Abstract
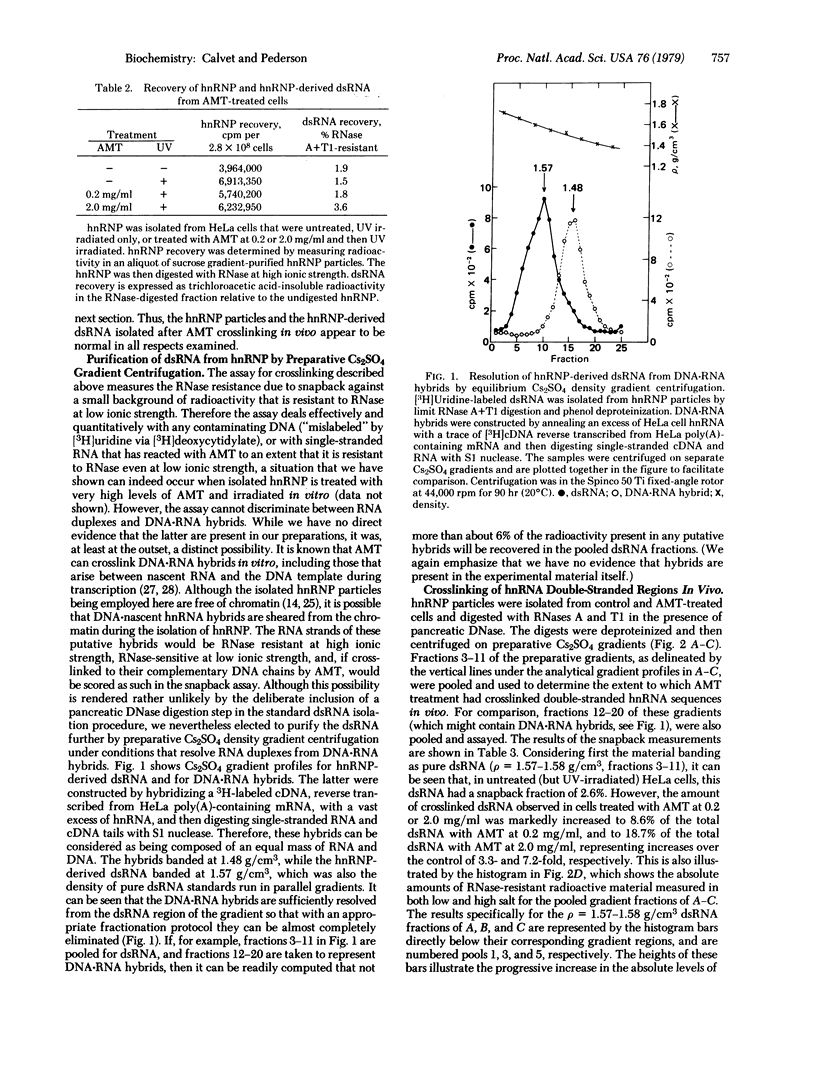
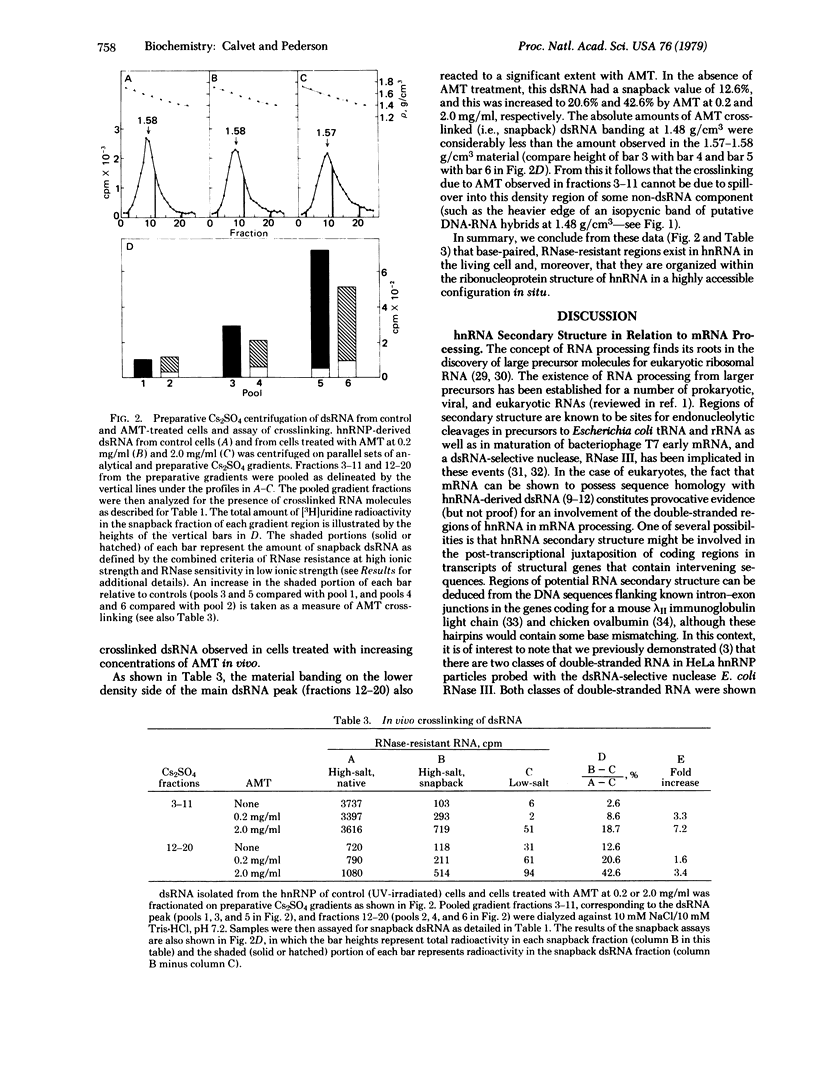
The psoralen derivative aminomethyltrioxsalen (AMT, 4'-aminomethyl-4,5',8-trimethylpsoralen) has been employed as a probe for heterogeneous nuclear RNA (hnRNA) double-stranded regions in experiments with living HeLa cells. hnRNA ribonucleoprotein (hnRNP) particles were purified from untreated or AMT-treated cells after irradiation with 365-nm light, and double-stranded hnRNA regions (dsRNA) were isolated by RNase A + T1 digestion of hnRNP, followed by preparative Cs2SO4 isopycnic centrifugation. The purified, hnRNP-derived dsRNA was then assayed for interstrand crosslinks by measurement of its "snapback" to RNase-resistant form after thermal denaturation. By this procedure, the amount of crosslinked dsRNA was found to be increased 3- to 7-fold in cells exposed to AMT in vivo. The levels of crosslinking in vivo compared favorably with those observed in model experiments with pure dsRNA in vitro. These results establish that double-stranded hnRNA regions exist in the living cell, and they further demonstrate that these base-paired regions are organized as rather accessible sites within the nucleus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvet J. P., Pederson T. Nucleoprotein organization of inverted repeat DNA transcripts in heterogeneous nuclear RNA-ribonucleoprotein particles from HeLa cells. J Mol Biol. 1978 Jul 5;122(3):361–378. doi: 10.1016/0022-2836(78)90195-x. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Secondary structure of heterogeneous nuclear RNA: two classes of double-stranded RNA in native ribonucleoprotein. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3705–3709. doi: 10.1073/pnas.74.9.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., O'Malley B. W., Robertson M. A., Staden R., Tanaka Y., Brownlee G. G. Nucleotide sequence homology at 12 intron--exon junctions in the chick ovalbumin gene. Nature. 1978 Oct 12;275(5680):510–513. doi: 10.1038/275510a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Cech T., Potter D., Pardue M. L. Electron microscopy of DNA cross-linked with trimethylpsoralen: a probe for chromatin structure. Biochemistry. 1977 Nov 29;16(24):5313–5321. doi: 10.1021/bi00643a024. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Hall S. H., Crouch R. J. Isolation and characterization of two enzymatic activities from chick embryos which degrade double-stranded RNA. J Biol Chem. 1977 Jun 25;252(12):4092–4097. [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Specific nucleotide sequences in HeLa cell inverted repeated DNA: enrichment for sequences found in double-stranded regions of heterogeneous nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):591–601. doi: 10.1016/0022-2836(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Evans R., Wilson M., Salditt-Georgieff M., Darnell J. E. Oligonucleotides in heterogeneous nuclear RNA: similarity of inverted repeats and RNA from repetitious DNA sites. Biochemistry. 1978 Jul 11;17(14):2776–2783. doi: 10.1021/bi00607a012. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Evans R., Wilson M., Salditt-Georgieff M., Darnell J. E. Oligonucleotides in heterogeneous nuclear RNA: similarity of inverted repeats and RNA from repetitious DNA sites. Biochemistry. 1978 Jul 11;17(14):2776–2783. doi: 10.1021/bi00607a012. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Isolation and characterization of ribonucleoprotein particles containing heterogeneous nuclear RNA. Methods Cell Biol. 1978;17:377–399. doi: 10.1016/s0091-679x(08)61155-3. [DOI] [PubMed] [Google Scholar]
- Kronenberg L. H., Humphreys T. Double-stranded ribonucleic acid in sea urchin embryos. Biochemistry. 1972 May 23;11(11):2020–2026. doi: 10.1021/bi00761a005. [DOI] [PubMed] [Google Scholar]
- Naora H., Whitelam J. M. Presence of sequences hybridisable to dsRNA in cytoplasmic mRNA molecules. Nature. 1975 Aug 28;256(5520):756–759. doi: 10.1038/256756a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Groner Y., Hurwitz J. Isolation and purification of double-stranded ribonuclease from calf thymus. J Biol Chem. 1977 Jan 25;252(2):483–491. [PubMed] [Google Scholar]
- Pederson T. Chromatin structure and the cell cycle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2224–2228. doi: 10.1073/pnas.69.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Perry R. P. THE CELLULAR SITES OF SYNTHESIS OF RIBOSOMAL AND 4S RNA. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E. RNA processing and the control of gene expression. Brookhaven Symp Biol. 1975 Jul;(26):240–266. [PubMed] [Google Scholar]
- Ryskov A. P., Farashyan V. R., Georgiev G. P. Ribonuclease-stable base sequences specific exclusively for giant dRNA. Biochim Biophys Acta. 1972 Apr 12;262(4):568–572. doi: 10.1016/0005-2787(72)90502-3. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Kramerov D. A., Georgiev G. P. The structural organization of nuclear messenger RNA precursor. I. Reassociation and hybridization properties of double-stranded hairpin-like loops in messenger RNA precursor. Biochim Biophys Acta. 1976 Oct 4;447(2):214–229. doi: 10.1016/0005-2787(76)90344-0. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Tokarskaya O. V., Georgiev G. P., Coutelle C., Thiele B. Globin mRNA contains a sequence complementary to double-stranded region of nuclear pre-mRNA. Nucleic Acids Res. 1976 Jun;3(6):1487–1498. doi: 10.1093/nar/3.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskov A. P., Yenikolopov G. N., Limborska S. A. Complementary regions of the nuclear precursor of messenger RNA. FEBS Lett. 1974 Oct 1;47(1):98–102. doi: 10.1016/0014-5793(74)80434-5. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., LATHAM H., DARNELL J. E. Demonstration of an unstable RNA and of a precursor to ribosomal RNA in HeLa cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:240–248. doi: 10.1073/pnas.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B. K., Schlessinger D. Separation and characterization of two activities from HeLa cell nuclei that degrade double-stranded RNA. J Biol Chem. 1978 Jul 10;253(13):4537–4543. [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Mapping of sequences with 2-fold symmetry on the simian virus 40 genome: a photochemical crosslinking approach. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1363–1367. doi: 10.1073/pnas.74.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Photochemical crosslinking of transcription complexes with psoralen. I. Covalent attachment of in vitro SV40 nascent RNA to its double-stranded DNA template. Nucleic Acids Res. 1978 Apr;5(4):1429–1441. doi: 10.1093/nar/5.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Hsieh T. S., Wang J. C., Hearst J. E. Photochemical cross-linking of DNA-RNA helices by psoralen derivatives. J Mol Biol. 1977 Nov;116(4):661–679. doi: 10.1016/0022-2836(77)90265-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Wollenzien P. L., Youvan D. C., Hearst J. E. Structure of psoralen-crosslinked ribosomal RNA from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1642–1646. doi: 10.1073/pnas.75.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]


