Abstract
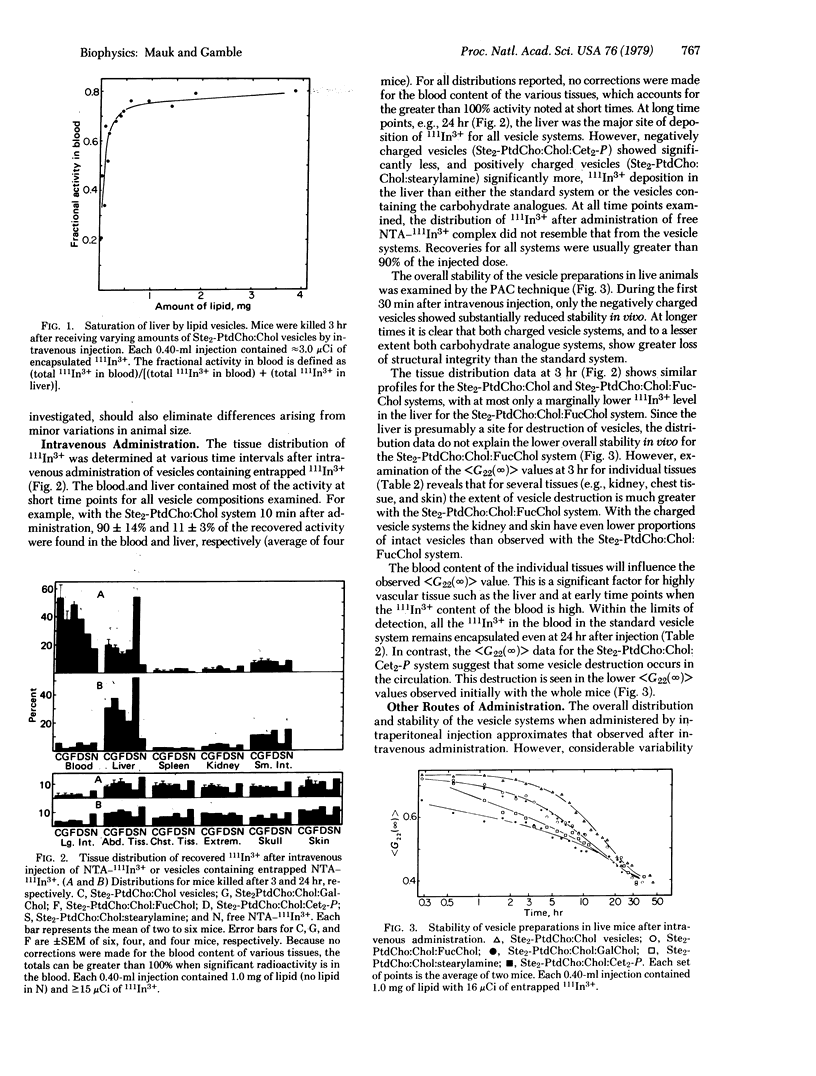
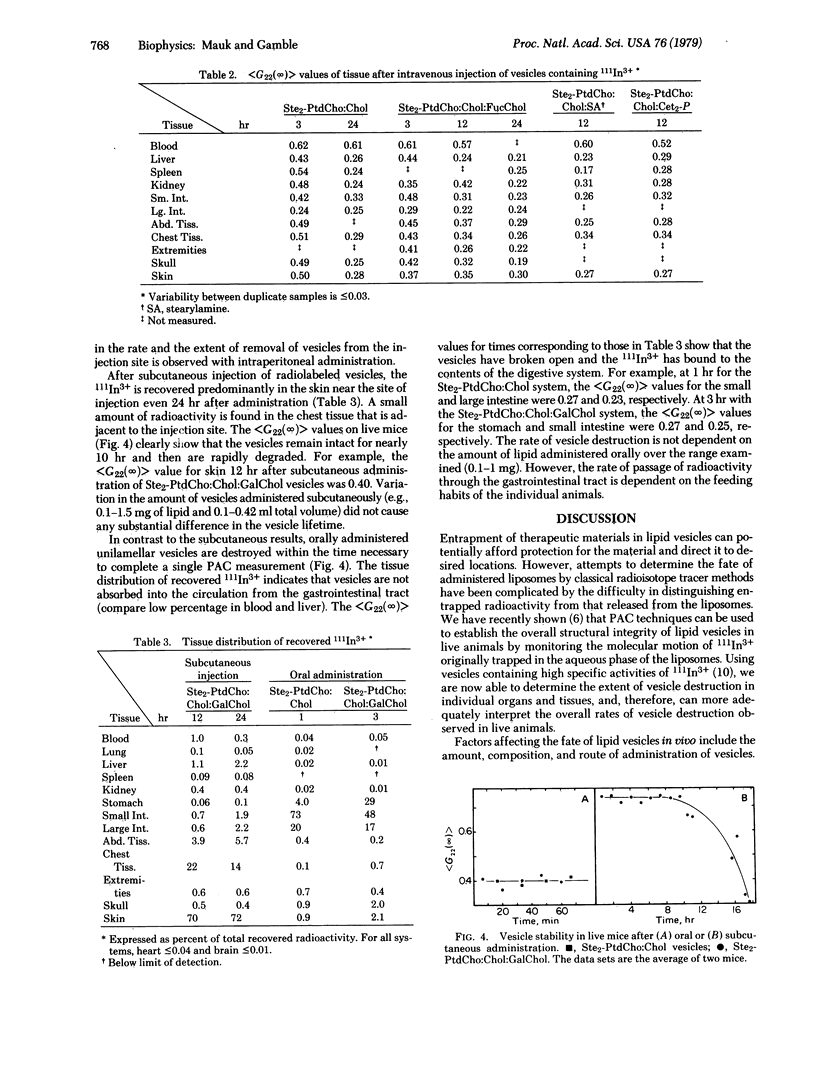
The rate of phospholipid vesicle disruption in specific tissues of the mouse was followed by gamma-ray perturbed angular correlation (PAC) spectroscopy. In these studies, high levels of 111In-nitrilotriacetic acid complex are contained in unilamellar vesicles consisting of distearoyl phosphatidylcholine, cholesterol, and small amounts of other lipids which modify the surface properties. The PAC technique monitors the extent of vesicle breakup by measuring a time-integrated perturbation factor, less than G22 (infinity) greater than. As the vesicles are broken open in vivo, the released 111In3+ ions quickly bind to macromolecules and the less than G22 (infinity) greater than value decreases substantially. After administration of vesicles by various routes (intravenous, intraperitoneal, subcutaneous, and oral), the radioactivity and less than G22 (infinity) greater than values were determined for several tissues at intervals up to 24 hr. We conclude from these data that (i) the PAC technique in conjunction with standard gamma counting methods provides unique information on the condition and location of vesicles in specific tissues, (ii) significant differences in vesicle integrity are found in various tissues, and (iii) both the means of administration and the presence of surface charge affect the vesicle stability and distribution. The carbohydrate analogues of cholesterol affect vesicle stability but not distribution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Weinstein J. N., Sharrow S. O., Henkart P. Liposome--lymphocyte interaction: saturable sites for transfer and intracellular release of liposome contents. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5603–5607. doi: 10.1073/pnas.74.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian R. W., Wriston J. C., Jr Influence of incorporated cerebrosides on the interaction of liposomes with HeLa cells. Biochim Biophys Acta. 1977 Dec 1;471(2):336–336. doi: 10.1016/0005-2736(77)90261-9. [DOI] [PubMed] [Google Scholar]
- Caride V. J., Taylor W., Cramer J. A., Gottschalk A. Evaluation of liposome-entrapped radioactive tracers as scanning agents. Part 1: Organ distribution of liposome (99mTc-DTPA) in mice. J Nucl Med. 1976 Dec;17(12):1067–1072. [PubMed] [Google Scholar]
- Fendler J. H., Romero A. Liposomes as drug carriers. Life Sci. 1977 Apr 1;20(7):1109–1120. doi: 10.1016/0024-3205(77)90481-7. [DOI] [PubMed] [Google Scholar]
- Goodwin D. A., Meares C. F., Song C. H. The study of 111 In-labeled compounds in mice, using perturbed angular correlations of gamma radiations. Radiology. 1972 Dec;105(3):699–702. doi: 10.1148/105.3.699. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Dapergolas G., Neerunjun E. D. Penetration of target areas in the rat by liposome-associated agents administered parenterally and intragastrically. Biochem Soc Trans. 1976;4(2):256–259. doi: 10.1042/bst0040256. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Neerunjun D. E., Hunt R. Fate of a liposome-associated agent injected into normal and tumour-bearing rodents. Attempts to improve localization in tumour tissues. Life Sci. 1977 Aug 1;21(3):357–369. doi: 10.1016/0024-3205(77)90516-1. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. Targeting of drugs. Nature. 1977 Feb 3;265(5593):407–411. doi: 10.1038/265407a0. [DOI] [PubMed] [Google Scholar]
- Hwang K. J., Mauk M. R. Fate of lipid vesicles in vivo: a gamma-ray perturbed angular correlation study. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4991–4995. doi: 10.1073/pnas.74.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawaczeck R., Kainosho M., Chan S. I. The formation and annealing of structural defects in lipid bilayer vesicles. Biochim Biophys Acta. 1976 Sep 7;443(3):313–330. doi: 10.1016/0005-2736(76)90032-8. [DOI] [PubMed] [Google Scholar]
- Leipert T. K., Baldeschwieler J. D., Shirley D. A. Applications of gamma ray angular correlations to the study of biological macromolecules in solution. Nature. 1968 Nov 30;220(5170):907–909. doi: 10.1038/220907a0. [DOI] [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall I. R., Dunnick J. K., Goris M. L., Kriss J. P. In vivo distribution of vesicles loaded with radiopharmaceuticals: a study of different routes of administration. J Nucl Med. 1975 Jun;16(6):488–491. [PubMed] [Google Scholar]
- Meares C. F., Sundberg M. W., Baldeschwieler J. D. Perturbed angular correlation study of a haptenic molecule. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3718–3722. doi: 10.1073/pnas.69.12.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meares C. F., Westmoreland D. G. The study of biological macromolecules using perturbed angular correlations of gamma radiation. Cold Spring Harb Symp Quant Biol. 1972;36:511–516. doi: 10.1101/sqb.1972.036.01.065. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Weinstein J. N. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- Patel H. M., Ryman B. E. Orally administered liposomally entrapped insulin [proceedings]. Biochem Soc Trans. 1977;5(6):1739–1741. doi: 10.1042/bst0051739. [DOI] [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D. Lipid vesicles as carriers for introducing materials into cultured cells: influence of vesicle lipid composition on mechanism(s) of vesicle incorporation into cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1603–1607. doi: 10.1073/pnas.73.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouet A. Perspectives in cancer research. Increased selectivity of drugs by linking to carriers. Eur J Cancer. 1978 Feb;14(2):105–111. doi: 10.1016/0014-2964(78)90167-6. [DOI] [PubMed] [Google Scholar]



