Abstract
Objective:
We evaluated and compared a high-fibre diet leaflet, daily microenema and no preparation to establish how best to achieve consistent bowel preparation in prostate cancer patients being treated with radical radiotherapy.
Methods:
3 cohorts of 10 patients had different dietary interventions: no bowel preparation, high-fibre diet information leaflet and daily microenemas. The available cone beam CT (CBCT) scans of each patient were used to quantify interfractional changes in rectal distension (measured using average cross-sectional area—CSA), prostate shifts relative to bony anatomy compared with that at CT planning scan and rates of geometric miss (i.e. shifts of ≥5 mm). 85 CBCT scans were available in the pre-leaflet cohort, 89 scans in the post-leaflet, and 89 scans in the post-enema group.
Results:
Mean rectal CSA in the post-enema group was reduced compared with both pre-leaflet (p=0.010) and post-leaflet values (p=0.031). The magnitude of observed mean prostate shifts was significantly reduced in the post-enema group compared with the pre-leaflet group (p=0.014). The proportion of scans showing geometric miss (i.e. shift >5 mm) in the post-enema group (31%) was significantly lower than in the pre-leaflet (62%, p<0.001) or post-leaflet groups (56%, p<0.001).
Conclusion:
This study indicates microenema to be an effective measure to achieve reduction in rectal CSA, prostate shift and reduce geometric miss of ≥5 mm. A further prospective randomised study is advocated to validate the results.
Advances in knowledge:
The use of microenema is effective in reducing prostate shift and rectal CSA, consequently decreasing the incidence of geographical miss.
Patients receiving radical radiotherapy to the prostate can exhibit prostate shifts owing to rectal distension that can lead to geographical miss [1,2]. There is strong evidence that lack of adequate image-guided radiotherapy (IGRT) to correct for these shifts reduces biochemical and local control [3]. Increasingly, newer treatment techniques such as intensity-modulated radiotherapy (IMRT) and IGRT are used together in the treatment of prostate cancer, aiming to improve tumour control probability without increasing normal tissue toxicity [4]. Although the use of smaller expansion margins could reduce the incidence of toxicity, it will also increase the risk of geographical miss unless the IGRT protocol is sufficient to support the reduced margins. Although there are other possible daily variables in prostate radiotherapy, such as bladder filling, rectal distension is the single most important variable in causing prostate motion in the anteroposterior direction [5–7].
Although available image-guidance systems are able to correct the interfractional random set-up errors, the possibility of a more stable prostate owing to lower mobility of the rectum is still appealing because of the associated reduction of deformation effects on prostate and seminal vesicles, which cannot be corrected by rigid translations.
We identified our management of patients having radiotherapy to the prostate with rectal distension as inconsistent and in need of review. In a UK-wide survey in 2009, 40% of the responding centres routinely used some form of bowel preparation to reduce rectal distension. These strategies included simple dietary advice such as a high-fibre diet, prescription of laxatives or microenemas [8]. Fiorino et al [9] showed that use of daily enemas for rectal emptying efficiently minimised prostate motion, while further studies reinforced this finding, also demonstrating that the resulting improvements could lead to a reduction in rectal dose [10,11]. The existing local dietary protocol (no dietary advice or intervention) was identified as being in need of improvement, but at the time of writing, little published evidence on the comparative efficacy of these practices was found, so the optimal strategy was unclear. The relatively invasive nature of the daily enemas means that the benefits of such a strategy should be confirmed on a local population before its routine clinical adoption.
This work therefore aims to evaluate the impact of three different rectal strategies in an attempt to establish the best measure to achieve consistent results in terms of the consequent reductions in rectal distension and resulting movement of the prostate throughout treatment: (i) no dietary advice, (ii) dietary advice and (iii) use of daily microenemas.
MATERIALS AND METHODS
Patient selection and treatment
30 radical prostate patients previously treated to 74 Gy in 37 fractions were selected for this study. All patients had received neo-adjuvant hormones before commencing radical radiotherapy. Patients’ characteristics including age and risk groups based on TNM stage, Gleason score and prostate-specific antigen (PSA) at diagnosis in all three groups are shown in Table 1.
Table 1.
Patient characteristics, i.e. age and risk categories as defined by TNM stage, Gleason score and prostate-specific antigen (PSA) at diagnosis of the pre-leaflet, post-leaflet and post-enema groups
| Group | Age at presentation (years) | Risk category (n) | PSA level at presentation (ng ml−1) |
| Pre-leaflet group | Range 55.0–77.0 | High risk=3 | Range 8.50–31.20 |
| Median 70.5 | Intermediate risk=4 | Median=19.40 | |
| Low risk=2 | |||
| NA=1 | |||
| Post-leaflet group | Range 51.0–76.0 | High risk=3 | Range 6.90–13.00 |
| Median 62.0 | Intermediate risk=1 | Median=9.30 | |
| Low risk=4 | |||
| NA=2 | |||
| Post-enema group | Range 54.0–78.0 | High risk=2 | Range 3.46–18.60 |
| Median 71.0 | Intermediate risk=5 | Median=6.40 | |
| Low risk=3 |
NA, not available.
High risk=T3–T4 or PSA>20 ng ml−1 or Gleason score 8–10; intermediate risk=T2b–T2c or PSA 10–20 ng ml−1 or Gleason score 7; low risk=T1–T2a and PSA<10 ng ml−1 and Gleason score≤6.
In an effort to ensure reproducible bladder filling, all patients were asked to empty their bladder, then drink 4 cups of water (200 ml) and wait for 30 min before the planning CT scan and again prior to each day of treatment. All planning CT scans were acquired at 3-mm resolution in supine position and exported to ProSoma v. 3.2 (Oncology Systems Limited, Shrewsbury, UK) for delineation by a trained clinical oncologist. The rectum was outlined from the anus to the recto-sigmoid junction (definition previously followed by the authors in [12]), planned target volume (PTV1) included in prostate and the base of the seminal vesicles (typically 18–21 mm)+10 mm, PTV2 included the prostate +5 mm. PTV1 target isodose coverage was aimed at a minimum of 76% (of the prescribed dose of 74 Gy) with a ≥80% median dose (to PTV1 outside of PTV2). The PTV2 target isodose was aimed at 91% (minimum) with a ≥96% median dose (of the prescribed 74 Gy) to the PTV2. All patients were planned with forward-planned IMRT (field-in-field), treated on an Elekta Synergy® linear accelerator (Elekta AB, Stockholm, Sweden) and verified using cone beam CT (CBCT) image guidance. All patients underwent systematic set-up correction via offline imaging on Days 1–3 and then weekly, and all available CBCT scans (i.e. at least 8 per patient) were used in this analysis.
Owing to local changes in protocol over time (i.e. implementation of dietary information sheet in July 2009 and introduction of microenema from November 2010), the patients in the current study were necessarily treated at different periods. The patients in each group were randomly selected during a 4-week period following implementation of the dietary leaflet and microenema protocol to avoid selection bias. In all cases, the relevant imaging data were retrieved from the archive.
Sample 1—“pre-leaflet” group: these patients received no bowel preparation or dietary advice.
Sample 2—“post-leaflet” group: these patients were given an information sheet with details of a recommended dietary protocol (detailing how to increase fibre intake, fluid intake and meals/snack ideas—see Appendix, Figure A1) at the planning scan appointment and asked to follow the advice for at least 2 weeks before, and throughout, treatment unless advised to stop (e.g. owing to diarrhoea).
Figure A1.
Dietary protocol information leaflet for post-leaflet group.
Sample 3—“Post-enema” group: these patients received no dietary advice sheet but were requested to administer daily microlette microenemas before filling their bladders.
Image registration and segmentation
Retrospective registration of each CBCT image with the corresponding planning CT scan was carried out in ProSoma for the purposes of this study. Initial rigid registration was performed using the full data sets and resulted in an accurate registration to bony anatomy. Subsequently, a “clipbox” was defined around the prostate to restrict the rigid registration to this region, which allowed quantification of the required translational shift for soft-tissue registration from the difference between the two registrations.
Owing to the variability in CBCT scan length, the rectum was not always fully available for delineation on CBCT. Therefore, to ensure consistent delineation between all scans, the rectum was outlined from the slice above the top of the seminal vesicles to one slice below the apex of prostate on all CBCT and CT scans. Delineation was carried out by five observers, each of whom had been trained by the study lead. A sample of scans was subsequently checked by the study lead to ensure consistency.
Data analysis
Data were collected and analysed in an Excel spreadsheet (Microsoft® Corporation, Redmond, WA), with the left to right shift recorded as x (x +ve=left and x −ve= right), the anteroposterior shift as y (y +ve=anterior and y −ve=posterior) and superior–inferior shift as z (z +ve=superior and z −ve=inferior).
Rectal volume was recorded from the above outlines. The mean rectal cross-sectional area (CSA) was calculated for each CBCT scan by dividing the total rectal volume (including organ contents such as faeces and gas) by its length [9]. Relative CSA (CSA rel) was defined as the CSA at the time of CBCT scan divided by the CSA at the planning CT scan. The significance of differences in this variability of rectal volume on repeated CBCT scans was quantified using analysis of variance (ANOVA).
Daily shifts in prostate position were quantified relative to bony anatomy as the difference between the above registration results for bony anatomy and soft tissue. Statistical significance of differences between cohorts was quantified using ANOVA followed by Tukey’s post hoc test to identify significant pairwise differences. All shifts combined were calculated for each scan for each patient as the square root of (x2+y2+z2).
A geographical miss was defined as a prostate shift in any direction of ≥5 mm (taken in view of the PTV2 margin of 5 mm), with the significance of any difference between cohorts analysed using Fisher’s exact test. As per local protocol, the CBCT images carried out on Days 1–3 and then weekly were retrieved from the archive; however, a few scans (5 in pre-leaflet and 1 each in postleaflet and post-enema groups) were not retrievable from the archive, hence comparison was made of 84 vs 89 vs 89 scans performed in the pre-leaflet, post-leaflet and post-enema groups, respectively.
SPSS® PASW stats v. 18 (2009; SPSS, Inc., Chicago, IL) was used for statistical analysis. Parametric statistical analyses were carried out on the data, where possible. For the assumption of normality to hold, the standard deviation (SD) data were log transformed.
RESULTS
Geometric miss (i.e. prostate shift of >5 mm relative to bony anatomy) occurred on an average of 62% (53/85) of fractions imaged in pre-leaflet, 56% (48/89) of post-leaflet and 31% (28/89) of post-enema samples. The rate of prostate shift for the post-enema group was significantly lower (p<0.001, Fisher’s exact test) than for either of the other cohorts.
The left–right, anteroposterior and superoinferior mean prostate shifts relative to the position at the planning CT scan are shown in Table 2 for each cohort. While reductions in prostate shift are seen for the post-enema cohort, they do not reach statistical significance (p=0.081, 0.062 and 0.845 for x, y and z, respectively; ANOVA). The magnitude of the systematic vector shift was found to be significantly reduced for the post-enema cohort than for the pre-leaflet (p=0.014, Tukey’s post hoc test), although differences in the random position error (Table 3, right) were not (p=0.162; ANOVA). The variation of x, y and z shift was significant for x-direction only (SD of x shift: p=0.018, SD of y shift: p=0.630, and SD of z shift: p=0.343). On pairwise comparison of all three groups, the variation in the post-enema values was less than that in the pre-leaflet values (p=0.013, Tukey’s post hoc test) showing greater stability in the prostate position.
Table 2.
Left–right, anteroposterior, superoinferior and combined arithmetic mean of mean prostate shift in pre-leaflet, post-leaflet and post-enema groups
| Shift | Pre-leaflet group | Post-leaflet group | Post-enema group |
| Left–right | −2.4 | −0.6 | −1.3 |
| Anteroposterior | 3.5 | 3.1 | 0.7 |
| Superioinferior | 0.5 | 0.8 | 0.9 |
| All shifts combined | 7.9a | 6.5 | 5.1a |
Significant difference (p<0.05) between pre-leaflet group and post-enema group.
Table 3.
Left–right, anteroposterior, superioinferior and combined geometric mean of mean standard deviation prostate shift in pre-leaflet, post-leaflet and post-enema groups
| Shift | Pre-leaflet group | Post-leaflet group | Post-enema group |
| Left–right | 3.1a | 2.1 | 1.6a |
| Anteroposterior | 3.3 | 2.7 | 2.6 |
| Superioinferior | 2.8 | 1.8 | 2.3 |
| All shifts combined | 3.0 | 2.0 | 2.5 |
SD, standard deviation.
Significant difference (p<0.05) between pre-leaflet group and post-enema group.
Table 4 depicts the values for the mean rectal CSA, showing lower values for the post-enema group than others that were found to be significant (p=0.007; ANOVA). On comparison of the mean CSA values, post-enema values were less than both pre-leaflet values (p=0.010) and post-leaflet values (p=0.031) (Tukey’s post hoc test). A positive trend was observed in the variability of the bowel volume on repeated CBCT scans than on planning scans when tested by mean CSA rel; however, it was not significant (p=0.133; Kruskal–Wallis).
Table 4.
Geometric mean of mean rectal CSA (cm2) and range of mean values of CSA (cm2)
| Rectal CSA | Pre-leaflet group | Post-leaflet group | Post-enema group |
| Geometric mean of mean CSA (cm2) | 12.5a | 11.5b | 7.0a,b |
| Range mean CSA (cm2) | 8.0–31.4 | 6.2–23.0 | 3.9–14.5 |
CSA, cross-sectional area.
Significant difference (p<0.05) between pre-leaflet group and post-enema group.
Significant difference (p<0.05) between post-leaflet group and post-enema group.
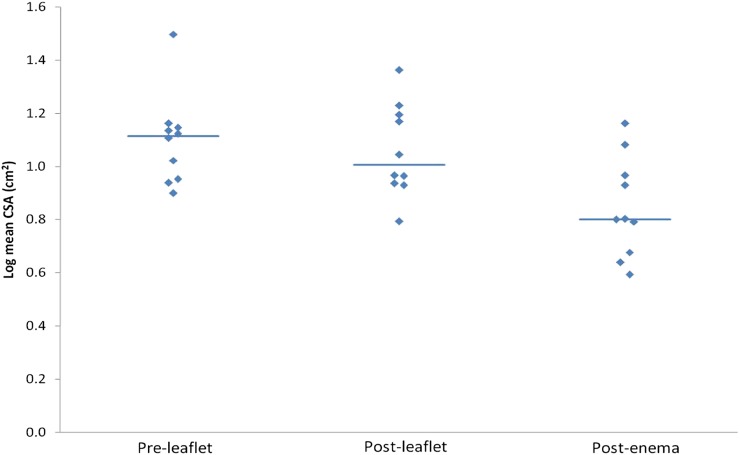
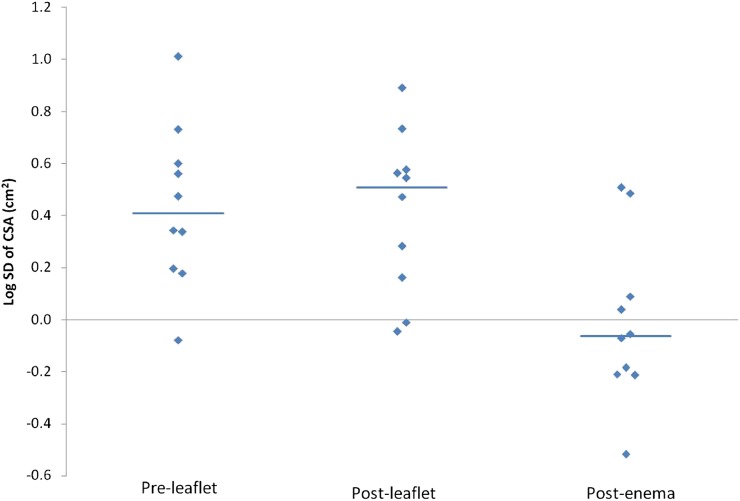
Figures 1 and 2 depict the range of log mean values of the CSA and log standard deviation of CSA. The variability of the CSA and CSA rel was significant (SD of CSA: p=0.005, SD of CSA rel: p=0.014, tested after log transformation; ANOVA). When variability of the CSA values (as depicted in Table 5) was compared amongst all three groups: (i) SD of CSA: post-enema values were less than both pre-leaflet values (p=0.009) and post-leaflet values (p=0.013), (ii) SD of CSA rel: post-enema values were less than both pre-leaflet values (p=0.017) and post-leaflet values (p=0.049) (Tukey's post hoc test).
Figure 1.
Graph shows values of log mean cross-sectional area (CSA) in pre-leaflet, post-leaflet and post-enema groups.
Figure 2.
Graph shows values of log standard deviation of cross-sectional area (CSA) in pre-leaflet, post-leaflet and post-enema groups.
Table 5.
Geometric mean of SD of rectal CSA and range of SD values of CSA
| Rectal CSA | Pre-leaflet group | Post-leaflet group | Post-enema group |
| Geometric mean of SD of CSA (cm2) | 2.7a | 2.6b | 1.0a,b |
| Range SD CSA (cm2) | 0.8–10.2 | 0.9–7.8 | 0.3–3.2 |
CSA, cross-sectional area; SD, Standard deviation.
Significant difference (p<0.05) between pre-leaflet group and post-enema group.
Significant difference (p<0.05) between post-leaflet group and post-enema group.
DISCUSSION
Our study was conducted to establish the best method to achieve reduction in the prostate shift and rectal CSA using bowel preparation leaflet and microenema vs no preparation. The results of this study have demonstrated microenemas to be effective in reduction in organ motion, rectal CSA and hence prostate shift. Tables 2 and 3 show the prostate shifts in the x, y and z directions as well as combined shifts showing a trend towards decreasing shift with the use of bowel preparation. However, the shift in the anteroposterior direction in our study was not found to be significant (p=0.06), with a trend towards decreased shift, possibly owing to a small sample size. Most studies have shown prostate motion either primarily in the anteroposterior and superior–inferior directions or primarily in the anterior direction with increasing rectal distension [13–16]. The authors did not feel that the non-significant anteroposterior shift in our study was related to the patient characteristics shown in Table 1; however; this could possibly be owing to the study being underpowered.
A reduction in the frequency of prostate shifts of >5 mm was achieved in our study with the use of microenema as compared with other strategies (no preparation vs bowel preparation leaflet) on daily IGRT scans, implying a more reproducible daily set-up and a more accurate delivery of intended radical dose to the target volume. These results agree with those of Palombarini et al [17], which showed greatest intra- and interpatient variability in prostate position in the AP direction with 70% displacements within a 5-mm margin. The authors stressed the importance of using internal organ motion reduction strategies such as regular rectum emptying, which has also been shown in studies by Fiorino et al [9] and Stasi et al [11]. Bylund et al [18] used data from daily megavoltage CT (MVCT) imaging to analyse the consequences of alternative strategies for the management of interfraction prostate motion, finding that laxatives or rectal balloons had no effect on systematic error in prostate position but random error (included bony misalignment as well as internal prostate motion) could decrease in the anteroposterior direction by up to 50%.
In prostate cancer, rectal distension is a well-recognised factor responsible for prostate motion: in a study by Padhani et al [19], rectal distension caused significant displacements of the prostate gland in the anteroposterior direction in 29% of patients, and in 16% of patients the movement was >5 mm, which was shown using cine MRI (in real time) over a time period similar to that used for daily fractionated radiotherapy treatments. Engels et al [20] identified two distinct groups of patients with no bowel preparation: a stable group and an unstable group based on the extent of observed rectal distension [CSA (mean ± SD) of 6.6±2.1 cm2 vs 9.5±3.7 cm2 (p<0.01), respectively], based on MVCT planning imaging. This study demonstrates the association of rectal filling with prostate displacement, with a mean anteroposterior prostate displacement of 0.4±2.4 mm in the stable group vs −2.4±6.1 mm in the unstable group (p<0.01).
De Crevoisier et al [3] showed that a distended rectum in prostate radiotherapy patients was related to an increased risk of biochemical and local failure (p=0.0009). In a retrospective analysis of 127 patients, researchers concluded that an empty rectum on planning CT and throughout a course of radical radiotherapy ensures reproducible patient set-up. They found rectal CSA >11.2 cm2, an independent predictor of increased risk of biochemical failure. In our study, 6/10 pre-leaflet sample, 4/10 post-leaflet sample and 2/10 post-enema sample groups had a rectal CSA of >11.2 cm2, although estimation of biochemical control rates was beyond the scope of our study.
We compared the results of our study with those of De Crevoisier et al [3] and Stillie et al [21] (Table 6). In the De Crevoisier et al study, no bowel prep was given to the patients, whereas in the Stillie et al study, dietary advice was given. However, if patients had irregular bowel movements for 7 days before planning scan and during radiotherapy, then ispaghula husk [Fybogel; Reckitt Benckiser Healthcare (UK) Limited, Kinacton Doon Themes, UK] was used to empty the rectum, an intervention that was seen to reduce rectal volume in most patients. The results in Table 6 reinforce the conclusion of the current work that use of daily microenemas is associated with significantly reduced rectal CSA than no bowel preparation vs bowel preparation leaflet. The results showed greater consistency in rectal volume, i.e. CSA with most consistent results in the post-leaflet group, which was found to be significant. The variability of the rectal volume on repeated CBCT was also significantly less in the post-enema group than in others when compared with the planning scan.
Table 6.
Comparison of the range and median of mean cross-sectional area (CSA) of the post-enema group in our study (microenema use; n=10) with the De Crevoisier et al [3] (no bowel preparation, n=127) and Stillie et al [21] (dietary advice/fibogel n=89) studies
Heemsbergen et al [22] recommended the routine use of mild laxative and dietary regimen over no intervention to reduce rectal gas on planning scan and throughout the course of radiotherapy treatment. 549 patients were tested with anorectal volume of ≥90 cm3 and ≥25% of treatment time diarrhoea and mean anorectal CSA (factors defining geometric miss) to predict freedom from any failure (FFF) and freedom from clinical failure (FFCF). Their results showed significantly decreased tumour control in patients with a large rectum filling (FFF, p=0.02; FFCF, p=0.01) visible on the planning CT scan.
Modern radiotherapy techniques allow for the possibility of a reduction in the dose to normal tissue and tumour dose escalation. However, exploitation of these gains is limited by poor understanding of factors causing internal organ motion; therefore, methods should be devised to minimise this and the associated risk of geographical miss.
Several methods have been investigated to minimise rectal distension and reduce the associated prostate motion, but unfortunately there is limited evidence and no randomised controlled trials demonstrating whether any particular method works significantly better than others. A study by Darud et al [23] compared a group of patients following bowel-emptying protocol (using both low-fibre diet and enemas) with those using no rectal preparation. The patients on empty rectum protocol (15/32) were advised to follow a low-fibre diet regimen starting 2 days before the CT scan and to take two tablespoons of milk of magnesia at bedtime to ensure bowel movement in the morning. This was to continue throughout the duration of the treatment or until the development of Grade 2 gastrointestinal side effects. The study showed that empty rectum protocol had larger displacements in the inferior direction (p=0.02) and tended to have larger displacements over the course of treatment (p=0.04). There was no statistically significant trend in the anteroposterior direction. The authors recommended the use of more stringent bowel emptying regimes such as stronger laxatives or an enema to stabilise the prostate gland. Similarly, Ogino et al [13] showed that active rectal gas removal by inserting the index finger and washing the rectums to evacuate the rectal gas before the planning CT scan and each treatment fraction resulted in a significant reduction in the rectal CSA (p=0.01) and reduced prostate motion during a course of radiotherapy (p=0.02).
With increasing use of IGRT, smaller margins are being used; however, this does not automatically generate better clinical results. It is interesting to note that neither De Crevoisier et al [3] nor Ogino et al [13] used IGRT or implanted fiducial markers to identify the interfraction prostate motion. Hence, it can be argued that there may have been even greater variability of prostate motion between the interfraction position of the prostate than with modern IGRT techniques.
Careful consideration to the margins ought to be given when using fiducials and image-guided techniques for treatment verification to encompass inter- and intrafraction organ motion. Budiharto et al [24] reported on intrafraction prostate motion during online IMRT in prostate cancer patients. All patients underwent rectal preparation protocol and drank 300 ml of water after voiding prior to simulation. However, during treatment, no specific bowel or bladder preparation instructions were followed. The study showed that even with IGRT and online correction, at least 21% of cases showed a prostate shift of >5 mm when the radiotherapy fraction delivery time exceeded 450 s. Similarly, Engels et al [25] has shown significantly lower biochemical disease-free survival in patients with fiducial markers than in those with none [5-year freedom from biochemical failure (FFBF); 58% compared with 91% (p=0.02)]. This was consequent on significantly reduced margins (3 mm in the left–right and 5 mm in the anteroposterior and craniocaudal directions). They also showed that the rectal CSA of 16 cm2 was associated with a significantly impaired FFBF. Hence, bowel preparation is desirable even with the use of fiducials and IGRT to prevent geographical miss when smaller margins are used. In our study, CBCT-based IGRT was used, which is increasingly common practice in the UK. It is important, however, to highlight here that IGRT is still not widely available across all UK centres and the use of fiducial markers is even more limited. This calls for efficient and cost-effective strategies that aim to reduce rectal CSA consequent upon reduction in prostate shift.
A study similar to ours was recently reported by Graf et al [26] showing reduction in prostate motion with the use of information sheets as well as enemas in addition to fiducial markers. The authors concluded that bowel preparation could be at least as good as marker-based image guidance to achieve constant anatomy and comes as a cost-effective and less time-consuming strategy than invasive marker insertion.
The limitation of our study is acknowledged in that ideally a sample size calculation should have been performed to improve the validity of the results, and therefore it is not possible to draw any strong conclusions. The results, however, does suggest use of microenemas to be an effective bowel preparation strategy. We are planning to follow up our study with a quality of life questionnaire on an extended cohort of patients to assess the clinical impact of the use of microenema with regard to late toxicity and feasibility of use of the daily enemas.
CONCLUSIONS
The use of daily microenemas leads to significant reduction in rectal CSA and the incidence of prostate positional errors ≥5 mm (i.e. geographical miss) in the treatment of radical prostate cancer. The authors intend to collate the data for quality of life with the microenema use and further toxicity data related to its use. The authors would welcome a larger randomised trial on the comparative clinical efficacy of different dietary interventions, which are critical in day-to-day radiotherapy practice and the long-term improvement of tumour control and normal tissue toxicity rates with or without the use of IGRT.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of Arpita Karina and Dr Cheng Boon to this study.
APPENDIX
REFERENCES
- 1.Balter JM, Sandler HM, Lam K, Bree RL, Lichter AS, ten Haken RK. Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys 1995;31:113–18 10.1016/0360-3016(94)00382-U [DOI] [PubMed] [Google Scholar]
- 2.Crook JM, Raymond Y, Salhani D, Yang H, Esche B. Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother Oncol 1995;37:35–42 [DOI] [PubMed] [Google Scholar]
- 3.De Crevoisier R, Tucker SL, Dong L, Mohan R, Cheung R, Cox JD, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:965–73 10.1016/j.ijrobp.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 4.Dawson LA, Sharpe MB. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol 2006;7:848–58 10.1016/S1470-2045(06)70904-4 [DOI] [PubMed] [Google Scholar]
- 5.Pinkawa M, Siluschek J, Gagel B, Demirel C, Asadpour B, Holy R, et al. Influence of the initial rectal distension on posterior margins in primary and postoperative radiotherapy for prostate cancer. Radiother Oncol 2006;81:284–90 10.1016/j.radonc.2006.10.028 [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Crean D, Mageras GS, Lyass O, Happersett L, Ling CC, et al. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol 1999;50:225–34 [DOI] [PubMed] [Google Scholar]
- 7.Beard CJ, Kijewski P, Bussière M, Gelman R, Gladstone D, Shaffer K, et al. Analysis of prostate and seminal vesicle motion: implications for treatment planning. Int J Radiat Oncol Biol Phys 1996;34:451–8 [DOI] [PubMed] [Google Scholar]
- 8.McNair HA, Wedlake L, McVey GP, Thomas K, Andreyev J, Dearnaley DP. Can diet combined with treatment scheduling achieve consistency of rectal filling in patients receiving radiotherapy to the prostate? Radiother Oncol 2011;101:471–8 10.1016/j.radonc.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Fiorino C, Di Muzio N, Broggi S, Cozzarini C, Maggiulli E, Alongi F, et al. Evidence of limited motion of the prostate by carefully emptying the rectum as assessed by daily MVCT image guidance with helical tomotherapy. Int J Radiat Oncol Biol Phys 2008;71:611–17 10.1016/j.ijrobp.2008.01.048 [DOI] [PubMed] [Google Scholar]
- 10.Nijkamp J, Pos FJ, Nuver TT, de Jong R, Remeijer P, Sonke JJ, et al. Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results. Int J Radiat Oncol Biol Phys 2008;70:75–82 10.1016/j.ijrobp.2007.05.046 [DOI] [PubMed] [Google Scholar]
- 11.Stasi M, Munoz F, Fiorino C, Pasquino M, Baiotto B, Marini P, et al. Emptying the rectum before treatment delivery limits the variations of rectal dose—volume parameters during 3DCRT of prostate cancer. Radiother Oncol 2006;80:363–70 10.1016/j.radonc.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Foppiano F, Fiorino C, Frezza G, Greco C, Valdagni R, AIRO National Working Group on Prostate Radiotherapy The impact of contouring uncertainty on rectal 3D dose-volume data: results of a dummy run in a multicenter trial (AIROPROS01-02). Int J Radiat Oncol Biol Phys 2003;57:573–9 [DOI] [PubMed] [Google Scholar]
- 13.Ogino I, Uemura H, Inoue T, Kubota Y, Nomura K, Okamoto N. Reduction of prostate motion by removal of gas in rectum during radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:456–66 10.1016/j.ijrobp.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Beard CJ, Kijewski P, Bussière M, Gelman R, Gladstone D, Shaffer K, et al. Analysis of prostate and seminal vesicle motion: implications for treatment planning. Int J Radiat Oncol Biol Phys 1996;34:451–8 [DOI] [PubMed] [Google Scholar]
- 15.Roeske JC, Forman JD, Mesina CF, He T, Pelizzari CA, Fontenla E, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys 1995;33:1321–9 10.1016/0360-3016(95)00225-1 [DOI] [PubMed] [Google Scholar]
- 16.Dawson LA, Mah K, Franssen E, Morton G. Target position variability throughout prostate radiotherapy. Int J Radiat Oncol Biol Phys 1998;42:1155–61 [DOI] [PubMed] [Google Scholar]
- 17.Palombarini M, Mengoli S, Fantazzini P, Cadioli C, Degli EC, Frezza GP. Analysis of inter-fraction setup errors and organ motion by daily kilovoltage cone beam computed tomography in intensity modulated radiotherapy of prostate cancer. Radiat Oncol 2006;80:363–70 10.1186/1748-717X-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bylund KC, Bayouth JE, Smith MC, Hass AC, Bhatia SK, Buatti JM. Analysis of interfraction prostate motion using megavoltage cone beam computed tomography. Int J Radiat Oncol Biol Phys 2008;72:949–56 10.1016/j.ijrobp.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 19.Padhani AR, Khoo VS, Suckling J, Husband JE, Leach MO, Dearnaley DP. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys 1999;44:525–33 [DOI] [PubMed] [Google Scholar]
- 20.Engels B, Tournel K, Soete G, Storme G. Assessment of rectal distention in radiotherapy of prostate cancer using daily megavoltage CT image guidance. Radiother Oncol 2009;90:377–81 10.1016/j.radonc.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Stillie AL, Kron T, Fox C, Herschtal A, Haworth A, Thompson A, et al. Rectal filling at planning does not predict stability of the prostate gland during a course of radical radiotherapy if patients with large rectal filling are re-imaged. Clin Oncol (R Coll Radiol) 2009;21:760–7 10.1016/j.clon.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Heemsbergen WD, Hoogeman MS, Witte MG, Peeters ST, Incrocci L, Lebesque JV. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 Gy versus 78 Gy. Int J Radiat Oncol Biol Phys 2007;67:1418–24 10.1016/j.ijrobp.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 23.Darud M, Giddings A, Keyes M, McGahan C, Tyldesely S. Evaluation of a protocol to reduce rectal volume and prostate motion for external beam radiation therapy of the prostate. J Med Imaging Radiat Oncol 2010;41:12–19 [DOI] [PubMed] [Google Scholar]
- 24.Budiharto T, Slagmolen P, Haustermans K, Maes F, Junius S, Verstraete J, et al. Intrafractional prostate motion during online image guided intensity-modulated radiotherapy for prostate cancer. Radiother Oncol 2011;98:181–6 10.1016/j.radonc.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 25.Engels B, Soete G, Verellen D, Storme G. Conformal arc radiotherapy for prostate cancer: increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys 2009;74:388–91 [DOI] [PubMed] [Google Scholar]
- 26.Graf R, Boehmer D, Nadobny J, Budach V, Wust P. Appropriate patient instructions can reduce prostate motion. Radiat Oncol 2012;7:125 10.1186/1748-717X-7-125 [DOI] [PMC free article] [PubMed] [Google Scholar]