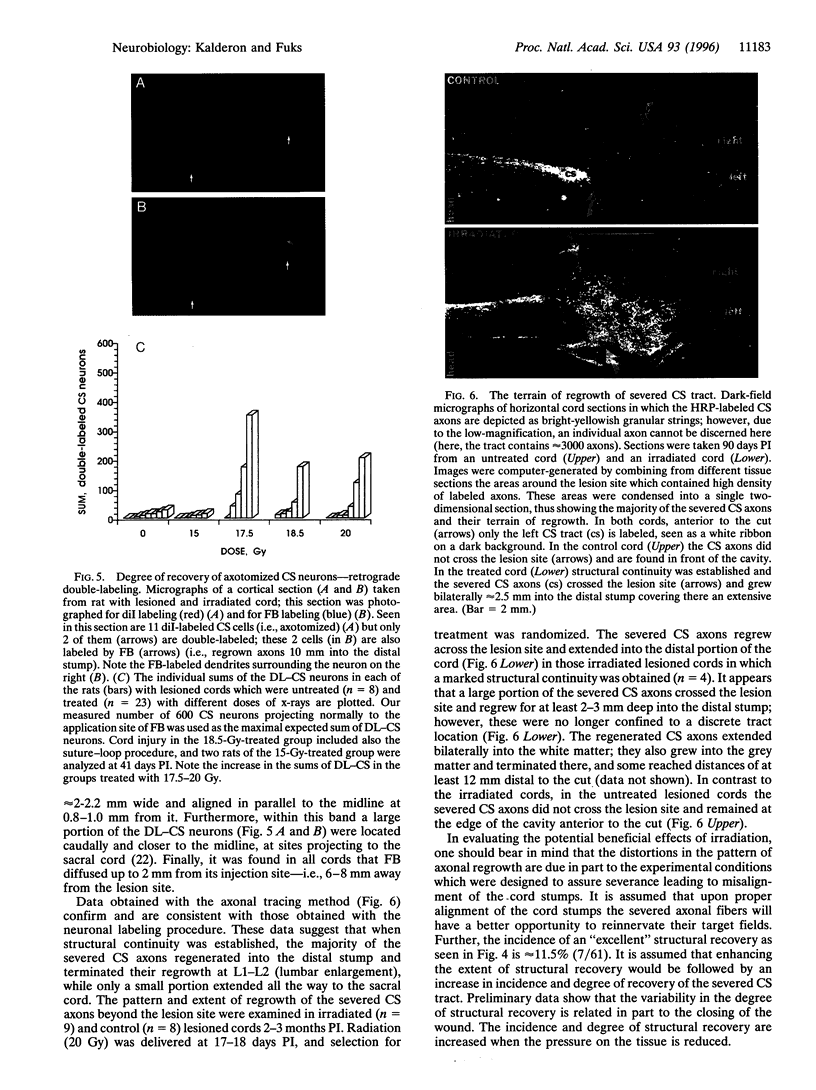
Mechanical injury to the adult mammalian spinal cord results in permanent morphological disintegration including severance/laceration of brain-cord axons at the lesion site. We report here that some of the structural consequences of injury can be averted by altering the cellular components of the lesion site with x-irradiation. We observed that localized irradiation of the unilaterally transected adult rat spinal cord when delivered during a defined time-window (third week) postinjury prevented cavitation, enabled establishment of structural integrity, and resulted in regrowth of severed corticospinal axons through the lesion site and into the distal stump. In addition, we examined the natural course of degeneration and cavitation at the site of lesion with time after injury, noting that through the third week postinjury recovery processes are in progress and only at the fourth week do the destructive processes take over. Our data suggest that the adult mammalian spinal cord has innate mechanisms required for recovery from injury and that timed intervention in certain cellular events by x-irradiation prevents the onset of degeneration and thus enables structural regenerative processes to proceed unhindered. We postulate that a radiation-sensitive subgroup of cells triggers the delayed degenerative processes. The identity of these intrusive cells and the mechanisms for triggering tissue degeneration are still unknown.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Anderson W. J. Experimental reorganization of the cerebellar cortex. I. Morphological effects of elimination of all microneurons with prolonged x-irradiation started at birth. J Comp Neurol. 1972 Nov;146(3):355–406. doi: 10.1002/cne.901460305. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M., Kuypers H. G., Catsman-Berrevoets C. E., Loewe H., Dann O. Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci Lett. 1980 May 15;18(1):25–30. doi: 10.1016/0304-3940(80)90208-6. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F., Patterson R. C. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975 Oct;4(5):573–585. doi: 10.1007/BF01351538. [DOI] [PubMed] [Google Scholar]
- Bresnahan J. C. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J Neurol Sci. 1978 Jun;37(1-2):59–82. doi: 10.1016/0022-510x(78)90228-9. [DOI] [PubMed] [Google Scholar]
- Brown L. T., Jr Projections and termination of the corticospinal tract in rodents. Exp Brain Res. 1971 Oct 25;13(4):432–450. doi: 10.1007/BF00234340. [DOI] [PubMed] [Google Scholar]
- Guth L., Barrett C. P., Donati E. J., Anderson F. D., Smith M. V., Lifson M. Essentiality of a specific cellular terrain for growth of axons into a spinal cord lesion. Exp Neurol. 1985 Apr;88(1):1–12. doi: 10.1016/0014-4886(85)90109-8. [DOI] [PubMed] [Google Scholar]
- Hicks S. P., D'Amato C. J. Locating corticospinal neurons by retrograde axonal transport of horseradish peroxidase. Exp Neurol. 1977 Aug;56(2):410–420. doi: 10.1016/0014-4886(77)90357-0. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986 Jul;103(1):171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Bezhadian M. A., Caldwell R. B. Astrocytes modulate retinal vasculogenesis: effects on endothelial cell differentiation. Glia. 1995 Sep;15(1):1–10. doi: 10.1002/glia.440150102. [DOI] [PubMed] [Google Scholar]
- Kakulas B. A. The clinical neuropathology of spinal cord injury. A guide to the future. Paraplegia. 1987 Jun;25(3):212–216. doi: 10.1038/sc.1987.37. [DOI] [PubMed] [Google Scholar]
- Kalderon N., Alfieri A. A., Fuks Z. Beneficial effects of x-irradiation on recovery of lesioned mammalian central nervous tissue. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10058–10062. doi: 10.1073/pnas.87.24.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon N. Differentiating astroglia in nervous tissue histogenesis/regeneration: studies in a model system of regenerating peripheral nerve. J Neurosci Res. 1988 Oct-Dec;21(2-4):501–512. doi: 10.1002/jnr.490210241. [DOI] [PubMed] [Google Scholar]
- Kalderon N., Fuks Z. Severed corticospinal axons recover electrophysiologic control of muscle activity after x-ray therapy in lesioned adult spinal cord. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11185–11190. doi: 10.1073/pnas.93.20.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian J. G., Guth L., Donati E. J. Origin of the connective tissue scar in the transected rat spinal cord. Exp Neurol. 1981 Jun;72(3):698–707. doi: 10.1016/0014-4886(81)90018-2. [DOI] [PubMed] [Google Scholar]
- Leong S. K. Localizing the corticospinal neurons in neonatal, developing and mature albino rat. Brain Res. 1983 Apr 11;265(1):1–9. doi: 10.1016/0006-8993(83)91327-6. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W., Alexander S. W., Miller F. D., Bisby M. A. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci. 1991 Aug;11(8):2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullan J., Artieda J. Somatotopy of the corticospinal neurons in the rat. Neurosci Lett. 1981 Jan 1;21(1):13–18. doi: 10.1016/0304-3940(81)90049-5. [DOI] [PubMed] [Google Scholar]
- Vahlsing H. L., Feringa E. R. A ventral uncrossed corticospinal tract in the rat. Exp Neurol. 1980 Nov;70(2):282–287. doi: 10.1016/0014-4886(80)90027-8. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M., Villegas-Pérez M. P., Bray G. M., Aguayo A. J. Persistent retrograde labeling of adult rat retinal ganglion cells with the carbocyanine dye diI. Exp Neurol. 1988 Oct;102(1):92–101. doi: 10.1016/0014-4886(88)90081-7. [DOI] [PubMed] [Google Scholar]
- Wrathall J. R., Pettegrew R. K., Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985 Apr;88(1):108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]