Abstract
A second-generation solar disinfection (SODIS) system (pouch) was constructed from food-grade, commercially available packaging materials selected to fully transmit and amplify the antimicrobial properties of sunlight. Depending upon the season, water source, and challenge organism, culturable bacteria were reduced between 3.5 and 5.5 log cycles. The system was also capable of reducing the background presumptive coliform population in nonsterile river water below the level of detection. Similar experiments conducted with a model virus, the F-specific RNA bacteriophage MS2, indicated that the pouch was slightly less efficient, reducing viable plaques by 3.5 log units in comparison to a 5.0 log reduction of enterotoxigenic Escherichia coli O18:H11 within the same time period. These results suggest that water of poor microbiological quality can be improved by using a freely available resource (sunlight) and a specifically designed plastic pouch constructed of food-grade packaging materials.
Though estimates vary widely, it is thought that exposure to water of poor microbiological quality results in the deaths of 10,000 to 20,000 children every day (7). Most of this human suffering and loss is borne by developing countries where the availability of clean drinking water is highly unpredictable due to a variety of socioeconomic, environmental, and geopolitical factors. In order to reduce or eliminate this needless suffering, the development and dissemination of inexpensive, reliable water disinfection systems should be considered a priority in global efforts to improve pediatric health. As sunlight is freely available and has been identified as the most important environmental factor responsible for the normal inactivation of fecal indicator bacteria and bacteriophages in both seawater (18) and freshwater supplies (19), it represents an ideal energy resource for use in the purification of microbiologically contaminated water. Numerous investigations have demonstrated the effectiveness of the solar disinfection (SODIS) of water and established it as an emerging water treatment process meriting continued development (21).
Efficient, large-scale SODIS systems have been designed that decontaminate drinking water by relying on heat alone (10) or on a combination of heat and light (21). A potential drawback of these systems is the significant investment of resources required for apparatus construction. Simpler, less expensive methods have relied on the use of polyethylene (PET) bags (placed on a black or steel surface) or PET soft drink bottles (clear or partially blackened). Such systems have proven effective in the disinfection of river or pond water contaminated with fecal coliforms and Vibrio cholerae, where 140 min of exposure to sunlight was sufficient to reduce culturable bacteria 3.0 log units, an effect that has been attributed to heating and UV-A radiation (21). In a controlled clinical trial involving 206 Maasai children, the exposure of drinking water in 1.5-liter plastic bottles to sunlight for a full day reduced the incidence of severe diarrhea over a 3-month period by 26% (2), revealing that pediatric health can be improved even with a nonoptimized, simple, and inexpensive water purification system. Given these results, it is reasonable to assume that a more effective, second-generation SODIS system could be constructed from food-grade packaging materials specifically selected to transmit and amplify the physiochemical properties of sunlight. The aim of this study was the development and evaluation of such a system.
SODIS pouches were constructed by fusing a UV-transmitting upper layer to one of two surfaces: (i) metallized plastic to reflect light or (ii) an absorptive, black plastic to increase water temperature. A reflective pouch was assembled by bonding two food-grade packaging films: (i) Liquiflex 7131-K, a transparent multilayer composite of 60-gauge (100 gauge = 1 mil or 1/1000 in.) biaxially oriented nylon (for strength), adhesive, and 3-mil linear low-density PET (for sealing) (Curwood, Inc., Oshkosh, Wis.) and (ii) Curlam Grade 9019-K, a reflective multilayer composite of 48-gauge PET (for strength), adhesive, metallized 48-gauge PET, adhesive, and 3-mil linear low-density PET (for sealing) (Curwood, Inc.). An absorptive pouch was assembled by bonding the identical transparent upper layer to a food-grade packaging film consisting of 48-gauge PET-ink (100% black)-adhesive-3-mil linear low-density PET (for sealing) (Curwood, Inc.). The pouch materials were cut to the dimensions of 20 by 28 cm and bonded by heat sealing near the edge on three sides by using a bench scale impulse sealer (Clamco Corp., Cleveland, Ohio). The individual pouches were filled with 1.0 liter of water, and after most of the residual air was removed, they were heat sealed on the fourth side. When placed horizontally on a flat surface, they assumed a lens-like, convex configuration (approximately 4.0 cm in depth).
The initial SODIS experiments were conducted at the Nestlé Product Technology Center, New Milford, Conn. (latitude, 41°34′43"N; longitude, 73°24′45"W) between 20 October and 13 November 1998, dates that are characterized by significant declines in both temperature and cumulative solar radiation (insolation) in the New England states. Natural spring water (Poland Spring Water Co., Poland, Maine) was autoclaved (20 min at 121°C and 15 psig), cooled, transferred in 1.0-liter volumes to individual pouches, and inoculated at a rate of approximately 3.0 × 106 CFU/ml with an overnight culture of one of the following: Escherichia coli FDA strain Seattle 1946 (ATCC 25922), Staphylococcus aureus 485 FDA, Salmonella enterica serovar Typhimurium ATCC 13311, or Shigella sonnei (ATCC 11060). The challenge organisms were injected by syringe in a 1.0-ml volume of culture broth through a 12-mm rubber septum attached to an outer edge of the upper pouch surface. The SODIS pouches were placed in full sunlight for 6 h, during which time they were shielded from the wind by being placed within a three-sided cardboard box (49.5 cm wide by 49.5 cm deep by 33.5 cm high) lined with aluminum foil on the sides and bottom, as this has been reported to enhance the efficiency of solar systems (15). Nontreated controls were maintained in the same environment but shielded from sunlight beneath an inverted cardboard box (49.5 cm wide by 49.5 cm deep by 33.5 cm high). Environmental and water temperatures were recorded by thermocouple or thermometer. Aliquots of contaminated water were withdrawn periodically, serially diluted in peptone water, and pour plated in Difco plate count agar (PCA) (Becton Dickinson and Co., Sparks, Md.) and/or Difco brain heart infusion agar for the estimation of viable bacteria. All plates were incubated for 48 h at 37°C before determination of the number of colony-forming units.
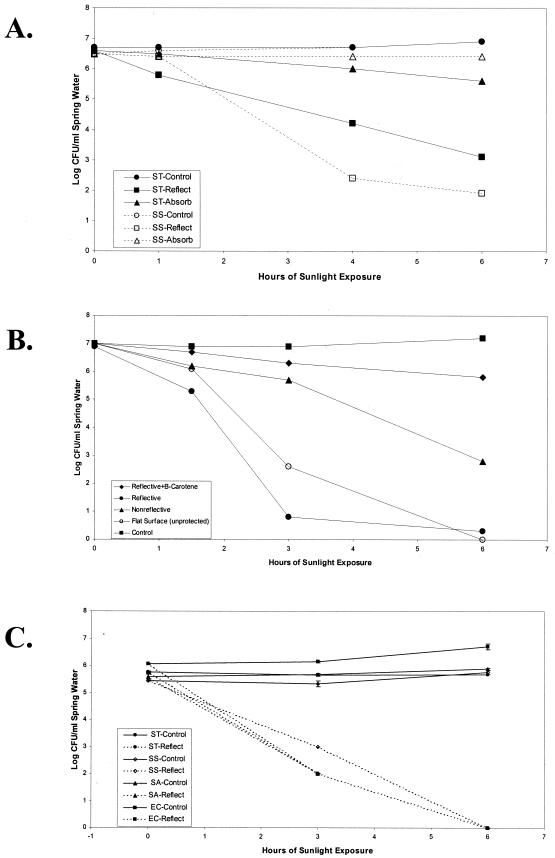
Over the course of 6 h, the culturable population of Salmonella serovar Typhimurium declined no more than 1.0 log in the absorptive pouch, and the internal water temperature reached a maximum of only 35°C. The reflective pouch (maximum internal temperature, 27°C) proved much more effective as the detectable bacterial population declined in excess of 3.5 logs relative to the control (maximum internal temperature, 9°C), indicating that light reflection is more important than a modest temperature increase in bacterial inactivation (Fig. 1A). Shigella sonnei proved even more sensitive to sunlight, declining 4.5 logs in 6 h within the reflective pouch (maximum internal temperature, 18°C). In contrast, a comparable population contained in the absorptive pouch (maximum internal temperature, 21°C) appeared as stable as the control population (maximum internal temperature, 11°C) (Fig. 1A). These results supported preliminary findings according to which the absorptive pouch failed to provide a level of inactivation comparable to the reflective pouch even when the former reached a maximum internal temperature of 52°C versus 36°C in the latter (data not shown). As a result, the remainder of the study focused on the inactivation kinetics of sunlight as transmitted and amplified by the reflective pouch only.
FIG. 1.
Inactivation rates of bacteria contained in spring water in response to variation in the design of SODIS pouches. (A) Impact of major system components on the survival of target organisms when exposed in reflective (Reflect) or absorptive (Absorb) pouches relative to controls protected from sunlight. (B) Impact of minor system components on the survival of E. coli ATCC 25922 in reflective pouches placed within a three-sided cardboard box lined with aluminum foil (Reflective), a comparable box without aluminum foil (Nonreflective), unprotected on the flat surface, or completely covered by a four-sided cardboard box (Control). An additional pouch containing β-carotene was similarly inoculated and placed in the reflective box (Reflective + B-carotene). (C) Reproducibility of the inactivation of four challenge organisms contained in reflective pouches exposed to sunlight (Reflect) or protected beneath a cardboard box (Control). ST, Salmonella serovar Typhimurium; SS, Shigella sonnei; EC, E. coli.
SODIS reportedly involves absorptive heating (11, 15), photooxidation, and direct photochemical effects (19) which can damage microorganisms through denaturation, oxygen radical production, and the breakage and UV cross-linking of nucleic acids. The antimicrobial effectiveness of sunlight is influenced by the physiochemical properties of the water such as salinity, UV-absorbing organic materials (3), aeration (oxygen content) (14), and accessory physical features such as the presence of reflectors to increase water temperature (15). The next phase of the study, designed to identify the minimal system components necessary for effective SODIS, incorporated a single challenge organism (E. coli ATCC 25922) and only reflective pouches. Identical pouches were placed as follows: (i) in a standard reflective box, (ii) in a standard reflective box with the addition of 0.29 mM water-soluble β-carotene as an antioxidant (Sigma Chemical Company, St. Louis, Mo.), (iii) in a cardboard box with no reflective lining (nonreflective), (iv) on a flat surface (unprotected), and (v) under an inverted cardboard box (control). The efficacy of the reflective SODIS pouch was confirmed as E. coli counts declined almost 7.0 logs when the reflective pouch (maximum internal temperature, 31°C) was placed within a reflective box during a 6-h exposure (external temperature, 25°C) (Fig. 1B). Comparable results were obtained when the pouch (maximum internal temperature, 29°C) was placed unprotected on the same surface, indicating that environmental shielding and the presence of external reflectors had no impact on effectiveness. In contrast, shielding the plastic pouch within a nonreflective cardboard box actually reduced the level of disinfection by almost 3.0 logs. This decline is most likely due to the blocking and absorption of scattered light that might otherwise have reached the pouch. Lastly, the addition of β-carotene to water reduced the effectiveness of sunlight against E. coli by almost 6.0 log units. This reduction in efficacy could be attributed to the antioxidative activity of β-carotene, quenching the reactive species produced by the solar photooxidation of dissolved oxygen (H2O2, superoxide, and hydroxyl radical). This hypothesis is supported by reports that dissolved oxygen is a critical factor in the rapid SODIS of drinking water inoculated with fecal indicator bacteria (E. coli and Enterococcus faecalis) (13) or naturally contaminated with fecal coliforms and fecal streptococci (14). As the addition of β-carotene also produced a reddish opacity, an alternative explanation for the decline in efficiency could be the simple absorption of light before it could reach the challenge microorganism. Additional experimentation would be required to fully evaluate the scientific merit of these alternative explanations. Taken together, the results cited above simplify the ultimate design of the SODIS pouch as no additional packaging materials or concepts need be incorporated for effective water disinfection other than a resealable closure.
As reproducibility and effectiveness are critical prerequisites for any water disinfection system intended for human use, additional reflective pouch trials, consisting of three replicates of four target organisms, were conducted in May 2000. E. coli, Salmonella serovar Typhimurium, Shigella sonnei, and S. aureus were inoculated into sterile spring water at concentrations varying between 2.8 × 105/ml and 1.2 × 106/ml and placed under full sunlight for 6 h (11:00 a.m. through 5:00 p.m.) where maximum reflective pouch temperatures reached 34°C. The populations of all four challenge organisms declined in logarithmic fashion during the 6-h exposure and were essentially undetectable at the end of the experiment (Fig. 1C). With the exception of Salmonella serovar Typhimurium, the control populations increased slightly during the same time, possibly due to the incorporation of a small amount of medium (1.0 ml) with the inoculation. These data confirm the antimicrobial effectiveness of the new reflective SODIS pouch when the water to be treated is low in visible particulate matter.
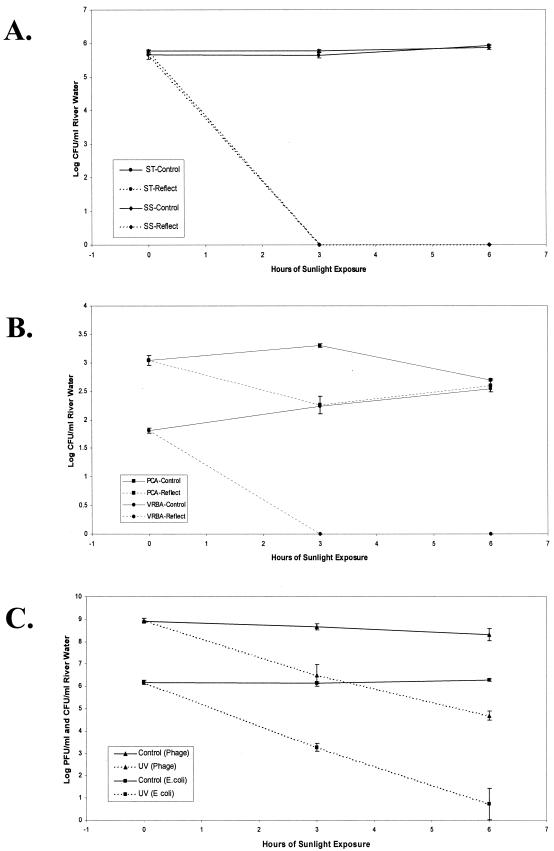
As the quality and composition of drinking water are expected to vary considerably and influence the efficacy of SODIS systems, the remaining experiments were conducted with water obtained from a natural source (the Housatonic River, New Milford, Conn.) containing a variety of particulate, mineral, and organic matter. River water collected in May 2000 was allowed to settle for 30 min, sterilized in 1.0-liter volumes (as described above), and cooled rapidly. The river water was mechanically reaerated by shaking, transferred aseptically to reflective pouches (in triplicate), inoculated with either Salmonella serovar Typhimurium or Shigella sonnei, and exposed to 6 h of sunlight (12:30 p.m. to 6:30 p.m.; maximum internal temperature, 35°C). The apparent antimicrobial effectiveness of the system was even greater with river water than with spring water, as the culturable populations of both challenge organisms declined 5.5 log units within 3 h, becoming essentially undetectable by the end of exposure (Fig. 2A). The increased rate of inactivation was most likely due to the natural increase in solar radiation that is typical of late spring in the New England states and not to an inherent difference in the physiochemical or antimicrobial properties of the water source. This interpretation is supported by the relative stability (culturability) that was observed in the Salmonella serovar Typhimurium and Shigella sonnei control populations maintained in the same river water.
FIG. 2.
SODIS of various test organisms contained in Housatonic River water and exposed to sunlight in a reflective pouch. (A) Inactivation of Salmonella serovar Typhimurium (ST) and Shigella sonnei (SS) inoculated in sterilized water and placed in direct sunlight (Reflect) or protected beneath a cardboard box (Control). (B) Inactivation of the native microflora of nonsterile river water exposed to sunlight (Reflect) or protected beneath a cardboard box (Control), where total viable bacteria and presumptive coliforms were enumerated on PCA and VRBA, respectively. (C) Inactivation of MS2 bacteriophage (ATCC 15597-B1) and E. coli O18:H11 (ATCC 35401) inoculated in sterilized river water and exposed to sunlight (UV) or protected beneath a cardboard box (Control). All values cited above are presented as the means with two standard deviations.
It is possible that the solar sensitivity of laboratory-grown bacteria may differ from that of diarrheal agents sporadically present in water supplies. In order to address this issue, the antimicrobial effectiveness of the reflective pouch against the background microflora of a natural water source (the Housatonic River) was evaluated. Nonsterile river water was collected as described above, transferred in 1.0-liter volumes to reflective pouches (in triplicate), and periodically evaluated for the presence of total background microflora (on PCA) and presumptive coliforms (on Difco violet red bile agar [VRBA]) during a 6-h exposure to sunlight. As was observed previously with the specific challenge organisms (E. coli, Salmonella serovar Typhimurium, and Shigella sonnei), the detectable population of presumptive coliforms (growth on VRBA) declined in excess of 1.5 logs in 3 h and was unrecoverable thereafter (Fig. 2B). This result supports the hypothesis that the reflective pouch is capable of inactivating a subset of the microbial population often associated with waterborne, diarrheal disease. In contrast, the total microbial population (growth on PCA) declined less than 1.0 log units within 3 h and then rebounded slightly by 6 h. This rebound phenomenon could result from spore germination and/or a disproportionate increase in sunlight-resistant microorganisms in response to a decline in competition. Although solar UV radiation is known to limit bacterial spore longevity in the natural environment (20), spores are typically 1.0 to 2.0 orders of magnitude more resistant to UV light than vegetative cells (reviewed in reference 17).
In order to evaluate the potential contributions of spore germination and/or other microbiological phenomena to the rebound effect seen in the total population, the survivors from a second river water source were evaluated following exposure to sunlight. River water (Milwaukee River; latitude, 43°01′32"N; longitude, 87°54′10"W), treated for 7 h in reflective pouches as described above, was dilution plated at intervals to both PCA (total count) and BBL VRBA plus 4-methylumbelliferyl-β-D-glucuronide (Becton Dickinson and Co.) (presumptive coliforms). As with the Housatonic River water, the presumptive coliform population declined 2.0 logs in 7 h and detectable E. coli were eliminated (data not shown). During the same exposure interval, the counts on PCA declined from 4.6 × 103 to 1.7 × 103 CFU/ml, and microscopic evaluation of 10 predominant colony types revealed that the majority of the surviving bacteria displayed a filamentous morphology like that of Hyphomicrobium spp. Hyphal, budding bacteria like Hyphomicrobium, which are common in surface water supplies and waste treatment plant effluent (9), are not considered a health threat. It is possible that the filamentous structure protected internal bacteria from the full effects of solar radiation and that these bacteria were subsequently released by the shaking that precedes sample withdrawal. Three of the remaining colony types (30.0%) proved to be gram-positive rods and were presumed Bacillus spp. The presumptive bacilli were streaked to the surface of Difco nutrient agar (Becton Dickinson), grown overnight at 30°C, transferred to API 50 CHB/E medium (bioMérieux, Inc., Hazelwood, Mo.) and evaluated for carbohydrate metabolism by an API 50 CH strip (bioMérieux, Inc.) according to the manufacturer's instructions. The carbohydrate usage patterns indicated that two of the colonies were most likely Bacillus pumilus (99.9%) and that the third was Bacillus megaterium (99.8%). As with the hyphal bacteria, minimal spore survival and growth during the SODIS treatment of water is unlikely to constitute a significant threat of diarrheal illness. More importantly, the presumptive coliforms, which are more representative of diarrheal agents, were unrecoverable following solar exposure.
Acute gastroenteritis is one of the most common illnesses reported in the United States, and the application of molecular diagnostic methods suggest that the enteric calciviruses, or Calciviridae (formerly referred to as the Norwalk family of viruses), are the dominant etiologic agents (reviewed by Glass et al. in reference 6). Since these single-stranded RNA Norwalk-like viruses are suspected to cause 90% of the acute viral gastroenteritis worldwide (5, 8) and are second only to rotavirus as causative agents of pediatric diarrhea (12), candidate disinfection systems should be evaluated for efficacy against this class of infectious agent. Bypassing the technical difficulty of estimating viable populations of enteric viruses, bacteriophages have served as both indicators and surrogates in the evaluation of water quality (4).
The effectiveness of the reflective pouch was evaluated against a model virus of the Leviviridae family, the F-specific RNA bacteriophage MS2 (ATCC 15597-B1), between January and March 2002. MS2 was propagated according to the instructions of the American Type Culture Collection (Manassas, Va.) against the host E. coli strain C3000 (ATCC 15597) grown in Difco tryptic soy broth, serially diluted, and enumerated in tryptic soy soft-overlay agar (0.75%) amended to contain CaCl2 (11 mM). The concentrated MS2 suspension (2.7 × 1011 PFU/ml) was diluted in pouches containing sterile Housatonic River water to a final concentration of 8.1 × 108 PFU/ml (three replicates), exposed to full sunlight for a 6-h period (∼9:00 a.m. to ∼3:00 p.m.), and periodically evaluated for bacteriophage viability (infectivity). In order to compare the effectiveness of the SODIS pouch against the MS2 bacteriophage relative to a bacterial contaminant, additional pouches of sterile river water were inoculated with an overnight culture of enterotoxigenic E. coli serotype O18:H11 (ATCC 35401) and exposed to full sunlight as with the model virus.
Viable MS2 bacteriophage, calculated as plaque-forming units generated against a confluent lawn of E. coli ATCC 15597 host cells, were found to decline 2.0 logs within 3 h and 3.5 logs by 6 h, relative to the populations of the control pouches (Fig. 2C), suggesting that the reflective pouch would effectively inactivate viral agents associated with pediatric diarrhea. Since MS2 belongs to genotype I of the F-specific RNA bacteriophages, the most environmentally resistant serogroup (16), and the presence of such bacteriophage populations has been positively correlated with that of enteric viruses and other pathogens (1), MS2 represents an appropriate surrogate for inactivation studies targeting viral diarrheal agents. In comparison, the viability of the enterotoxigenic E. coli strain (ATCC 35401) declined in excess of 5.0 logs during the same interval (Fig. 2C), suggesting that specific bacterial contaminants are inactivated more quickly than viral ones.
Taken together, the results of this study confirm that water can be effectively treated for bacterial and viral agents by using a freely available resource (sunlight) and a pouch constructed of food-grade packaging materials carefully selected to transmit and amplify the effects of solar radiation. As the system was functional during the cool weather of New England (Connecticut) and during seasons of declining (fall) and reduced (winter) total sunlight, it is highly likely that the system will function at least as well, if not better, in climates of higher average insolation. These are the developing regions of the world where water quality is consistently poor and the risk to infant health is greatest. Increasing the availability of inexpensive SODIS systems in such regions can be expected to have a positive influence on pediatric health through reductions in infant morbidity and mortality associated with exposure to waterborne bacterial and viral pathogens.
Acknowledgments
We thank Elaine Wedral for unwavering support, Mary Sullivan, Andy Corlett, and Melissa Spadanuda for excellent technical assistance, and both Gene Clyde and Jane Leedle for critical review of the manuscript.
We also thank the many representatives of Curwood (a Bemis company) for providing the packaging materials used in this study and technical advice on their selection.
REFERENCES
- 1.Borrego, J. J., M. A. Moriñigo, A. de Vicente, R. Cornax, and P. Romero. 1987. Coliphages as an indicator of faecal pollution in water. Its relationship with indicator and pathogenic microorganisms. Water Res. 21:1473-1480. [Google Scholar]
- 2.Conroy, R. M., M. Elmore-Meegan, T. Joyce, I. K. G. McGuigan, and J. Barnes. 1996. Solar disinfection of drinking water and diarrhoea in Maasai children: a controlled field trial. Lancet 348:1695-1697. [DOI] [PubMed] [Google Scholar]
- 3.Davies, C. M., and L. M. Evison. 1991. Sunlight and the survival of enteric bacteria in natural waters. J. Appl. Bacteriol. 70:265-274. [DOI] [PubMed] [Google Scholar]
- 4.Durán, A. E., M. Muniesa, X. Méndez, F. Valero, F. Lucena, and J. Jofre. 2002. Removal and inactivation of indicator bacteriophages in fresh waters. J. Appl. Microbiol. 92:338-347. [DOI] [PubMed] [Google Scholar]
- 5.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of small round structured viruses in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 6.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric calciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254-S261. [DOI] [PubMed] [Google Scholar]
- 7.Gleick, P. H. 2001. Making every drop count. Sci. Am. 284:41-45. [Google Scholar]
- 8.Green, K. Y. 1997. The role of human calciviruses in epidemic gastroenteritis. Arch. Virol. Suppl. 13:153-165. [DOI] [PubMed] [Google Scholar]
- 9.Holm, N. C., C. G. Gliesche, and P. Hirsch. 1996. Diversity and structure of Hyphomicrobium populations in a sewage treatment plant and its adjacent receiving lake. Appl. Environ. Microbiol. 62:522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen, A. J. F., K. Nøhr, H. Sørensen, and F. Boisen. 1998. Decontamination of drinking water by direct heating in solar panels. J. Appl. Microbiol. 85:441-447. [DOI] [PubMed] [Google Scholar]
- 11.Joyce, T. M., K. G. McGuigan, M. Elmore-Meegan, and R. M. Conroy. 1996. Inactivation of fecal bacteria in drinking water by solar heating. Appl. Environ. Microbiol. 62:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang, X. L., J. Joensuu, and T. Vesikari. 1999. Human calcivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr. Infect. Dis. J. 18:420-426. [DOI] [PubMed] [Google Scholar]
- 13.Reed, R. H. 1997. Solar inactivation of faecal bacteria in water: the critical role of oxygen. Lett. Appl. Microbiol. 24:276-280. [DOI] [PubMed] [Google Scholar]
- 14.Reed, R. H., S. K. Mani, and V. Meyer. 2000. Solar photo-oxidative disinfection of drinking water: preliminary field observations. Lett. Appl. Microbiol. 30:432-436. [DOI] [PubMed] [Google Scholar]
- 15.Safapour, N., and R. H. Metcalf. 1999. Enhancement of solar water pasteurization with reflectors. Appl. Environ. Microbiol. 65:859-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaper, M., A. E. Durán, and J. Jofre. 2002. Comparative resistance of phage isolates of four genotypes of F-specific RNA bacteriophages to various inactivation processes. Appl. Environ. Microbiol. 68:3702-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setlow, P. 1988. Resistance of bacterial spores to ultraviolet light. Comments Mol. Cell. Biophys. 5:253-264. [Google Scholar]
- 18.Sinton, L. W., R. K. Finley, and P. A. Lynch. 1999. Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65:3605-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinton, L. W., C. H. Hall, P. A. Lynch, and R. J. Davies-Colley. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages in waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slieman, T. A., and W. L. Nicholson. 2001. Role of dipicolinic acid in survival of Bacillus subtilis spores exposed to artificial and solar UV radiation. Appl. Environ. Microbiol. 67:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommer, B., A. Mariño, Y. Solarte, M. L. Salas, C. Dierolf, C. Valiente, D. Mora, R. Rechsteiner, P. Setter, W. Wirojanagud, H. Ajarmeh, A. Al-Hassan, and M. Wegelin. 1997. SODIS—an emerging water treatment process. Aqua 46:127-137. [Google Scholar]