Abstract
A 37-year-old woman, gravida 2, para 1 presented in the outpatient ward with dyspnoea and tachycardia of unknown origin. The physical examination was unremarkable. Echocardiography revealed an intracardiac mass protruding through the tricuspid orifice into the right ventricle during diastole. The patient was admitted to the intensive care unit with the suspicion of vena cava thrombosis with intracardiac expansion. An abdominal sonography showed a mass in the uterus, presumed to be a benign tumour, with extension into the vena cava inferior. Owing to the extent of the mass in the right atrium and the risk for pulmonary embolism, after interdisciplinary discussion, a decision to remove the atrial mass was made. The case was managed by a two-stage procedure. Pathological examination of the intracardiac portions of the tumour revealed a benign tumour that consisted of proliferating smooth muscle fibres without abnormal mitotic activity.
Background
Intravascular leiomyomatosis (IVL) of uterine origin is a rare disease. Extension to the right heart is exceptional. Leiomyomatosis is a benign tumour arising from either the uterine venous wall or uterine leiomyoma. We present an interesting case of IVL of uterine origin with intracardiac extension in a pregnant patient (gestational age 28 weeks).
Case presentation
A 37-year-old woman, gravida 2, para 1 presented in the outpatient ward with dyspnoea and tachycardia of unknown origin. On physical examination, the patient was haemodynamically stable (normal respiratory rate 22 breath/min, blood pressure 100/60 mm Hg and a pulse rate 80 bpm). The physical examination was unremarkable. The 12 lead ECG was normal. Blood pressure was also within the normal range. Patient history was unremarkable despite the abdominal laparoscopic myomectomy performed 3 years before hospital admission.
Echocardiography revealed an intracardiac mass protruding through the tricuspid orifice into the right ventricle during diastole (figure 1). The patient was admitted to the intensive care unit with the suspicion of vena cava thrombosis with intracardiac expansion. Because of the gestational age (28 week), anticoagulation with subcutaneous low molecular weight heparin was started. Under rigorous control of the target anti-Xa activity the patient was monitored. Subsequent echocardiographic controls showed an unchanged status of the cardiac mass. No pulmonary or cardiovascular deterioration occurred during the hospitalisation.
Figure 1.
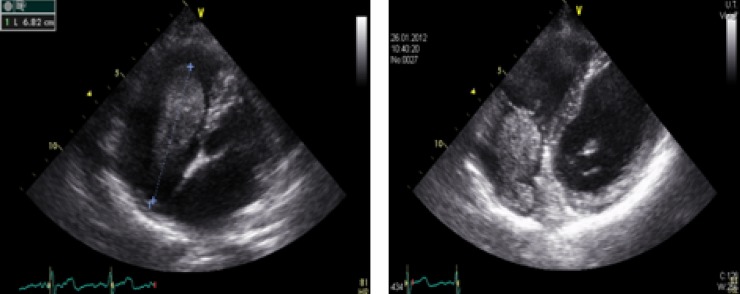
Intracardiac mass protruding through the tricuspid orifice into the right ventricle during diastole.
Owing to the extent of the mass in the right atrium and the risk of pulmonary embolism, after interdisciplinary discussion, a decision to remove the atrial mass was made.
The postoperative course was uneventful.
Investigations
Biochemical and haematological investigations were normal. The echocardiography revealed an intracardiac mass protruding through the tricuspid orifice into the right ventricle during diastole. The lower extremity Doppler sonography has excluded deep venous thrombosis. The abdominal sonography showed a mass in the uterus (lateral wall of the uterus 6×9 cm) presumed to be a benign tumour, with extension into the vena cava inferior. The sonographic fetometry showed a normal development of the fetus, a normal quantity of amniotic fluid and no proof of fetal malformation.
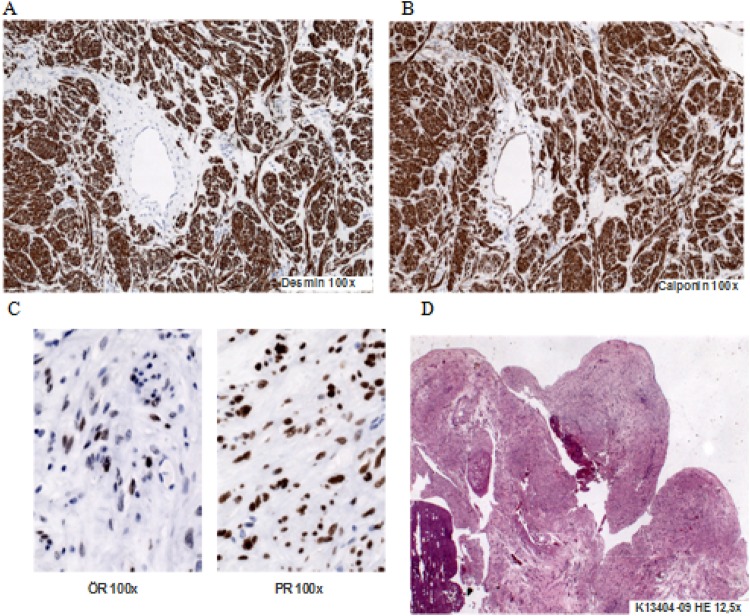
Pathological examination of the intracardiac portions of the tumour revealed a cylindrical mass (5×2.8×2.8 cm; figure 2D) occupying most of the right atrium and extending in the right ventricle during diastole. The histological examination showed a strong expression of actin, desmin, calponin and caldesmon (figure 2A,B). The tumour consisted of proliferating smooth muscle fibres without abnormal mitotic activity (<1% rate of proliferation).
Figure 2.
Pathological examination of the intracardiac tumour.
Differential diagnosis
Endolymphatic stromal myosis
Low grade leiomyosarcoma
Disseminated peritoneal leiomyomatosis
Benign metastasing leiomyoma.
Treatment
The case was managed by a two-stage procedure. First, heart surgery conducted under extracorporeal circulation to remove intracardiac and the upper vena caval portions of the tumour. Six months after delivery, a second stage abdominal surgery was performed to remove the remaining portion of the tumour.
Outcome and follow up
Two months after the first operation the patient gave birth to a healthy child (3208 kg, 51 cm, Apgar 9/9/10). The second stage abdominal surgery was performed six months after delivery. Control echocardiography did not reveal recurrent intracardiac mass and demonstrated a good long-term result after cardiac surgery (figure 3). A long-term follow-up is required because of the tumour’s potential to recur.
Figure 3.
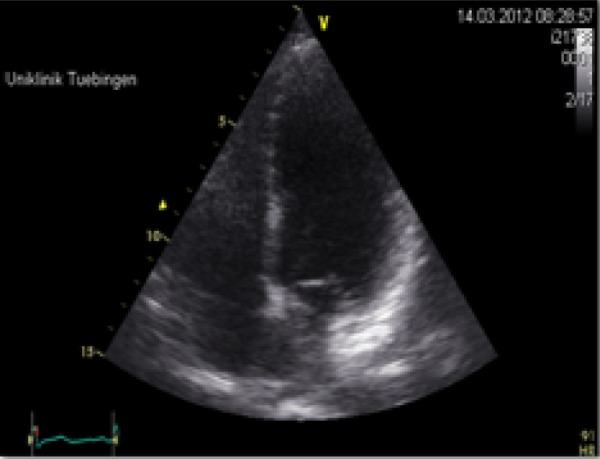
No recurrence of intracardiac mass.
Discussion
IVL is a rare uterine tumour. It usually does not invade the walls of these vessels but in some cases can be firmly fixed to the wall.1 IVL was first described by Birch-Hirschfield in 1896.2
IVL occurs in a middle-aged woman and with a history of hysterectomy in more than half of the cases.3 In literature there are till now 200 cases described, 68 of which reported intracardiac extension.4 Patients may remain asymptomatic until intracardiac growth leads to cardiac insufficiency, pulmonary embolism or sudden cardiac death.5
When the tumour reaches the right heart, patients can show symptoms of a cardiac insufficiency such as dyspnoea on exertion, shortness of breath, orthopnea, abdominal distension or syncopal episodes. This tumour can extend till the right ventricle and cause an obstruction of the right ventricle filling. Tricuspidal valve insufficiency is generally associated with symptoms of right-sided heart failure such as oedema, ascites, hepatomegaly, jugular venous distension, upper abdominal discomfort (from a congested liver) and fatigue (diminished cardiac output). Because of its rare occurrence, this tumour can be misdiagnosed and treated improperly.
For a precise diagnosis an echocardiography, venography, CT or MRI can be very helpful. In our case the MRI was not possible because of claustrophobia.
Because of the severe complications produced by the extension of the intracardiac tumour an urgent removal was essential. The surgery is a treatment of choice. A complete removal of the tumour is required because recurrences are frequent despite the benign nature of the tumour.
Surgical resection is considered to be the standard of care for the treatment of IVL. The first total resection of an intracardiac IVL was reported in 1982.6 In literature 2 modalities of removing the tumour are described: either a two-stage procedure involving resection of the infradiaphragmatic tumour and intrathoracic tumour at two different times,7 or a single stage operation that has the advantage of excision of the tumour at once.
Because of possible adhesions to the vena caval wall, a two-stage procedure was preferred in this case. Despite surgery, recurrence has been reported in some cases.8
A long term follow-up is required because of the tumour’s potential to recur. Intravenous leiomyomatomas are hormone-dependent tumours so that cytoplasmic oestradiol and progesterone receptors have been reported in a leiomyoma spreading into the heart9 (figure 2C).
By incomplete tumour resection or in the case of recurrence antioestrogen therapy may be useful. The efficacy of antioestrogens, such as tamoxifen, has been controversially discussed.5 Bilateral oophorectomy is essential in severe cases.
In our particular case the immunohystochemical analysis has shown a low grade of receptor for oestrogen (only 30–40%) while progesterone receptor was strongly expressed (80%; figure 2C). It is tempting to speculate that this tumour massively expanded during pregnancy by hormonal influence although diagnostic findings before pregnancy are missing.
This is the first case ever described in literature so far. In case of unclear intracardiac tumour, treating gynaecologists and cardiologists should be aware of this differential diagnosis.
Learning points.
By unclear intracardiac tumour, leiomyomatosis must be taken into account as a differential diagnosis.
Hormonal changes during pregnancy can facilitate, in rare situations, the occurrence of uncommon tumours with cardiac involvement.
The therapeutic strategy in a case of leiomyomatosis with cardiac involvement should be surgery with complete tumour resection.
Footnotes
Contributors: SC was involved in data collection and developing of the manuscript draft. KR was involved in the data collection, review the manuscript and helped with figure composition and formatting. GT and MG contributed to the writing and the critical revision of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lam PM, Lo KW, Yu MM, et al. Intravenous leiomyomatosis with atypical histologic features: a case report. Int J Gynecol Cancer 2003;2013:83–7 [DOI] [PubMed] [Google Scholar]
- 2.Arif S, Ganesan R, Spooner D. Intravascular leiomyomatosis and benign metastazing leiomyoma: an unusual case. Int J Gynecol Cancer 2006;2013:1448–50 [DOI] [PubMed] [Google Scholar]
- 3.Lam PM, Lo KW, Yu MY, et al. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg 2004;2013:465–9 [DOI] [PubMed] [Google Scholar]
- 4.Rispoli P, Santovito D. A one stage approach to the treatment of intravenous leiomyomatosis extending to the right heart. J Vasc Surg 2010;2013:212–15 [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Shi E, Gu T. Intravenous leiomyomatosis with intracardiac extension: a report of two cases. J Card Surg 2011;2013:37–68 [DOI] [PubMed] [Google Scholar]
- 6. Ariza A, Cerra C, Hahn IS, et al. Intravascular leiomyomatosis of the uterus. A case report. Conn Med 1982;46:700–3. [PubMed] [Google Scholar]
- 7.Mazzola A, Gregorini R. Intracaval and intracardiac leiomyomatosis of uterine origin. Ann Vasc Surg 1986;2013:134–8 [DOI] [PubMed] [Google Scholar]
- 8.Shida T, Yoshimura M, Chihara H, et al. Intravenous leiomyomatosis of the pelvis with reextension into the heart. ANN Thorac Surg 1986;2013:104–6 [DOI] [PubMed] [Google Scholar]
- 9.Cameron AEP, Graham JC. Intracaval leiomyomatosis. Br J Obstet Gynecol 1983;2013:272–5 [DOI] [PubMed] [Google Scholar]