Abstract
This article reviews the technique, basic science principles and applications of integrated single photon emission CT (SPECT)-CT in musculoskeletal radiology. A review of the current evidence on the topic was undertaken, and selected clinical cases from the authors' institution have been used for illustration. SPECT-CT is a technology with emerging applications that offers technical advantages to image fusion of separately acquired SPECT and CT studies. The prevailing evidence indicates that there may be benefit in adding SPECT-CT to conventional imaging algorithms during the evaluation of some malignant and benign musculoskeletal conditions. SPECT-CT can improve both sensitivity and specificity by reducing equivocal interpretation in comparison to planar scintigraphy or SPECT alone. The evidence base for SPECT-CT in musculoskeletal radiology is still evolving. There is a lack of evidence comparing SPECT-CT with MRI in many key indications, and further research is required in these areas.
A wide range of pathological conditions may affect the musculoskeletal system, including infection, trauma and malignant disease. Advances in MRI, multidetector CT (MDCT) and high-frequency ultrasound have provided considerable improvements in imaging musculoskeletal disease over the past decade. There are, however, limitations associated with each of these techniques. MRI image acquisition remains relatively time consuming and, particularly in musculoskeletal radiology, image quality can be degraded significantly by metal artefact from internal fixation and prostheses. MDCT offers exquisite characterisation of bone and can rapidly generate high-quality three-dimensional reconstructions; however, the contrast resolution for soft tissues is limited and the modality can also suffer metal-related artefact, although like MRI, imaging parameters can be adjusted to reduce this artefact. High-frequency ultrasound, although operator dependent, provides excellent spatial and contrast resolution for superficial structures but is suboptimal for deeper structures and is unable to penetrate cortical bone.
Bone scintigraphy remains an important and highly sensitive tool to the musculoskeletal radiologist but this is frequently criticized for its lack of specificity. Radionuclide imaging, however, may be the only modality to demonstrate pathology before it becomes evident on anatomical imaging. Developments in gamma camera technology now allow high-resolution imaging with shortened scan times, and single photon emission CT (SPECT) imaging has become commonplace in the UK. SPECT is able to increase sensitivity further and improves lesion localisation, but the paucity of anatomical markers on radionuclide imaging remains a constant challenge. Thus, the importance of correlating anatomical and functional imaging has become increasingly recognised [1].
Use of a combined SPECT/CT system allows for sequential acquisition of both anatomical and functional information with a high degree of image fusion accuracy.
In this article, we briefly review the technique, basic science principles and technical challenges of SPECT-CT, and discuss the applications of this hybrid modality in musculoskeletal radiology.
TECHNIQUE AND BASIC SCIENCE
Before the introduction of dedicated SPECT-CT cameras, coregistration software algorithms for fusion of anatomical with functional imaging were developed with varying degrees of success in the 1980s [1]. More recently, automated software for image coregistration has become commercially available and has shown success in fusing brain images [2]. Software coregistration of neck and body imaging has encountered a number of challenges that are yet to be overcome. Firstly, motion artefacts markedly affect image fusion in the neck and body when CT and SPECT images are acquired separately [1,3]. Secondly, there are a few landmarks present on functional imaging that can be correlated with reference points on anatomical imaging. Furthermore, the chest and abdomen are mobile structures whose variation during respiration and patient positioning introduces further complexity to correct image alignment [4]. Developments in three-dimensional elastic transformations or non-linear warping have further improved the accuracy of image fusion. Accuracy for software-based image coregistration has been reported in the range of approximately 5–7 mm [5].
Hasegawa et al [6] are widely credited with the innovation behind the first simultaneous SPECT/CT system by successfully combining the hardware components into an integrated system and developing algorithms for SPECT attenuation correction using CT. The first commercial SPECT/CT was launched in 1999, combining a dual-detector variable-angle gamma camera with a low-dose X-ray tube mounted on the same gantry [7,8]. Although the main purpose of the CT was to provide a good attenuation map, it also generated interpretable anatomical images with a patient dose significantly lower than that of conventional CT scanners by a factor of up to five times [9]. The benefits of using CT for attenuation correction as opposed to a radionuclide transmission source include less noise, faster acquisition, the lack of influence on the CT data by the SPECT radionuclide and avoidance of the need to replace decayed transmission sources [10]. However, the potential disadvantage comes from sequential acquisition of the CT and then the potential allowance of SPECT data sets for misregistration in functional images from patient motion artefact [2]. In addition to superior attenuation correction, integrated SPECT/CT provides added value by generating coregistered images that are obtained during the same study [11,12]. This affords better control of patient position and easier access to both sets of images, and provides patient convenience in attending for a single study [2]. Coregistration accuracy for SPECT-CT has been reported to be 3 mm or better based on phantom studies [2]. Since the first commercial model, manufacturers have produced a number of SPECT-CT hybrid systems with different CT capabilities ranging from single-slice low-dose CT systems to multislice systems capable of low-dose CT or standard-dose diagnostic CT. In general, images are obtained by a low-dose CT area valuable for anatomical localization and attenuation correction purposes, but morphology detail is better demonstrated by diagnostic quality (i.e. multislice) CT [13]. This could be especially important in the musculoskeletal system, where CT characterisation rather than mere localisation may provide valuable supplementary information to the radionuclide data. This raises as yet unanswered questions on the appropriate CT protocols in terms of radiation burden for particular clinical applications.
APPLICATIONS IN MUSCULOSKELETAL RADIOLOGY
Malignant disease in the axial skeleton
The vertebral column and pelvis are the most frequent sites of metastatic bone disease because of their high red marrow content [14], and, consequently, accurate detection of metastases at these sites is of critical clinical importance in staging of cancer patients. The detection of metastases in the spine may be complicated by the presence of coexisting pathological conditions, such as severe degenerative disease and compression fractures, which can make firm diagnosis of malignant disease at this site difficult [15] (Figures 1 and 2).
Figure 1.
Patient with prostate cancer and suspected bony metastases showing low-grade uptake involving the right lateral aspect of the posterior elements of L4/L5 level. On the single photon emission CT-CT images of the lumbar sacral spine, appearances of L4/L5 correspond to the right-sided facet joint level, where there are degenerative changes noted on the CT study. Scan appearances are in keeping with degenerative changes. There is no evidence of bony metastases.
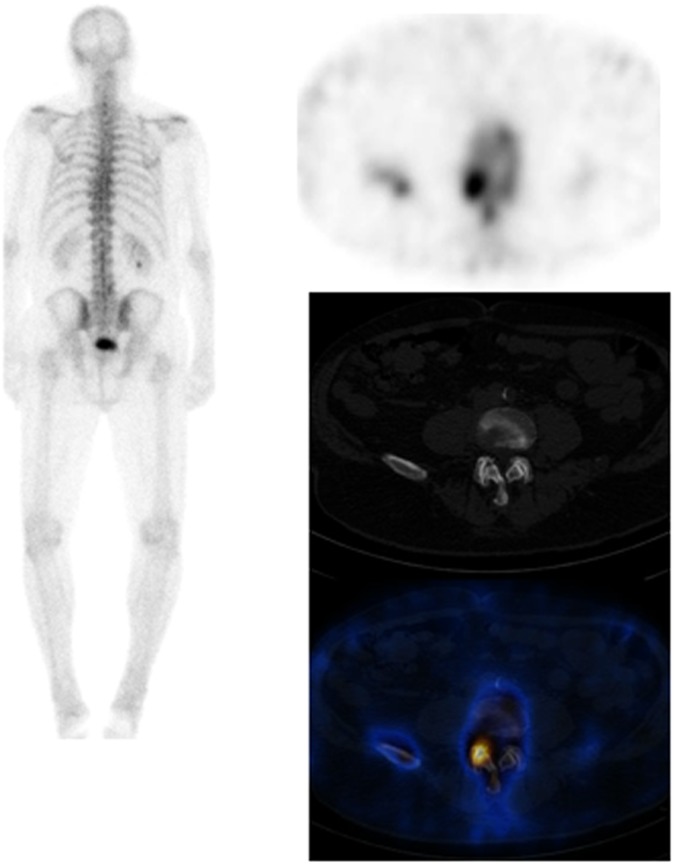
Figure 2.
Patient with a 2-year history of breast cancer, a background of degenerative spine disease and left total-hip replacement presenting with pain in the right sacroiliac area failing to settle 4 months following a fall. On the planar images, there is intense tracer uptake seen in both sacroiliac regions, right more than left. Low-grade tracer uptake is seen in both shoulders; left tenth rib posteriorly and lower lumbar spine. Note is made of left hip prosthesis. On the single photon emission CT-CT, the avid tracer uptake is localised to the right sacral ala. The right side of the sacrum shows more sclerosis than the left side with vertical linear lucency. Scan appearances in keeping with a right sacral ala fracture.
Considerable loss of bone density is required to detect bone metastases by plain radiography [16]. There have been advances in MDCT enabling high-resolution imaging of cortical and trabecular bone with shortened acquisition times. However, the sensitivity of MDCT remains significantly lower than that of MRI in the detection of vertebral metastases [17], mainly owing to the ability of MRI to detect bone marrow signal changes. It should be noted that MRI is regarded less sensitive than CT in demonstrating cortical destruction [18,19]. In cases where the SPECT component of the study shows focal uptake suggestive of malignancy and no bony abnormality is evident on the CT images, further correlation with MRI may be required. The most widely used bone radiopharmaceuticals are technitium-99m (99mTc)-labelled phosphate containing compounds, such as 99mTc methylenediphosphonate (99mTc-MDP) and 99mTc hydroxydiphosphonate. Phosphate analogue uptake is dependent not only on osteoblastic activity but also on several factors, which include (1) bone turnover and osteoblastic activity, (2) the non-linear relationship between blood flow and vascularity, and (3) poorly understood microenvironmental factors affecting calcium/phosphate deposition and intracellular calcium flux. Localisation is not specific to a malignant process. Another well-recognised drawback of this group of radiopharmaceuticals is that although osteolytic metastases may induce an osteoblastic reaction, the sensitivity for small lytic metastases is low, and false negatives may be found related to lesions in myeloma.
Overall, the sensitivity and specificity of planar bone scintigraphy for bone metastases is widely variable, depending on technique, patient population and analysis (e.g. lesion-based vs patient-based assessment of accuracy). Atypical patterns of uptake and coexisting benign bone pathologies present a challenge in differentiating benign from malignant disease with planar bone scintigraphy alone. Several studies have reported improved diagnostic accuracy with the addition of SPECT, particularly in the vertebral column through improved tracer localisation [20–22]. As a general rule, uptake in the vertebral body or the pedicle alone is more likely to represent malignant disease than a pattern of uptake isolated to the vertebral body periphery or at the facet joints [23,24]. However, even with SPECT, spatial resolution is often insufficient for precise localisation of bone lesions, and correlation with anatomical imaging is often required.
Currently, the imaging investigations for most patients with suspected bone metastases comprise planar bone scintigraphy with SPECT of indeterminate areas if available (Figure 3). Indeterminate lesions undergo further imaging evaluation with plain radiographs, CT or MR. MR is particularly sensitive for marrow involvement and may demonstrate bone metastases before loss of bone density occurs [25].
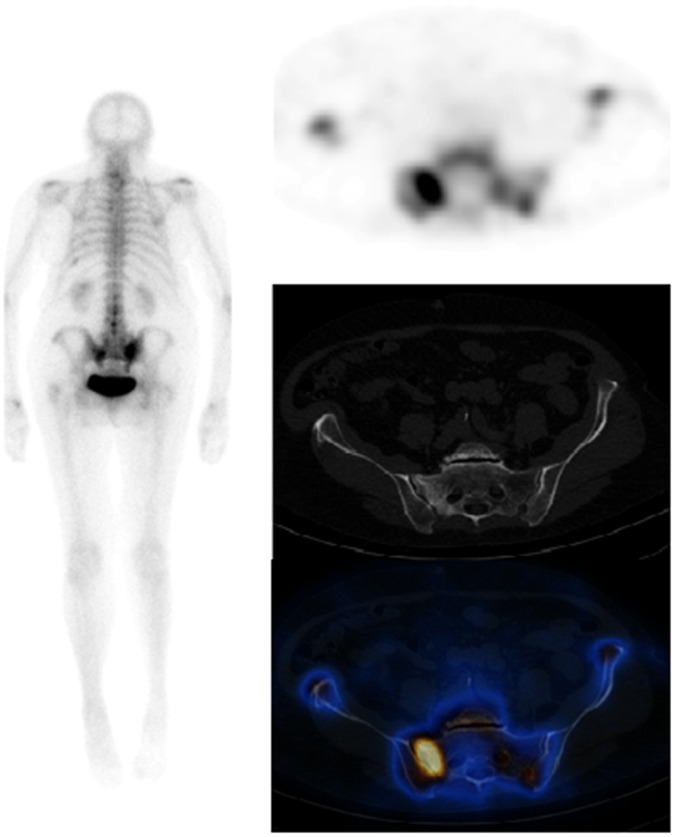
Figure 3.
Single photon emission CT (SPECT)-CT performed to characterise the nature of lesion in L1 found on MRI (right). The patient was experiencing right-sided low back and leg pain within the lateral thigh consistent with L4 sensory radiculopathy. On MRI, a low T1 and T2 signal focus within the body of L1 was identified, which was thought to be benign, e.g. a bone island; however, MR could not completely exclude metastatic disease. There was also evidence of osteophytes and Modic changes noted. On SPECT, however, no abnormality is noted within L1 to suggest underlying metastatic pathology. On the planar images, there is focal increased tracer uptake seen, involving the left lateral aspect of L3/L4 vertebra with further patchy increased tracer uptake seen involving the lumber spine, shoulders, right elbow, left knee and both feet. The focally intense area at L3/L4 corresponds to osteophytic changes at this level in keeping with degenerative changes.
Comparison studies of fused SPECT and CT data vs SPECT alone or side-by-side reading of separate scintigraphic and CT images in patients with known or suspected cancer have shown the fused data set to improve diagnostic confidence in differentiating malignant from benign bone lesions [26,27] (Figure 2). Results from Strobel et al [27] showed that in a series of 37 patients with 42 focal bone lesions, a specific diagnosis was made with planar scintigraphy in 64% of cases, SPECT in 86% and SPECT fused with CT in all cases. Several small studies have shown the value of SPECT-CT over SPECT alone in differentiating benign and malignant disease. A prospective series involving 47 patients with 104 equivocal lesions showed that SPECT-CT was able to correctly classify 85% of indeterminate scintigraphic bone lesions compared with only 36% when using SPECT alone [28]. A retrospective evaluation of 44 patients with 52 indeterminate lesions on SPECT reported that 48 (92%) of the 52 lesions were subsequently classified using SPECT-CT [29]. These results are comparable to those obtained at the authors' institution with a definitive diagnosis made on SPECT-CT in 87% (83/95) of indeterminate foci in 48 patients compared with only 30% using SPECT alone [30]. An additional benefit noted in this study was that SPECT-CT was able to identify additional malignant lesions in 21% (10/48) of the patients [30]. A further study at the authors' institution retrospectively reviewed 40 consecutive prostate cancer patients who were examined with 99mTc-MDP whole-body planar bone scintigraphy, SPECT and SPECT-CT [31]. After reading the planar study and SPECT scans, reviewers rated 61% of lesions as equivocal [31]. After reading the SPECT-CT scans, only 8% of lesions were rated as equivocal, 24% were rated as malignant and 68% as benign [31]. The addition of SPECT-CT resulted in a significant reduction of equivocal reports and improved diagnostic confidence [31].
The results from the small studies published to date indicate that there may be a role for the addition of localised SPECT-CT to the imaging of patients with known or suspected cancer by improving diagnostic confidence in differentiating benign from malignant bone lesions. This may potentially lead to a reduction in additional investigations such as MRI with consequent benefits in pathway efficiency and limitation of patient anxiety. Any discussion comparing MRI with SPECT-CT should take account that the radiation dose in SPECT-CT comprises the dose from the SPECT radiopharmaceutical (typically 3–4 mSv for a 99mTc-MDP whole-body bone scan) and the dose from the CT portion of the study. Although the concept of a “representative” dose can be misleading owing to the wide variety of CT systems, applications and protocols in use, it should be borne in mind that the effective dose in a typical patient undergoing CT of the chest, abdomen and pelvis may be approximately 15 mSv. The quality of the current evidence on this topic is limited to small single-centre studies, and the true impact on clinical outcomes is as yet undefined, particularly as the performance analysis so far has been focused on lesions rather than patient outcomes. Clearly, further prospective studies are required, structured with the aim of quantifying the impact on patient management of this emerging technology. In particular, comparative studies of SPECT-CT vs MRI would be helpful. It is worth noting, however, that of the 8% of lesions that Romer et al reported to be indeterminate after imaging with SPECT-CT, all were in the ribs and scapulae [29]. The studies so far have been performed on various SPECT-CT systems using a variety of dose protocols, with most being low-dose CT protocols. It remains to be seen whether high-dose CT would provide additional diagnostic benefit over low-dose protocols to justify the increased radiation burden. It should be noted that in spite of the variety of different SPECT-CT systems used in the studies so far from low-dose protocols to multislice SPECT-CT, the range of performance in characterising indeterminate lesions was only 86–92%, suggesting that low-dose CT may suffice in most cases. Furthermore, many oncology patients will have had a recent diagnostic CT and whether side-by-side comparison of this with SPECT is comparable to SPECT/CT has yet to be determined. Also, the potential of diagnostic contrast-enhanced SPECT-CT imaging has not yet been explored fully and raises the possibility of streamlined pathways of investigation in selected patients such as those with locally advanced breast cancer.
Single photon emission CT-CT in benign musculoskeletal disease
The evidence base for the role of SPECT-CT in benign musculoskeletal pathology is still emerging. Utility has been demonstrated in a prospective series of 76 non-oncological patients with non-specific findings on planar imaging in which SPECT-CT reached a final diagnosis in 59% (45/76) of patients, obviating the need for further imaging [32]. In another 30% (23/76) of patients in the series, SPECT-CT was deemed to have guided additional imaging [32]. Linke et al [33] reported a series involving 71 patients with no history of cancer who had extremity pain. These patients were examined with three-phase bone scintigraphy and SPECT-CT. Planar scintigraphic SPECT images and SPECT-CT images were read independently from each other [33]. Four patients had no abnormality demonstrated in the extremities [33]. Of the 34 lesions thought to represent osteoarthritis on planar and SPECT images, 7 were reclassified as fractures and 1 was reclassified as a benign tumour on SPECT-CT. Of the 15 lesions initially thought to represent osteomyelitis, 4 were reclassified as osteoarthritis, 4 as fracture and 1 as soft-tissue inflammation [33]. Among eight fracture diagnoses, two were diagnosed as osteomyelitis and two as osteoarthritis using SPECT-CT [33]. In 1 of the 10 patients with an initial diagnosis of tumour, the diagnosis was changed to trauma based on SPECT-CT findings, and in another patient in this group, the diagnosis was changed to osteoarthritis [34]. Overall, SPECT-CT led to diagnostic category reclassification in 23 of 71 patients (p<0.01) [33].
Lower back pain
Bone scintigraphy can help localise metabolically active bone disease, and SPECT has been shown to provide diagnostically useful information not available from some other modalities (Figure 3), although recent published data for this indication are lacking. In a series of 34 patients with chronic back pain, 27 patients had lesions demonstrated on SPECT, of whom 24 (89%) had abnormal CT and 18 (67%) had abnormal radiography. SPECT demonstrated 54 lesions, of which only 20 (37%) were evident with planar imaging. 43 (80%) SPECT lesions correlated with an abnormal site on CT and 20 (37%) correlated with an abnormal site at radiography [34]. Although the use of facet joint injection remains a controversial topic, SPECT has been shown to be of use in identifying back pain and localising active facet joint disease, which may benefit from facet joint injection [35–37].
SPECT may be useful in patients with osteoporotic collapse, in whom back pain becomes chronic and in whom causes of pain other than fracture should be considered. For example, SPECT in some patients with osteoporotic vertebral collapse may demonstrate abnormal facet joint activity, hence identifying a site of pain generation that might benefit from specific treatment to this area [38,39]. It should be noted, however, that there are no head-to-head studies comparing SPECT-CT to MRI for this indication. Given the sensitivity of this modality for bone marrow oedema and the lack of associated ionising radiation, MRI will almost certainly remain the mainstay of imaging for back pain, with SPECT-CT reserved for problem-solving cases or for those patients in whom MR is contraindicated.
Applications of single photon emission CT-CT in the lower limb
Hip and groin
MRI is the best modality in the evaluation of unexplained hip pain, especially if occult fracture is a possibility and particularly given its ability to simultaneously evaluate the labrum, joint and soft tissues. At the authors' institution, bone scintigraphy is sometimes used in the assessment of patients with unexplained hip pain, mostly if MRI is contraindicated or equivocal (Figure 4). The use of SPECT-CT in selected cases has led to increased specificity compared with bone scintigraphy alone in diagnoses that may have otherwise been attributed to degenerative disease such as femoral acetabular impingement syndrome [40]. Uptake in the superior femoral neck in association with degenerative uptake of the superior hip joint on bone SPECT-CT has been reported to be suggestive of either cam or mixed cam–pincer impingement [41]. For general background information on femoroacetabular impingement, the reader is directed to the article by Tannast et al [42].
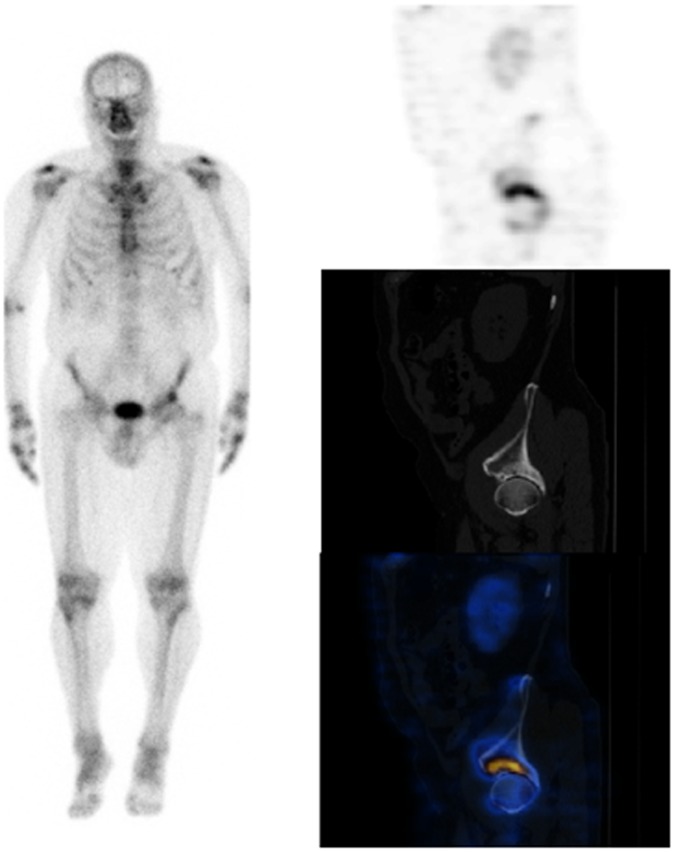
Figure 4.
Patient presenting with left buttock and groin pain. There is focal uptake of tracer in the superior aspect of the left acetabulum. On the single photon emission CT-CT, the focal uptake of tracer is in the superolateral aspect of the left hip, where the CT component shows articular and cystic changes in the acetabulum. There is also increased tracer uptake at left greater trochanter. The scan findings are consistent with degenerative changes at the hip. There is no evidence of significant facet joint inflammation in the lower spine, and the uptake at the left greater trochanter is suggestive of enthesopathy/trochanteric bursitis.
Foot and ankle
The foot and ankle represent a complex group of articulations, and each imaging modality has its own benefits and limitations in this region. Planar 99mTc-MDP bone scintigraphy has a high sensitivity but low specificity as localisation of focal uptake is challenging. In general, increased uptake in the extremities is often dismissed as degenerative, but SPECT-CT allows more specific diagnoses such as impingement syndromes (Figure 5), osteochondral defects and trauma to be made confidently. A SPECT-CT foot and ankle series, from the authors' institution in an unselected group of patients, including unexplained foot pain and post-surgical patients, demonstrated additional diagnostic information in 81% (25/31) of patients and a potential change in management in 62% of patients [43]. A comparison study of SPECT-CT with MRI vs MRI alone for osteochondral lesions of the talus in 25 patients showed that stand-alone SPECT-CT review changed treatment decision making in 48%, and 52% of treatment decisions were changed with SPECT-CT and MRI interpretation combined [44]. A further study has shown a significant correlation between positive 99mTc-dicarboxypropane diphosphonate (DPD)-SPECT-CT scans, showing increased pathological uptake within an osteochondral lesion of the ankle joint, and pain resolution after diagnostic ankle infiltration with local anaesthetics [45]. The utility of SPECT-CT to detect early degenerative changes in the varus and valgus malaligned hindfoot before conventional scintigraphy and CT scans has also been recently suggested [46]. Other potential applications described for SPECT-CT in the foot and ankle include post-operative evaluation of joint fusion, the assessment of infection post-arthrodesis, Achilles tendonitis, bursitis and plantar fasciitis, along with stress fracture (Figure 6), tarsal coalition and painful accessory bone syndromes [47–50], although MRI or ultrasound are likely to remain the primary modality for imaging in these indications. SPECT-CT may provide a valuable technique for the evaluation of continuing pain in the context of joint fusion with in situ hardware, as sites of altered metabolic activity on the bone scan would allow a more focused examination of the area on the CT study [47]. This may improve the accuracy of identifying non-union or malunion or subjacent arthritis as the cause for continuing pain [47] (Figure 7). Although the role of bone scintigraphy remains limited in patients with Achilles tendonitis and related pathologies, SPECT-CT may provide useful coincidental imaging information for the clinician [47]. The SPECT study would demonstrate the metabolic abnormalities associated with the calcaneus (oedema/enthesophyte, trauma etc.), whereas CT may be useful in demonstrating the associated soft-tissue abnormalities with the Kager's fat pad and/or the retrocalcaneal bursa [49]. It is likely that SPECT-CT may find a more prominent role to play in the early diagnosis and management of patients with suspected stress fractures, given the ability to direct attention to subtle areas of CT abnormality that might be easily overlooked without superimposed uptake abnormality being present. There may be a potential role for the use of SPECT-CT in tarsal coalition and painful accessory bone syndromes, although no studies to date have compared this technique with CT alone or MR imaging. At the authors' institution, we have found SPECT-CT particularly useful in the assessment of foot and ankle pathology after surgery when imaging with CT and MRI may be limited by metallic artefact [43].
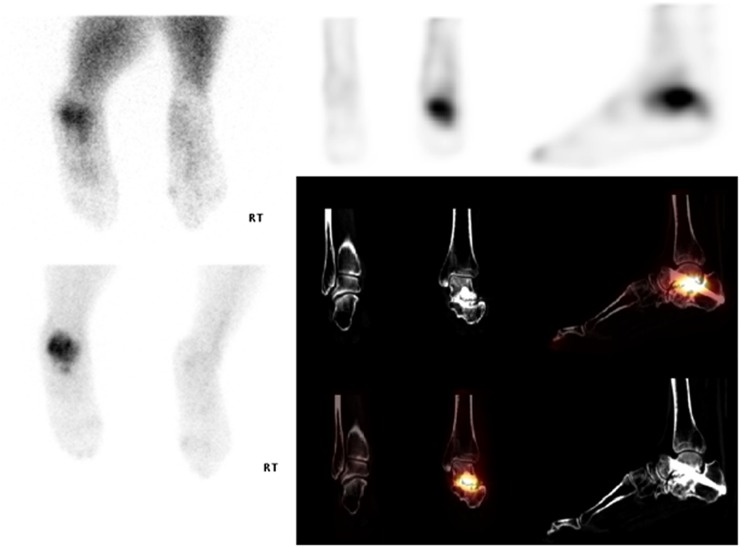
Figure 5.
Patient with suspected neuropathic joint or chronic regional pain syndrome (CPRS). On CT, there is sclerosis and subarticular cyst formation seen along the anterior cortex of the talus with associated ossification. On single photon emission CT-CT (bottom right), there is avid tracer uptake seen along the margins of the anterior aspect of the tibiotalar articulation and seen on the planar images (bottom left) within the lateral gutter. There is no evidence of neuropathic arthropathy or CRPS. The appearances reflect chronic degeneration of the left ankle, with associated hard anterior impingement.
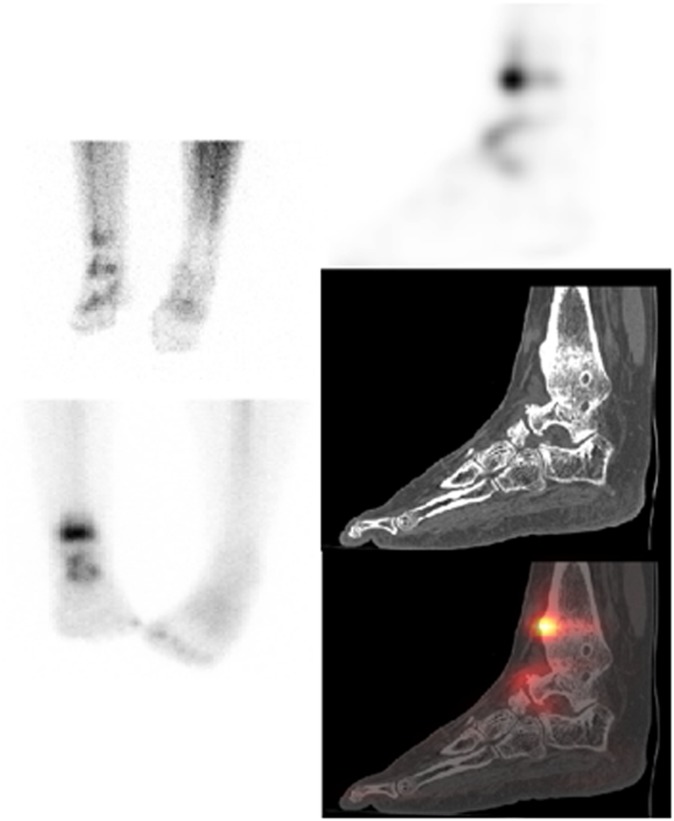
Figure 6.
Patient post-tibiotalar fusion demonstrating persistent uptake at the medial aspect of the right tibiotalar fusion highly suggestive of ongoing active bone turnover associated with incomplete bony union. There is a metabolically active fracture of the distal tibia and fibula, likely to be of insufficiency type with CT features suggestive of chronicity.
Figure 7.
Patient with left subtalar joint fusion but persistent pain. On the delayed views of the feet (top right and bottom left), there is increased tracer uptake seen involving the left ankle. On the early blood pool images (top left), there is increased vascularity seen to this site. On the single photon emission CT-CT images within the left foot, the increased area of uptake corresponds to the intended subtalar fusion. There is persistent joint space noted with significant degenerative changes. The appearances are in keeping with non-union of the intended subtalar fusion.
Knee
Although MRI remains the gold standard for evaluation of internal derangement of the knee, a number of studies have reported the utility of SPECT in the evaluation of meniscal tears and injury of cruciate and collateral ligaments; however, in practice, structural correlation is essential to understand the significance of the SPECT findings [51–54]. SPECT-CT may be useful in patients with a problematic painful knee, with diagnoses including osteoid osteoma, implant loosening and pain after anterior cruciate ligament repair (Figure 8); patella maltracking/subluxation, osteochondral defects or subchondral fracture [55] (Figure 9). There is, however, no evidence to suggest that SPECT-CT adds any additional or superior information to MRI for these indications.
Figure 8.
Patient with a painful knee post-left anterior cruciate ligament (ACL) reconstruction and medial meniscectomy. Single photon emission CT (SPECT)-CT dual phase knees, delayed phase whole-body and SPECT CT show that the epicentre of focal metabolic activity localises to the medial tibial spine and extends posteriorly along the medial aspect of the intercondylar notch. In addition, there is a moderately increased circumferential uptake associated with the medial tibial plateau. Both regions demonstrate degenerative changes as manifested by subchondral sclerosis, cortical irregularity, joint space loss and osteophytosis on CT. The focal hyperaemic uptake, very close to the intercondylar notch of the attachment of the ACL, is suggestive of possible disease related to the ACL reconstruction on a background of active degenerative changes and osteophyte fragmentation.
Figure 9.
Patient with bilateral knee pain without history of trauma. Proton density fat saturated MR (top right) had shown a focal abnormal signal within the lateral femoral condyle and proximal tibia of the right knee with a differential of avascular necrosis (AVN), infarct or metabolic cause. On the single photon emission CT-CT, there is focal increased uptake of tracer seen in the right lateral femoral condyle, which corresponds to an area of faint sclerosis on the CT. Scan appearances in the right knee indicate a healing subchondral fracture likely secondary to AVN, and the increased uptake at this site correlates to the marrow oedema seen on MRI. Uptake in the tibial plateau is likely secondary to the medial meniscal degeneration.
Applications of single photon emission CT-CT in the upper limb
Published studies evaluating the use of SPECT-CT in the upper limb are very limited. Utility of SPECT-CT has been reported in hand and wrist imaging and in imaging the shoulder.
Coregistration of bone scans with plain radiography has been reported as useful in the evaluation of hand pain/trauma, but the technique is challenging [56] and is not a widely used practice. Huellner et al [57] recently reported a pilot retrospective study of 21 patients with non-specific hand and wrist pain, comparing SPECT-CT with MRI. MRI yielded a higher sensitivity of 86% vs 71% for SPECT-CT but a lower specificity of 20% vs 100% [57]. This pilot study supports the use of SPECT-CT as a problem-solving tool in patients with non-specific hand and wrist pain and reflects our own experience with SPECT-CT in the hand and wrist. Typically, planar imaging is performed followed by SPECT-CT only on the injured hand/wrist unless abnormal uptake is seen in both hands and wrists on the planar images. SPECT-CT can also be used to assess for evidence of avascular necrosis (Figure 10).
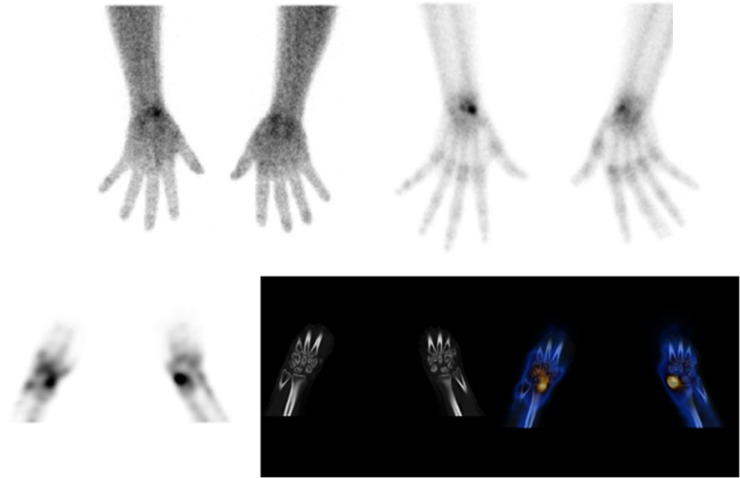
Figure 10.
Single photon emission CT (SPECT) in a patient with sickle-cell disease presenting with bilateral wrist pain, performed to assess vascularity of carpal bones for avascular necrosis ± evidence of degenerative changes. There is increased vascularity in the scaphoid bilaterally on the blood pool images (top left) with more marked uptake in the left scaphoid on delayed images (top right and bottom left). SPECT-CT shows preservation of the alignment and joint spaces between radio-ulna, ulna-carpal, radio-carpal and intercarpal joints but evidence of bilateral avascular necrosis of the scaphoid.
Hirschmann et al [58] reported a series of four cases where SPECT-CT demonstrated clinical value in imaging of the shoulder for a variety of indications. 99mTc-labelled antigranulocyte antibodies used with SPECT-CT were used to distinguish recurrence of infection related to internal fixation from humeral head necrosis, allowing the patient to proceed to total shoulder arthroplasty [58]. Other cases guided shoulder arthroplasty revision surgery to the relevant components and sites localising abnormal uptake [58]. SPECT-CT was also reported as useful in one case at excluding infection post-arthroscopy, guiding the decision to proceed to total shoulder replacement and again, subsequently, excluding infection post-shoulder replacement and identifying the acromion as a site of possible pain generation, which responded to arthroscopic subacromial decompression [58].
Single photon emission CT-CT in the imaging of musculoskeletal infections
Accurate and early diagnosis of osteomyelitis remains a diagnostic challenge. This is particularly true in the diabetic foot or post-operative orthopaedic patient. In general, MRI and dual/triple phase bone scintigraphy are the modalities mostly used in diagnosing osteomyelitis. MRI may assess for the presence of osteomyelitis and associated complications such as abscess formation. However, phenomena such as non-specific bone marrow signal change can occasionally reduce specificity. CT is useful to assess for early cortical destruction, sequestration and involucra. The major limitations of radionuclide imaging are the interpretation and significance of non-specific uptake and the poor anatomical localisation of infection.
Bar-Shalom et al [59] examined the adjunctive role of SPECT-CT to 67 Ga or 111In-labelled white blood cell scintigraphy in a series of 82 patients assessed for known or suspected infectious processes. The precise anatomical localisation of uptake offered by SPECT-CT was useful in 48% of patients [59]. It was interesting to note that a higher incremental contributory value was achieved for SPECT-CT with 111In-labelled white blood cells than 67 Ga [59]. Horger et al [60] reported results of a study consisting of 27 patients using SPECT-CT immunoscintigraphy and 99mTc-labelled antigranulocyte antibodies in patients with chronic osteomyelitis. On a lesion basis, 19 true-positive, 1 false-positive and 9 true-negative results were demonstrated. SPECT-CT accurately demonstrated the location of all positive foci of uptake in the appendicular skeleton and identified a cold lesion in the axial skeleton [60]. Also differentiation between cellulitis, septic arthritis and osteomyelitis become possible, as well as distinguishing between cortical, corticomedullary and subperiosteal foci [60]. Although sensitivity was identical for SPECT and SPECT-CT (100%), it was noted that specificity improved from 78% to 89% with SPECT-CT [60]. It was suggested that combined SPECT-CT imaging improves the accuracy of immunoscintigraphy by allowing distinction between soft-tissue infection and bone involvement [60]. Filippi and Schillaci [61] presented their results of SPECT-CT in 99mTc-hexamethylpropyleneamine oxime (HMPAO)-labelled leukocyte scintigraphy for a series of 15 patients with suspected bone infection and 13 patients with suspected orthopaedic implant infection. 99mTc-HMPAO scintigraphy gave a true-positive result for infection in 18 of 28 patients and produced a true-negative result in 10 of 28 subjects [61]. SPECT-CT was able to provide accurate localisation of all positive foci in this series [61]. SPECT-CT was deemed to have added a significant clinical contribution in 10 of 28 patients (35.7%) [61]. SPECT-CT was reported as useful in a number of clinical scenarios being able to distinguish soft tissue from bone infection both in patients with osteomyelitis and in patients with orthopaedic implants, and diagnosing osteomyelitis in patients with altered morphology after trauma, and was able to localise infection to the synovium without prosthesis involvement in patients with knee implants [61]. Horger et al [62] published results comparing 99mTc-DPD SPECT-CT with three-phase bone scintigraphy with the aim of evaluating the contribution of SPECT-CT in the diagnosis and localisation of bone infection. The performance of SPECT-CT was later compared with visual correlation of SPECT with additional CT, X-ray or MRI studies [62]. Three-phase bone scan (including SPECT) accurately classified 7 lesions as positive for osteomyelitis and 11 lesions as negative [62]. Six studies were read as false positive, five as equivocal and two as false negatives [62]. The sensitivity and specificity of bone scan were determined to be 78% and 50%, respectively. SPECT-CT gave true-positive results in 7 patients and a true-negative result in 19 patients [62]. Also noted were two false-positive and two false-negative results. One study was equivocal giving overall SPECT-CT performance of 78% sensitivity and 86% specificity [62]. Interestingly, an additional benefit of SPECT-CT vs visual fusion of SPECT with X-ray, CT or MRI could not be confirmed [62].
Two studies have described the utility of SPECT-CT in evaluation of the diabetic foot. Heiba et al [63] reported results for the diagnosis and localisation of infection from a method that combined three-phase bone scan and leukocyte scanning, and if needed, leukocyte scanning using SPECT-CT. Blood flow/pool acquisition was obtained, then leukocyte reinjection and on the following day, dual isotope three-phase bone scan and leukocyte scanning with planar and SPECT-CT techniques [63]. Leukocyte/bone marrow scanning SPECT-CT was obtained on the following day if images raised suspicions of mid- or hindfoot osteomyelitis [63]. Diagnostic confidence was significantly higher for SPECT-CT than for the other modalities [63]. In a smaller series by Filippi et al [64], 17 patients with suspected diabetic foot infection were assessed with planar leukocyte scanning followed by SPECT-CT. SPECT-CT was able to change the interpretation in 53% of suspected sites [64]. Osteomyelitis was excluded in six cases [64]. Bone infection was demonstrated in one case, and in three cases, both bone and soft-tissue infection were demonstrated [64]. Notably, SPECT-CT did not significantly add value when patients had negative scan results [64].
Other miscellaneous cases in which SPECT-CT has shown considerable impact on patient management at our institution include the diagnosis of tuberculosis in a young female patient with back pain referred for bone scintigraphy [65] and the diagnosis of heterotopic ossification in an amputation stump suspected of having osteomyelitis clinically and scintigraphically [66].
In summary, SPECT-CT using 99mTc-DPD, 67 Ga, 111In-labelled leukocytes, 99mTc-labelled antigranulocyte antibodies have been shown to raise diagnostic confidence, improve specificity and may add a significant clinical contribution in the diagnosis of musculoskeletal infection. However, the benefits compared with reading SPECT side by side with other modalities have not been demonstrated. SPECT-CT usefulness has been described in the evaluation of the diabetic foot for infection, but interestingly, SPECT-CT did not change results for those patients with negative planar scans.
CONCLUSIONS
The evidence base for SPECT-CT in musculoskeletal radiology is still evolving and often compares SPECT-CT with SPECT or planar scintigraphy alone. The quality of evidence on this topic is summarised in Table 1 based on guidance from the Oxford Centre for Evidence-Based Medicine [67]. Results from studies so far indicate the addition of SPECT-CT to the imaging of patients with known or suspected cancer improved diagnostic confidence in differentiating benign from malignant lesions and may reveal more lesions than detected with other modalities. There is a paucity of comparative studies of MRI and SPECT-CT and SPECT-CT vs fused separate SPECT and CT studies. Particularly, this gap in the latter group for the oncology patient who may have numerous CT studies previously needs to be addressed to justify additional ionising radiation exposure. Our practice suggests that for most indications a low-dose CT protocol provides sufficient information for diagnostic purposes, although no head-to-head studies have been undertaken as yet to confirm this.
Table 1.
Evidence quality for single photon emission CT (SPECT)-CT in musculoskeletal radiology based on Oxford Centre of Evidence-Based Medicine guidelines [67]
| Indication | Study | Design | Level of evidence |
| Malignant disease in the axial skeleton | Utsunomiya et al [26] | 45 patients with 42 metastatic foci and 40 benign foci. Two independent readers scoring diagnostic confidence for each lesion for bone scintigraphy alone and bone scintigraphy and CT images viewed together and, finally, bone scintigraphy, CT images and fused images | Level II |
| All underwent planar scintigraphy and SPECT and CT directed by SPECT findings. SPECT and CT images then were digitally combined. The three types of images were evaluated separately for lesion visibility, diagnostic performance and diagnostic certainty. | Strobel et al [27] | Prospective study of 37 patients with 42 focal skeletal lesions | Level II |
| Horger et al [28] | 47 patients with 104 equivocal lesions on bone scintigraphy. Findings of bone scintigraphy (planar and SPECT), SPECT+CT or radiography and SPECT-CT were compared with regard to location and benignity | Level II | |
| Romer et al [29] | 272 patients with malignancy underwent bone scintigraphy. 112 (41%) required further investigation by SPECT because of diagnostic uncertainty with standalone whole-body planar scintigraphy. For 57 of these patients, inline CT over the body region of interest accompanied SPECT; the remaining 55 subjects underwent only SPECT for logistic reasons. The 57 SPECT/CT studies were retrospectively reported by readers blinded to the clinical pretest probability and the planar scan findings. 52 lesions were rated as indeterminate on the SPECT images. Afterwards, the corresponding SPECT/CT images were assessed and the previous indeterminate findings were reclassified either as definitely benign, indeterminate or definitely malignant | Level II | |
| Barwick et al [30] | 48 oncological patients were retrospectively reviewed, comparing diagnostic confidence between SPECT-CT and SPECT alone | Levels III/IV | |
| Helyar et al [31] | Retrospective review of 40 consecutive prostate cancer patients who underwent whole-body planar bone scintigraphy, SPECT and SPECT-CT. The images were reported by two independent reviewers and interreviewer agreement was evaluated. Each abnormal focus of increased uptake was scored using a four-tier scale of diagnostic confidence. In total, 50 lesions on planar bone scintigraphy in the 40 patients were evaluated | Level III | |
| Benign musculoskeletal disease | Even-Sapir et al [32] | 76 consecutive non-cancer patients with non-specific bone scan findings, which needed further morphologic data for interpretation. Bone scan indications were pain (n=61), trauma (n=7), suspected infection or inflammation (n=6) and fever of unknown origin (n=2). The reviewing physicians commented on whether SPECT-CT reached a final diagnosis and whether additional imaging was warranted | Level III |
| Linke et al [33] | 71 non-cancer patients with extremity pain underwent 3-phase bone scan and SPECT-CT. Planar and SPECT images and planar scintigraphic and SPECT-CT images were interpreted separately from each other. The results were classified as: normal, trauma, tumour, osteomyelitis and degenerative disease. The additional diagnostic value of skeletal SPECT/CT for extremity pain was assessed | Level III | |
| Hip and groin | Mulholland et al [40] | Demonstration of femoroacetabular impingement syndrome in a single case using SPECT-CT | Levels IV/V |
| Foot and ankle | Gnanasegaran et al [43] | SPECT-CT series of 31 patients with unexplained foot pain and post-surgical patients compared with SPECT alone assessed for additional diagnostic information and potential change in management | Level IV |
| Leumann et al [44] | MRI and SPECT-CT of 25 patients were analysed by 3 orthopaedic surgeons blinded to the study. Defined criteria was used for cartilage, subchondral bone plate and subchondral bone, including bone marrow signal change on MRI and activity on SPECT-CT. For MRI alone, SPECT-CT alone and the combined correlation, the treatment decision had to be defined | Level IV | |
| Wiewioski et al [45] | Correlation between pain from osteochondral lesions and pathological uptake seen on SPECT-CT in 15 patients and response to diagnostic local anaesthetic infiltration into the ankle joint | Level IV | |
| Knupp et al [46] | 27 patients with varus or valgus hindfoot were assessed using radiography, plain CT, bone scan and SPECT-CT. The degree of deformity, stage of osteoarthritis and level of bone scan and SPECT-CT activity were measured | Levels IV/V | |
| Mohan et al [47] | Assessed the additional value of SPECT-CT in 16 patients referred from a specialist orthopaedic clinic and role in changing management of bony foot and ankle pathology | Levels IV/V | |
| Breunung et al [50] | Single case describing additional benefit of SPECT-CT in the investigation of heel pain | Levels IV/V | |
| Knee pain | Fernando et al [55] | Case review of selected single cases of SPECT-CT findings in painful knees | Levels IV/V |
| Upper limb | Huellner et al [57] | Retrospective study of 21 patients investigating non-specific pain of the hand or wrist. All patients underwent planar early-phase imaging and late-phase SPECT-CT in addition to MRI. Lesions were divided into causative and not causative pathologies on the basis of clinical follow-up. Oedema-like bone marrow MR signal changes were compared with focally increased SPECT-CT tracer uptake | Level IV |
| Hirschmann et al [58] | Case review of four selected cases demonstrating clinical value of SPECT-CT for shoulder pathology | Levels IV/V | |
| Musculoskeletal infection | Bar-Shalom et al [59] | 82 patients evaluated for known or suspected infection. 47 patients underwent 67 Ga SPECT/CT and 35 patients underwent 111In-labelled WBC SPECT/CT. Any additional information revealed by SPECT/CT as compared with planar and SPECT scintigraphy was recorded | Level IV |
| Horger et al [60] | 27 patients with suspected bone infection underwent immunoscintigraphy with 99mTc-labelled AGA. Planar images were acquired immediately, 4 h and 24 h post injection, and SPECT/CT was performed using a dual-head gamma camera fitted with a low-energy X-ray system. Accumulation of AGA in lesions was quantified, comparing uptake at 4 h and 24 h after injection. The validation was based on culture data derived from surgical or biopsy samples (20 lesions in 18 patients) or clinical follow-up without further therapy for more than 6 months (9 lesions) | Level II | |
| Filippi and Schillaci [61] | 99mTc-hexamethylpropyleneamine oxime (HMPAO) scintigraphy was performed on 28 patients. 15 with suspected bone infection and 13 with suspected orthopaedic implant infection. Planar scans were obtained 30 min, 4 h and 24 h after injection. SPECT-CT was acquired 6 h after injection. Results were matched with the results from surgery or cultures and clinical follow-up | Level II | |
| Horger et al [62] | 31 suspected bone infection patients with pathological findings on 3-phase bone scan underwent additional SPECT-CT. 99mTc-DPD was used for all patients. Bone scintigraphy findings (planar scans as well as SPECT) were categorised as positive, negative or equivocal for the presence of osteomyelitis. In a second step, they were compared with SPECT-CT and SPECT+CT/X-ray/MRI. Validation was achieved through surgery, biopsy or by clinical follow-up | Level II | |
| Heiba et al [63] | 213 patients with suspected diabetic foot infection. Blood flow/pool images were obtained followed by leukocyte reinjection and next day dual isotope 3 phase 99mTc HDP bone scan, 111In leukocyte scan (WBCs) planar and SPECT-CT. Bone marrow scanning/WBCs SPECT-CT was obtained on the following day when images were suspicious for mid-/hindfoot osteomyelitis. Diagnosis was classified as ostemyelitis, soft-tissue infection, both ostemyelitis/soft-tissue infection and other/no bony pathology by microbiology/pathology or clinical follow-up. Differentiation between various diagnostic categories and overall osteomyelitis diagnostic accuracy was assessed | Levels II/III | |
| Filippi et al [64] | 17 diabetic foot patients with 19 suspected infection locations were analysed. After 99mTc-HMPAO leukocyte labelling and administration, planar scans were obtained at 30 min, 4 h and 24 h. SPECT-CT was acquired at 6 h. The final diagnosis was established by clinical follow-up and by bone biopsy at 14 sites | Level II | |
| Gnanasegaran et al [65] | Single case report of usefulness of SPECT-CT in the diagnosis of spinal tuberculosis | Level IV | |
| Hassan et al [66] | Single case report of additional value of SPECT-CT in diagnosing heterotopic ossification around an amputation stump in a case of suspected osteomyelitis | Level IV |
AGA, antigranulocyte antibodies; HDP, hydroxydiphosphonate; WBC, white blood cell.
The evidence for the use of SPECT-CT in imaging of benign musculoskeletal pathology is very limited, but in small series, it has been shown to improve specificity, reduce equivocal interpretation and change the diagnostic category of some lesions. The studies, however, tend to lack robust reference standards. SPECT-CT is likely to remain an adjunctive problem-solving tool to MRI in cases of unexplained musculoskeletal pain and in patients post-orthopaedic surgery with metalwork in situ. Encouraging results using SPECT-CT have been demonstrated in imaging musculoskeletal infection and the diabetic foot, which remains a challenging area for the musculoskeletal radiologist. In particular, direct comparison studies of SPECT-CT with MR is required across a range of indications in musculoskeletal radiology, although the use of ionising radiation in SPECT-CT poses a challenge to the structure and approval of potential studies.
Further research is necessary to examine the influence on patient management and, wherever possible, should focus on measurable patient outcomes. Only by establishing evidence based on these outcomes can the cost effectiveness of this technique ultimately be determined for a range of different indications.
REFERENCES
- 1.O'Connor MK, Kemp BJ. Single-photon emission computed tomography/computed tomography: basic instrumentation and innovations. Semin Nucl Med. 2006;36:258–66 10.1053/j.semnuclmed.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Bybel B, Brunken RC, DiFilippo FP, Neumann DR, Wu G, Cerquiera MD. SPECT/CT imaging: clinical utility of an emerging technology. Radiographics 2008;28:1097–113 10.1148/rg.284075203 [DOI] [PubMed] [Google Scholar]
- 3.Perault C, Schwartz C, Wampach H, Liehn JC, Delisle MJ. Thoracic and abdominal SPECT-CT image fusion without external markers in endocrine carcinomas. The Group of Thyroid Tumoral Pathology of Champagne-Ardenne. J Nucl Med 1997;38:1234–42 [PubMed] [Google Scholar]
- 4.Buck AK, Nekolla S, Ziegler S, Beer A, Krause BJ, Herrmann K, et al. SPECT/CT. J Nucl Med 2008;49:1305–19 10.2967/jnumed.107.050195 [DOI] [PubMed] [Google Scholar]
- 5.Forster GJ, Laumann C, Nickel O, Kann P, Rieker O, Bartenstein P. SPET/CT image co-registration in the abdomen with a simple and cost-effective tool. Eur J Nucl Med Mol Imaging 2003;30:32–9 10.1007/s00259-002-1013-0 [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa BH, Wong KH, Iwata K, Barber WC, Hwang AB, Sakdinawat AE, et al. Dual-modality imaging of cancer with SPECT/CT. Technol Cancer Res Treat 2002;1;449–58 [DOI] [PubMed] [Google Scholar]
- 7.Townsend DW, Cherry SR. Combining anatomy and function: the path to true image fusion. Eur Radiol 2001;11:1968–74 10.1007/s003300101007 [DOI] [PubMed] [Google Scholar]
- 8.Lang TF, Hasegawa BH, Liew SC, Brown JK, Blankespoor SC, Reilly SM, et al. Description of a prototype emission-transmission computed tomography imaging system. J Nucl Med 1992;33:1881–7 [PubMed] [Google Scholar]
- 9.Bocher M, Balan A, Krauaz Y, Shrem Y, Lonn A, Wilk M, et al Gamma camera-mounted anatomical x-ray tomography: technology, system characteristics and first images. Eur J Nucl Med 2000;27:619–27 [DOI] [PubMed] [Google Scholar]
- 10.Seo Y, Wong KH, Sun M, Franc BL, Hawkins RA, Hasegawa BH. Correction of photon attenuation and collimator response for a body-contouring SPECT/CT imaging system. J Nucl Med 2005;46:868–77 [PubMed] [Google Scholar]
- 11.Von Schulthess GK. Integrated modality imaging with PET-CT and SPECT-CT: CT issues. Eur Radiol 2005;15(Suppl. 4):D121–6 [DOI] [PubMed] [Google Scholar]
- 12.Roach PJ, Schembri GP, Ho Shon IA, Bailey EA, Bailey DL. SPECT/CT imaging using a spiral CT scanner for anatomical localization: impact on diagnostic accuracy and reported confidence in clinical practice. Nucl Med Commun 2006;27:977–87 [DOI] [PubMed] [Google Scholar]
- 13.Gnanasegaran G, Barwick T, Adamson K, Mohan H, Sharp D, Fogelman I. Multislice SPECT/CT in benign and malignant bone disease: when the ordinary turns in the extraordinary. Semin Nucl Med 2009;39:431–42 10.1053/j.semnuclmed.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy GT, Tubis M, Hiss J, Blahd WH. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA 1977;237:2504–6 [PubMed] [Google Scholar]
- 15.Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic and hybrid modalities. J Nucl Med 2005;46:1356–67 [PubMed] [Google Scholar]
- 16.Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol 1967;18:158–62 [DOI] [PubMed] [Google Scholar]
- 17.Buhmann KS, Becker C, Duerr HR, Reiser M, Baur-Melnyk A. Detection of osseous metastases of the spine: comparison of high resolution multi-detector CT with MRI. Eur J Radiol 2009;69:567–73 10.1016/j.ejrad.2007.11.039 [DOI] [PubMed] [Google Scholar]
- 18.Shih TT, Huang KM, Li YW. Solitary vertebral collapse: distinction between benign and malignant causes using MR patterns. J Magn Reson Imaging 1999;9:635–42 [DOI] [PubMed] [Google Scholar]
- 19.Spuentrup E, Buecker A, Adam G, et al. Diffusion-weighted MR imaging for differentiation of benign fracture edema and tumour infiltration of the vertebral body. AJR Am J Roentgenol 2001;176:351–8 10.2214/ajr.176.2.1760351 [DOI] [PubMed] [Google Scholar]
- 20.Gates GF. SPECT bone scanning of the spine. Semin Nucl Med 1998;28;78–94 [DOI] [PubMed] [Google Scholar]
- 21.Savelli G, Maffioli L, Maccauro M, De Deckere E, Bombardieri E. Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q J Nucl Med 2001;45;27–37 [PubMed] [Google Scholar]
- 22.Evan-Sapir E, Martin RH, Barnes DC, Pringle CR, Iles SE, Mitchell MJ. Role of SPECT in differentiating malignant from benign lesions in the lower thoracic and lumbar vertebrae. Radiology 1993;187:193–8 [DOI] [PubMed] [Google Scholar]
- 23.Bushell DL, Kahn D, Hutson B, Bevering CG. Utility of SPECT imaging for determination of vertebral metastases in patient with known primary tumours. Skeletal Radiol 1995;24:13–6 [DOI] [PubMed] [Google Scholar]
- 24.Comparison of bone single photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur J Nucl Med 1998;25:635–8 [DOI] [PubMed] [Google Scholar]
- 25.Daffner RH, Lupetin AR, Dash N, Deeb ZL, Sefczek RJ, Schapiro RL. MRI in the detection of malignant infiltration of bone marrow. AJR Am J Roentgenol 1986;146:353–8 10.2214/ajr.146.2.353 [DOI] [PubMed] [Google Scholar]
- 26.Utsunomiya D, Shiraishi S, Imuta M, Tomiguchi S, Kawanaka K, Morishita S, et al. Added value of SPECT/CT fusion in assessing suspected bone metastasis: comparison with scintigraphy alone and nonfused scintigraphy and CT. Radiology 2006;238:264–71 10.1148/radiol.2373041358 [DOI] [PubMed] [Google Scholar]
- 27.Strobel K, Burger C, Seifert B, Husarik DB, Soyka JD, Hany TF. Characterization of focal bone lesions in the axial skeleton: performance of planar bone scintigraphy compared with SPECT and SPECT fused with CT. AJR Am J Roentgenol 2007;188:W467–74 10.2214/AJR.06.1215 [DOI] [PubMed] [Google Scholar]
- 28.Horger M, Eschmann SM, Pfannenberg C, Storek D, Vonthein R, Claussen CD, et al. Evaluation of combined transmission and emission tomography for classification of skeletal lesions. AJR Am J Roentgenol 2004;183:655–61 10.2214/ajr.183.3.1830655 [DOI] [PubMed] [Google Scholar]
- 29.Romer W, Nomayr A, Uder M, Bautz W, Kuwert T. SPECT-guided CT for evaluating foci of increased bone metabolism classified as indeterminate on SPECT in cancer patients. J Nucl Med 2006;47:1102–6 [PubMed] [Google Scholar]
- 30.Barwick T, Fernando R, Gnanasegaran G. The use of 99mTc-MDP SPECT/CT in the evaluation of indeterminate bone lesions on whole body planar imaging in cancer patients [abstract]. Eur J Nucl Med Mol Imaging 2008;35(Suppl. 2):S155 [Google Scholar]
- 31.Helyar V, Mohan HK, Barwick T, Livieratos L, Gnanasegaran G, Clarke SE, et al. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imag. 2010;37:706–13 Epub ahead of print December 2009 10.1007/s00259-009-1334-3 [DOI] [PubMed] [Google Scholar]
- 32.Even-Sapir E, Flusser G, Lerman H, Lievshitz G, Metser U. SPECT/multislice low-dose CT: a clinically relevant constituent in the imaging algorithm of nononcologic patients referred for bone scintigraphy. J Nucl Med 2007;48:319–24 [PubMed] [Google Scholar]
- 33.Linke R, Kuwert T, Uder M, Forst R, Wuest W. Skeletal SPECT/CT of the peripheral extremities. AJR Am J Roentgenol. 2010;194:W329–35 10.2214/AJR.09.3288 [DOI] [PubMed] [Google Scholar]
- 34.Ryan PJ, Evans PA, Gibson T, Fogelman I. Chronic low back pain: comparison of bone SPECT with radiography and CT. Radiology 1992;182:849–54 [DOI] [PubMed] [Google Scholar]
- 35.Dolan AL, Ryan PJ, Arden NK, Stratton R, Wedley JR, Hamann W, et al. The value of SPECT scans in identifying back pain likely to benefit from facet joint injection. Br J Rheumatol 1996;35:1269–73 [DOI] [PubMed] [Google Scholar]
- 36.Pneumaticos SG, Chatziioannou SN, Hipp JA, Moore WH, Esses SI. Low back pain: prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology 2006;238:693–8 10.1148/radiol.2382041930 [DOI] [PubMed] [Google Scholar]
- 37.Holder LE, Machin JL, Asdourian PL, Links JM, Sexton CC. Planar and high-resolution SPECT bone imaging in the diagnosis of facet syndrome. J Nucl Med 1995;36:37–44 [PubMed] [Google Scholar]
- 38.Ryan PJ, Fogelman I. Osteoporotic vertebral fractures: diagnosis with radiography and bone scintigraphy. Radiology 1994;190:669–72 [DOI] [PubMed] [Google Scholar]
- 39.Ryan PJ, Evans P, Gibson T, Fogel I. Osteoporosis and chronic back pain: a study with single-photon emission computed tomography bone scintigraphy. J Bone Miner Res 1992;7:1455–60 10.1002/jbmr.5650071213 [DOI] [PubMed] [Google Scholar]
- 40.Mulholland NJ, Gnanasegaran G, Mohan HK, Vijayanathan S, Clarke SE, Fogelman I. Recognition of the femoroacetabular impingement syndrome on MDP SPECT CT. Clin Nucl Med 2008;33:125–7 10.1097/RLU.0b013e31815ef80f [DOI] [PubMed] [Google Scholar]
- 41.Bredella MA, Stoller DW. MR imaging of femoroacetabular impingement. MRI Clin North Am 2005;13:653–64 10.1016/j.mric.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 42.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis-what the radiologist should know. AJR Am J Roentgenol 2007;188:1540–52 10.2214/AJR.06.0921 [DOI] [PubMed] [Google Scholar]
- 43.Gnanasegaran G, Mohan HK, Sharp D. Single photon emission computed tomography-computed tomography in the management of foot pathology [abstract]. Nucl Med Commun 2008;29:482 [Google Scholar]
- 44.Leumann A, Valderrabano V, Plaass C, Rasch H, Studler U, Hintermann B, et al. A novel imaging method for osteochondral lesions of the talus – comparison of SPECT-CT with MRI. Am J Sports Med 2011;39:1095–101 10.1177/0363546510392709 [DOI] [PubMed] [Google Scholar]
- 45.Wiewioski M, Pagenstat G, Rasch H, Jacob AL, Valderrabano V. Pain in osteochondral lesions. Foot Ankle Spec 2011;4:92–9 10.1177/1938640010395749 [DOI] [PubMed] [Google Scholar]
- 46.Knupp M, Pagenstert GI, Barg A, Bolliger L, Easley ME, Hintermann B. SPECT-CT compared with conventional imaging modalities for the assessment of the varus and valgus malaligned hindfoot. J Orthop Res 2009;27:1461–66 10.1002/jor.20922 [DOI] [PubMed] [Google Scholar]
- 47.Mohan HK, Gnanasegaran G, Vijayanathan S, Fogelman I. SPECT/CT in imaging foot and ankle pathology—the demise of other coregistration techniques. Semin Nucl Med 2010;40:41–51 10.1053/j.semnuclmed.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 48.Langroudi B, Mohan H, Gnanasegaran G. SPECT-CT in the assessment of bony foot pathology. J Nucl Med 2007;48(Suppl. 2):122P17204708 [Google Scholar]
- 49.Mohan H, Holker P, Gnanasegaran G. The applicability of SPECT-CT in directing the management of bony foot and ankle pathology. Eur J Nucl Med Mol Imaging 2007;34(Suppl. 2):S166 [Google Scholar]
- 50.Breunung N, Barwick T, Fernando R, Gnanasegaran G, Vijayanathan S, Hosahalli M, et al. Additional benefit of SPECT-CT in investigating heel pain. Clin Nucl Med 2008;33:705–06 10.1097/RLU.0b013e318184b987 [DOI] [PubMed] [Google Scholar]
- 51.Ryan PJ, Chauduri R, Bingham J, Fogelman I. A comparison of MRI and bone SPET in the diagnosis of knee pathology. Nucl Med Commun 1996;17:125–31 [DOI] [PubMed] [Google Scholar]
- 52.Ryan PJ, Reddy K, Fleetcroft J. A prospective comparison of clinical examination, MRI, bone SPECT, and arthroscopy to detect meniscal tears. Clin Nucl Med 1998;23:803–6 [DOI] [PubMed] [Google Scholar]
- 53.Cook GJ, Ryan PJ, Clarke SE, Fogelman I. SPECT bone scintigraphy of anterior cruciate ligament injury. J Nucl Med 1996;37:1353–6 [PubMed] [Google Scholar]
- 54.Collier BD, Johnson RP, Carrera GF, Isitman AT, Veluvolu P, Knobel J, et al. Chronic knee pain assessed by SPECT: comparison with other modalities. Radiology 1985;157:795–802 [DOI] [PubMed] [Google Scholar]
- 55.Fernando RA, Panchadhar S, Barwick T. Initial experience of SPECT/CT in patients with problematic painful knee [abstract]. Eur J Nucl Med Mol Imag 2008;35(Suppl. 2):S379 [Google Scholar]
- 56.Mohamed A, Ryan P, Lewis M, Jarosz JM, Fogelman I, Spencer JD, et al. Registration bone scan in the evaluation of wrist pain. J Hand Surg 1997:22:161–6 [DOI] [PubMed] [Google Scholar]
- 57.Huellner MW, Bürkert A, Schleich FS, Schürch M, Hug U, von Wartburg U, et al. SPECT/CT versus MRI in patients with nonspecific pain of the hand and wrist—a pilot study. Eur J Nucl Med Mol Imag Jan 2012; 39:750–9 10.1007/s00259-011-2034-3 [DOI] [PubMed] [Google Scholar]
- 58.Hirschmann MT, Schmid R, Dhawan R, Skarvan J, Rasch H, Friederich NF, et al. Combined single photon emission computerized tomography and conventional computerized tomography: clinical value for the shoulder surgeons? Int J Shoulder Surg. 2011;5:72–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bar-Shalom R, Yefremov N, Guralnik L, Keidar Z, Engel A, Nitecki S, et al. SPECT/CT using 67Ga and 111In-labeled leukocyte scintigraphy for diagnosis of infection. J Nucl Med 2006;47:587–94 [PubMed] [Google Scholar]
- 60.Horger M, Eschmann SM, Pfannenberg C, Storek D, Vonthein R, Claussen CD, et al. The value of SPET/CT in chronic osteomyelitis. Eur J Nucl Med Mol Imag 2003;30:1665–73 10.1007/s00259-003-1321-z [DOI] [PubMed] [Google Scholar]
- 61.Filippi L, Schillaci O. Usefulness of hybrid SPECT/CT in 99mTc-HMPAO-labeled leukocyte scintigraphy for bone and joint infections. J Nucl Med 2006;47:1908–13 [PubMed] [Google Scholar]
- 62.Horger M, Eschmann SM, Pfannenberg C, Storek D, Vonthein R, Claussen CD, et al. Added value of SPECT/CT in patients suspected of having bone infection: preliminary results. Arch Orthop Trauma Surg 2007;127:211–21 10.1007/s00402-006-0259-6 [DOI] [PubMed] [Google Scholar]
- 63.Heiba SI, Kolker D, Mocherla B, Kapoor K, Jiang M, Son H, et al. The optimized evaluation of diabetic foot infection by dual isotope SPECT/CT imaging protocol. J Foot Ankle Surg. 2010;49:529–36 10.1053/j.jfas.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 64.Filippi L, Uccioli L, Giurato L, Schillaci O. Diabetic foot infection: usefulness of SPECT/CT for 99mTc-HMPAO-labeled leukocyte imaging. J Nucl Med. 2009;50:1042–6 10.2967/jnumed.108.059493 [DOI] [PubMed] [Google Scholar]
- 65.Gnanasegaran G, Barwick T, Milburn H, Vijayanathan S, Fogelman I. Tuberculosis of spine on Tc-99m MDP bone scan: additional role of SPECT-CT. Clin Nucl Med 2009;34:271–4 10.1097/RLU.0b013e31819e52e8 [DOI] [PubMed] [Google Scholar]
- 66.Hassan FU, Enayat M, Mohammed F, Vijayanathan S, Gnanasegaran G. Heterotrophic ossification in a patient suspected of having osteomyelitis: additional value of SPECT/CT. Clin Nucl Med. 2012;37:170–1 10.1097/RLU.0b013e31823e9ac0 [DOI] [PubMed] [Google Scholar]
- 67.www.cebm.net [homepage on the internet]. OCEBM Levels of Evidence Working Group*. “The Oxford 2011 Levels of Evidence”. Oxford, UK: Oxford Centre for Evidence-Based Medicine ; [updated 16 September 2013]. Available from:http://www.cebm.net/index.aspx?o=5653 [Google Scholar]