Abstract
The temporal and spatial distribution of Salmonella contamination in the coastal waters of Galicia (northwestern Spain) relative to contamination events with different environmental factors (temperature, wind, hours of sunlight, rainfall, and river flow) were investigated over a 4-year period. Salmonellae were isolated from 127 of 5,384 samples of molluscs and seawater (2.4%), and no significant differences (P < 0.05) between isolates obtained in different years were observed. The incidence of salmonellae was significantly higher in water column samples (2.9%) than in those taken from the marine benthos (0.7%). Of the 127 strains of Salmonella isolated, 20 different serovars were identified. Salmonella enterica serovar Senftenberg was the predominant serovar, being represented by 54 isolates (42.5%), followed by serovar Typhimurium (19 isolates [15%]) and serovar Agona (12 isolates [9.4%]). Serovar Senftenberg was detected at specific points on the coast and could not be related to any of the environmental parameters analyzed. All serovars except Salmonella serovar Senftenberg were found principally in the southern coastal areas close to the mouths of rivers, and their incidence was associated with high southwestern wind and rainfall. Using multiple logistic regression analysis models, the prevalence of salmonellae was best explained by environmental parameters on the day prior to sampling. Understanding this relationship may be useful for the control of molluscan shellfish harvests, with wind and rainfall serving as triggers for closure.
The waters of the highly populated coastal areas receive large quantities of treated and sometimes untreated wastewater discharged from human and industrial sources. In addition, rivers and rainfall can introduce enteric pathogens from distant sources into coastal waters (1, 14). The study of coastal pollution is further complicated by the dilution, survival, and resuspension of sediment-bound pathogens, which are all affected by continuous and often violent environmental fluctuations. Microbiological contamination studies of seawater offer only a snapshot of the effect of contamination events. On the other hand, bivalve molluscs accumulate and retain contaminants for various periods, and their analysis can provide evidence of past contamination events well after a contaminant has been diluted beyond the level of its detection in the overlying waters.
Salmonella enterica has been considered the causal agent of the largest number of enteric infections in the world (2). Raw foods and cross-contamination of ready-to-eat products are the main routes of Salmonella transmission (2, 7). Moreover, Salmonella has been identified in marine environments (1, 3, 8, 9, 13, 16, 26, 27, 28), which are typically contaminated by rivers or storm-generated discharges (1, 6, 14). Salmonella that is introduced into marine environments readily contaminates the fauna, especially molluscan shellfish, which concentrate the marine microflora via filter feeding. Even in coastal areas of high Salmonella incidence, outbreaks of Salmonella associated with the consumption of seafood are uncommon, and few serovars of clinical importance are identified from those of marine origin (1, 9, 28). Serovars of clinical origin may not survive as well as environmental strains (16, 28); this is an area that needs further study. The sources of salmonellae are poorly understood, and this is another data gap that is critical in assessing and controlling the risk associated with the presence of salmonellae in the marine environment.
The objectives of this study were to establish the geographic and temporal distribution of salmonellae in coastal waters in the Galicia region of Spain, to identify serovars, and to determine the relationship of environmental factors and Salmonella prevalence.
MATERIALS AND METHODS
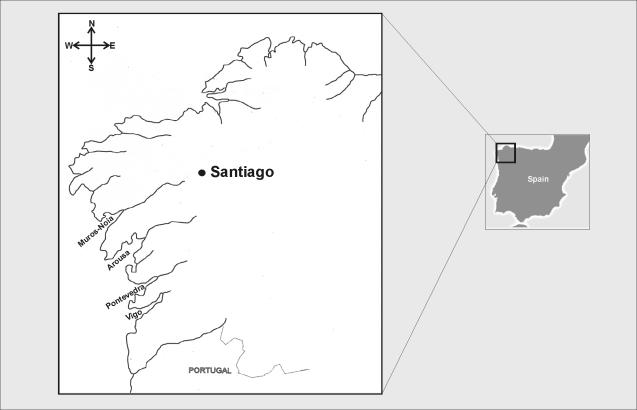
The study focused on the largest shellfish production areas of Galicia in northwestern Spain, which spread along the rias of Vigo, Pontevedra, Arousa, and Muros-Noia (Fig. 1). The rias are estuaries, similar to small fjords, which extend from west to east. They were formed by the sinking of riverbeds, so each ria possesses at least one river in its inland point, which is usually the main source of its freshwater. All the rias are subjected to heavy anthropogenic pressure caused by the high population density of their shores and the important industrial activity, mainly related to the fishing industry, located in the ports and along the coast. Mollusc cultivation is extensive in the estuarine portions of these rias, with production of mussels alone exceeding 200,000 tons per year.
FIG. 1.
Location of the coastal areas included in the present study.
Between January of 1999 and December of 2002, a total of 5,384 samples of molluscs and seawater from the mollusc production areas and the mollusc depuration plants located along the coast were analyzed (Table 1). Clams and cockles, which inhabit bottom sediments, were considered representative of the benthos, whereas mussels and oysters, which are grown on 15-m-long ropes hung from floating platforms, were considered representative of the water column. Overlying surface seawater samples from the corresponding shellfish production areas were also analyzed. Molluscs and seawater samples were collected weekly from 20 depuration plants. In 2002, seawater and mollusc samples collected weekly from 30 sites in the mollusc production zones associated with the four rias described above were also included in the study. For the shellfish analysis, about 1 kg of molluscs was placed in sterile plastic bags and sealed for transport to the laboratory. Surface seawater samples were collected in 500-ml sterile wide-mouth bottles. The samples were refrigerated with ice during the transport to the laboratory and were analyzed immediately upon arrival. At the laboratory, the molluscs were removed from the bags and washed in potable running water. Dead molluscs or those with broken shells were discarded.
TABLE 1.
Numbers of samples of molluscs and seawater analyzed and numbers of Salmonella isolates per year and ria
| Date | No. of samples | No. of samples per ria (% Salmonella isolates)
|
Total no. of isolations (%) | |||
|---|---|---|---|---|---|---|
| Vigo | Pontevedra | Arousa | Othera | |||
| 1999 | 1,134 | 92 (0) | 74 (2.7) | 754 (2.0) | 214 (0) | 17 (1.5) |
| 2000 | 916 | 44 (2.3) | 73 (5.5) | 623 (3.2) | 176 (0.6) | 26 (2.8) |
| 2001 | 1,043 | 71 (4.2) | 117 (1.7) | 613 (3.6) | 242 (0) | 27 (2.6) |
| 2002 | 2,291 | 228 (2.2) | 197 (2.5) | 1,473 (2.8) | 393 (1.5) | 57 (2.5) |
| Total | 5,384 | 435 (2.1) | 461 (2.8) | 3,463 (2.8) | 1,025 (0.7) | 127 (2.4) |
“Other” includes samples from coastal areas close to the rias of Galicia.
Salmonella analysis.
The presence of Salmonella was determined according to the ISO 6579 standard method (10). Twenty-five grams of shucked shellfish homogenate or seawater was added to 225 ml of buffered peptone water (Merck, Darmstadt, Germany), mixed for 90 s with a stomacher, and incubated at 37°C for 20 h. Ten milliliters of preenriched culture was then transferred to 100 ml of selenite cystine broth (Difco, Detroit, Mich.), and 0.1 ml was transferred to 10 ml of RV10 Rappaport-Vassiliadis broth (Difco), and then the mixtures were incubated at 37 and 42°C, respectively, for 24 h. The selective enrichment was streaked onto Hektoen enteric agar (Difco), phenol red-brilliant green agar (Difco), and bismuth sulfite agar (Difco) and incubated at 37°C for 24 h (if the growth was slight, the plates were reincubated for an additional 24 h). Typical colonies were selected and streaked onto nutrient agar and subjected to initial biochemical screening in TSI Agar (Difco). In cultures demonstrating a typical reaction of Salmonella (alkaline slant and acid butt, with or without the production of H2S), the presence of Salmonella was confirmed by biochemical tests on an API 20E strip (BioMérieux, Marcy l'Etoile, France).
Salmonella serotyping.
All Salmonella isolates were confirmed by serotyping. Serotyping was performed by seroagglutination using commercial antisera (Sanofi Diagnostic Pasteur and Difco). Polyvalent Salmonella O and H antisera were used to obtain a presumptive diagnosis, and then the definitive antigenic designation was determined using monovalent antisera.
Environmental parameters.
The environmental parameters examined in this study were temperature, wind, hours of sunshine per day, rainfall, and river flow. The daily ambient temperature was taken as the average of the temperatures registered in a day. Wind direction was measured as the time in hours that the wind blew in each of the four prevailing quadrants (northwest, northeast, southwest, and southeast) or as no wind (calm). Wind speed was measured in kilometers per day. Rainfall was measured in millimeters of precipitation per day, and river flow was calculated as the daily average volume of water from the Ulla River in cubic meters per second.
All the data except the river flow values were provided by the National Weather Institute of the Ministry of Environment and were collected from weather station number 1844 located in the Ria of Pontevedra (longitude, 8°36′59"W; latitude, 42°26′24"N; altitude, 107 m). River flow data was obtained from station 544 of the Ulla River (longitude, 4°20′15"W; latitude, 42°50′48"N) and provided by the Galicia-Coast Network, Department of Hydraulic Public Domain Management, Galician Water Department, Xunta of Galicia.
Salmonella isolation followed a seasonal pattern with the greatest incidence from October to December. For statistical analysis, Salmonella isolation was grouped into three periods (January to June, July to September, and October to December) and the season variable was included in the statistical analysis.
Statistical analysis.
The differences in the numbers of isolates by ria, year, and sample type were determined by the chi-square test, while the differences between numbers of isolates from the two growth habitats of the molluscs (benthic and in suspension) were evaluated by Fisher's exact test. To establish the dependency of the isolates on the environmental factors that could be directly related to contamination, Salmonella isolates were placed into two groups: Salmonella serovar Senftenberg, the predominant serovar (almost 50% of the isolates), and all other serovars (non-Senftenberg). Within the group excluding Salmonella serovar Senftenberg, we conducted a differential analysis of serovars with sufficient numbers of isolates for meaningful comparison (serovars Typhimurium and Agona); the rest of the serovars were grouped together (other serovars). The association between the various environmental factors and monthly numbers of Salmonella isolates was initially analyzed with Pearson correlation coefficients. To study the dependency relationships, we performed a survey using simple logistic regression analysis of the dependent variables (the different serovars of Salmonella) and each one of the environmental parameters included in this study. Once the significant variables at an individual level were selected, a multiple logistic regression model was conducted with the following equation:
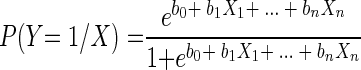 |
where P(Y = 1/X) is the model-predicted probability that the dependent variable (Y) is a positive response (with a value of 1), b0 through bn are the regression coefficients, and X0 through Xn are the independent variables (the subscript numbers are the numbers of coefficients or variables considered).
Due to the seasonal behavior of the Salmonella isolates, the influence of the environmental parameters on the isolates was studied in an analogous manner, with an analysis of the interaction of the parameters with the seasons being included. To do this, the initial survey was performed using multiple logistic regression analysis and taking into account the parameter itself, the season, and the interaction between them. Once the significant variables were selected, a multiple logistic regression analysis considering exclusively the selected variables was performed. In those cases where the relationship between an isolate and an environmental parameter or the season of isolation was not significant but the interaction between an isolate and both that parameter and the season was significant, due to the hierarchical principle, the variable without the interaction and the season was included in the final multiple analysis.
To analyze the relationship between the environmental values and the presence of Salmonella isolates, environmental data from the same day that the samples were collected and from each of the three previous days were used. The possible effect that the persistence of certain environmental factors could have on the contamination was also analyzed by using the accumulated values for the environmental parameters 2 and 3 days before the sample collection. In order to perform the multiple logistic regression analysis, the daily data for Salmonella were transformed to binary data, with values of 1 being the days that one or more isolates (detection) were found and values of 0 being the days in which no isolates (no detections) were obtained. Wald's backward stepwise analysis (POUT [probability out] = 0.10) was used, which initially included all the selected variables and in successive steps eliminated the nonsignificant variables.
All of the statistical analysis was effected with SPSS version 11.0.1 (SPSS Inc.), and the level of significance was set at a P of <0.05.
RESULTS
The overall incidence of Salmonella in the 5,384 samples analyzed was 2.4% (Table 1), with a total of 127 strains being isolated in the 4 years of study as follows: 17 (1.5%) in 1999, 26 (2.8%) in 2000, 27 (2.6%) in 2001, and 57 (2.5%) in 2002. No significant statistical differences were detected among the isolates obtained in the different years (P > 0.05). With respect to the incidence of Salmonella in the different species of molluscs and in seawater, the incidence was 0.8% in clams and 0.6% in cockles, whereas it was 3.1% in mussels and 2.5% in oysters. Of the 707 samples of seawater analyzed, 18 (2.5%) were positive for salmonellae. A significantly lower (P < 0.05) incidence of Salmonella (0.8%) was observed in shellfish inhabiting the benthic environment (clams and cockles) than in seawater or in oysters and mussels suspended from cultivation platforms (2.9%).
Among the 127 isolates of Salmonella, 20 different serovars were identified (Table 2). Nine of the isolated strains could not be serotyped and were registered as salmonellae. Salmonella serovar Senftenberg was the predominant serovar, being represented by 54 isolates, which accounted for 42.5% of the total number of isolated strains. The number of Salmonella serovar Senftenberg isolates with respect to other serovars varied by year; 70.6% of the isolates in 1999 and 63% in 2001 were serovar Senftenberg, but percentages were significantly (P < 0.05) lower in 2000 (23.1%) and 2002 (33.3%). Although Salmonella serovar Senftenberg was detected in all the rias, the number of isolates in the Ria of Arousa was significantly higher than that observed in the other rias. Salmonella serovar Typhimurium was the second most prevalent serovar, with 19 isolates (15%) over the entire study period. It was most prevalent in 2000, accounting for 34.6% of the isolates in that year, and it and Salmonella serovar Agona (34.6%) were the predominant serovars isolated in 2000. Serovar Typhimurium was isolated significantly more frequently in the Ria of Pontevedra than in other rias. The third most common serovar was serovar Agona, with 12 isolates, but it was detected only in 2000 and 2002 and was limited to the Ria of Arousa. The remaining 17 serovars accounted for 26% of the isolates (Table 2). Similar numbers of different serovars were identified for the first 3 years of the study (4, 5, and 7 serovars, respectively) but increased considerably in 2002 (Table 2), when 17 different serovars were detected, 10 of which had not been detected previously.
TABLE 2.
Distribution of the serovars among Salmonella strains isolated per year
| Serovar | No. of isolates (%) in yr:
|
Total (%) | |||
|---|---|---|---|---|---|
| 1999 | 2000 | 2001 | 2002 | ||
| Senftenberg | 12 (70.6) | 6 (23.1) | 17 (63) | 19 (33.3) | 54 (42.5) |
| Typhimurium | 0 | 9 (34.6) | 5 (18.5) | 5 (8.8) | 19 (15) |
| Agona | 0 | 9 (34.6) | 0 | 3 (8.3) | 12 (9.4) |
| Othera | 1 (5.9) | 0 | 0 | 8 (14) | 9 (7.1) |
| Litchfield | 0 | 0 | 0 | 4 (7) | 4 (3.1) |
| Enteritidis | 1 (5.9) | 0 | 1 (3.7) | 2 (3.5) | 4 (3.1) |
| Paratyphi B | 2 (11.8) | 0 | 1 (3.7) | 1 (1.7) | 4 (3.1) |
| Derby | 0 | 1 (3.8) | 1 (3.7) | 1 (1.7) | 3 (2.4) |
| Virchow | 0 | 0 | 0 | 3 (5.3) | 3 (2.4) |
| Anatum | 0 | 0 | 0 | 2 (3.5) | 2 (1.6) |
| Ohio | 1 (5.9) | 0 | 0 | 1 (1.7) | 2 (1.6) |
| Ndolo | 0 | 0 | 0 | 2 (3.5) | 2 (1.6) |
| Bredeney | 0 | 0 | 0 | 1 (1.7) | 1 (0.8) |
| Diarizonae | 0 | 1 (3.8) | 0 | 0 | 1 (0.8) |
| Idikan | 0 | 0 | 0 | 1 (1.7) | 1 (0.8) |
| II 18:z4,II 18:z23 | 0 | 0 | 1 (3.7) | 0 | 1 (0.8) |
| London | 0 | 0 | 0 | 1 (1.7) | 1 (0.8) |
| Muenchen | 0 | 0 | 0 | 1 (1.7) | 1 (0.8) |
| Panama | 0 | 0 | 0 | 1 (1.7) | 1 (0.8) |
| Tilburg | 0 | 0 | 1 (3.7) | 0 | 1 (0.8) |
| Isangi | 0 | 0 | 0 | 1 (1.7) | 1 (0.8) |
| Total | 17 | 26 | 27 | 57 | 127 |
“Other” includes salmonellae with indeterminate serovars.
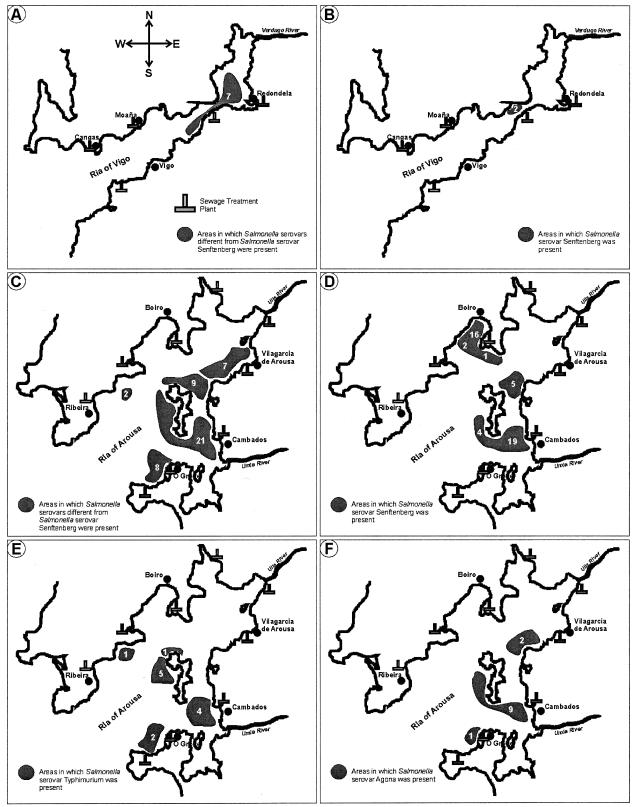
The incidence of Salmonella in the different rias was 2.1% in Vigo, 2.8% in Pontevedra, 2.8% in Arousa, and 0.7% in other coastal zones (Table 1). Isolation of Salmonella was significantly higher (P < 0.05) in the Ria of Arousa than in other rias. Figure 2 shows the geographic distribution of the isolates of the different serovars of Salmonella during the study period in the two most important rias (Arousa and Vigo). In the Ria of Arousa, Salmonella serovar Senftenberg was found in three zones (Fig. 2D) and had the greatest number of isolates in the vicinity of the coast. The majority of the isolates (n = 35) were detected at two coastal sites (Cambados and Boiro). In the Ria of Vigo, only two isolates of Salmonella serovar Senftenberg were found, and both were from the same site (Fig. 2B). Serovars other than Salmonella serovar Senftenberg were predominantly distributed along the south shores of these two rias (Arousa and Vigo) and usually at sites close to the mouths of the rivers (Fig. 2A and C). A different distribution pattern was observed for serovars Typhimurium and Agona in the Ria of Arousa (Fig. 2E and F). Salmonella serovar Agona, which was isolated exclusively in the Ria of Arousa, was found at three sites on the south shore, with eight isolates being collected in a single day. The distribution of Salmonella serovar Typhimurium was more widely dispersed than that of serovars Senftenberg and Agona. Cambados, on the south side of the Ria of Arousa, was the site with the greatest number and diversity of isolates.
FIG. 2.
Distributions of the different Salmonella serovars in the rias of Arousa and Vigo during the study period (1999 to 2002). (A) Distribution of the isolates of non-Senftenberg Salmonella serovars in the Ria of Vigo; (B) distribution of the serovar Senftenberg in the Ria of Vigo; (C) distribution of the non-Senftenberg Salmonella serovars in the Ria of Arousa; (D) distribution of the serovar Senftenberg in the Ria of Arousa; (E) distribution of the serovar Typhimurium in the Ria of Arousa; (F) distribution of the serovar Agona in the Ria of Arousa. The numbers in the shaded areas indicate the numbers of isolates in this area.
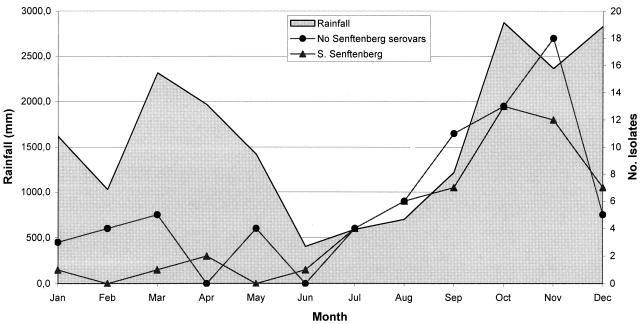
The isolation of Salmonella was seasonal, with higher detection frequencies in the summer (June to September; 30% of isolates) and fall (October to December; 53% of isolates) than in the winter and spring (Fig. 3). This pattern was applicable to all serovars except Salmonella serovar Typhimurium, which had a significantly higher incidence during the summer. The incidence of Salmonella serovar Senftenberg was lowest in February and May, when no isolates were detected (Fig. 3), while other serovars were detected least frequently in June, coinciding with the least rainfall (average of 403 mm/month). The maximum number of isolates was found in the months of October and November, both for Salmonella serovar Senftenberg (13 and 12 isolates, respectively) and for the rest of the serovars (13 and 18 isolates, respectively), coinciding with the months of maximum precipitation (averages of 2,875 and 2,365 mm/month, respectively). River flow did not coincide with rainfall, as maximum river flow was in January (monthly average, 865 m3/s) and the minimum was in September (monthly average, 65 m3/s).
FIG. 3.
Relationship between the monthly number of Salmonella serovar Senftenberg isolates and the number of isolates of the rest of the serovars, grouped with respect to the monthly precipitation average during the 4-year period of the study.
The study of the relationship and the daily values for the environmental parameters showed that rainfall presented a positive significant correlation (P < 0.01) with wind (Pearson correlation coefficient, 0.291), especially with southeast and southwest winds (0.475 and 0.322), and with river flow (0.369). Rainfall showed a negative significant correlation with the northeast winds (−0.335), northwest winds (0.254), calm winds (−0.161), hours of sunshine (−0.371), and temperature (−0.395), which are typical of stable weather. Isolates of serovars Senftenberg and Typhimurium were not correlated with any of the environmental parameters studied (Table 3). Serovar Agona isolates showed a significant correlation with rainfall of 0.317 (P < 0.05). The presence of isolates of the other serovars combined correlated significantly with southwest winds (0.633) and with rainfall (0.328) but showed a significant negative correlation with calm winds (−0.533) and with hours of sunshine (−0.323). Simple logistic regression analysis between the presence of different serovars of Salmonella and the various environmental parameters on the day of sample collection showed significant relationships (P < 0.05) between season and Salmonella serovar Senftenberg prevalence; wind and serovar Agona prevalence; river flow, season, and serovar Typhimurium prevalence; and southeast winds, calm winds, season, and the prevalence of remaining serovars. When multiple logistic regression models were constructed with the significant variables, most variables lost their significance, with only season remaining as the predominant factor related to the presence of the different isolates (Table 4). Only the prevalence of Salmonella serovar Senftenberg showed a significant relationship with temperature interacting with season. For all the isolates except serovar Senftenberg, there were significant relationships between their presence and wind, the interaction of wind with season, calm interacting with season, temperature, and the interaction of temperature with season. All the relationships were positive, except that of calm winds, which negatively influenced isolation frequency. When Salmonella isolation data were compared with environmental data for the days prior to sample collection (Table 4), data for the day before sample collection correlated best with overall Salmonella isolation frequency. However, excluding values for serovar Senftenberg, the sum of the values for the environmental parameters on the 3 days before the samples were collected was slightly higher. Data for the day before sample collection indicated significant relationships between the presence of Salmonella serovar Senftenberg and temperature, temperature interacting with season, and hours of sunshine interacting with season. Excluding values for serovar Senftenberg, there were significant relationships between the presence of isolates and calm winds, temperature, temperature interacting with season, rainfall interacting with season, and river flow interacting with season.
TABLE 3.
Pearson correlation coefficient values for the different serovars and the environmental parameters considered in this study
| Environmental parameter | Pearson correlation coefficient for indicated serovar(s)a
|
||||
|---|---|---|---|---|---|
| Senftenberg | Non-Senftenberg | Agona | Typhimurium | Others | |
| Northeast wind | −0.021 | 0.077 | −0.095 | 0.256 | 0.048 |
| Southeast wind | 0.190 | 0.262 | 0.166 | −0.073 | 0.255 |
| Southwest wind | 0.281 | 0.438** | −0.005 | −0.097 | 0.633** |
| Northwest wind | −0.228 | −0.100 | −0.095 | −0.090 | −0.015 |
| Calm wind | −0.145 | −0.391** | 0.002 | 0.025 | −0.533** |
| Wind (km/day) | 0.081 | 0.331** | 0.018 | −0.177 | 0.512** |
| Rainfall | 0.247 | 0.350** | 0.317* | −0.219 | 0.328* |
| River flow | −0.156 | 0.035 | 0.191 | −0.240 | 0.016 |
| Temp | 0.084 | 0.009 | −0.100 | 0.142 | 0.018 |
| No. of h of sunshine | −0.213 | −0.313** | −0.193 | 0.112 | −0.323** |
*, significant correlation (P < 0.05); **, significant correlation (P < 0.01).
TABLE 4.
Multiple logistic regression analysis describing the relationship between the different environmental parameters and the different Salmonella serovars
| Environmental parameter(s) |
P value (model P, <0.001) for environmental samples collected on indicated day(s)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Salmonella serovar Senftenberg isolates
|
Salmonella non-Senftenberg serovar isolates
|
|||||||||||
| 0 | 1 | 2 | 3 | Σ1,2 | Σ1,2,3 | 0 | 1 | 2 | 3 | Σ1,2 | Σ1,2,3 | |
| Intercept | <0.001** | 0.002** | <0.001** | 0.002** | <0.001** | <0.001** | 0.016* | 0.133 | 0.334 | 0.165 | 0.643 | 0.130 |
| Wind | 0.024* | 0.155 | 0.042* | |||||||||
| Wind and season | 0.042* | 0.532 | 0.279 | |||||||||
| Southeastern wind | 0.603 | |||||||||||
| Southeastern wind and season | 0.085 | |||||||||||
| Southwestern wind | 0.723 | |||||||||||
| Southwestern wind and season | 0.311 | |||||||||||
| Northwestern wind | 0.693 | 0.816 | ||||||||||
| Northwestern wind and season | 0.775 | 0.339 | ||||||||||
| Calm wind | 0.876 | 0.014* | 0.004** | 0.068 | 0.031* | |||||||
| Calm wind and season | 0.039* | 0.252 | 0.615 | 0.731 | 0.691 | |||||||
| No. of h of sunshine | 0.054 | 0.795 | 0.674 | 0.100 | 0.036* | 0.100 | 0.339 | 0.003** | ||||
| No. of h of sunshine and season | 0.018* | 0.062 | 0.061 | 0.080 | 0.557 | 0.956 | 0.026* | 0.792 | ||||
| Temp | 0.662 | 0.019* | 0.114 | 0.045* | 0.120 | 0.113 | 0.034* | 0.040* | 0.011* | 0.017* | 0.004** | 0.015* |
| Temp and season | <0.001** | 0.002** | <0.001** | <0.001** | <0.001** | <0.001** | 0.001** | 0.001** | <0.001** | 0.015* | <0.001** | 0.029* |
| Rainfall | 0.715 | |||||||||||
| Rainfall and season | 0.020* | |||||||||||
| River flow | 0.778 | 0.656 | 0.154 | 0.982 | 0.048* | |||||||
| River flow and season | 0.034* | 0.045* | 0.102 | 0.051 | 0.255 | |||||||
| Season | 0.748 | 0.697 | 0.242 | 0.909 | 0.279 | 0.299 | 0.956 | 0.419 | 0.371 | 0.062 | 0.641 | 0.076 |
| R2 of Nagelkerke | 0.138 | 0.192 | 0.121 | 0.136 | 0.149 | 0.148 | 0.146 | 0.146 | 0.118 | 0.129 | 0.146 | 0.151 |
Significance values show dependence between environmental parameters and Salmonella isolates. *, P < 0.05; **, P < 0.01. Environmental dates considered were the same day that the sample was collected (0), 1 the day before the sample was collected (1), 2 two days before the sample was collected (2), and 3 three days before the sample was collected. Σ1,2 is the sum of values from the 2 days before sample collection; Σ1,2,3 is the sum of values from the 3 days before sample collection. Salmonella non-Senftenberg serovar isolates are all the serovar isolates different from serovar Senftenberg isolates.
DISCUSSION
The results of this study indicate minor year-to-year variation in the prevalence of Salmonella in the rias, with an overall incidence of 2.7%. Salmonellae have been reported to be considerably more prevalent on the coasts of Portugal and the northeast coast of Spain, where values for the incidence of salmonellae in seawater of 41%, 72% (3), and 26% (16) were reported.
The Salmonella diversity in the present study as reflected in the identification of 20 serovars among the 127 strains of Salmonella is typical of what was found in previous studies. In a study by Wilson and Moore (27), 17 different serovars were identified among the 37 strains isolated, while a study in Portugal found 17 different serovars among 45 Salmonella isolates (3). In a study of coastal waters in Japan, 18 different serovars were identified among 251 strains (26). The serovars in the present study were notably different than those most frequently identified in clinical, animal, or food samples in Spain. According to the data from the Spanish Boletín Epidemiológico Semanal, between 1996 and 1999 the five Salmonella serovars most frequently isolated from clinical samples were Enteritidis, Typhimurium, Hadar, Virchow, and Brandenburg (18, 22, 23, 24). Isolates from animal samples were more varied, and the predominant serovars were Typhimurium, Enteritidis, and Abortusovis (19, 20, 21, 25). In environmental samples, serovars Enteritidis, Typhimurium, and Hadar were identified most often (19, 20, 21, 25). These frequency patterns of Salmonella serovar isolates differ notably from the data obtained in the present study, where serovars Senftenberg, Typhimurium, and Agona made up 67% of the isolates. The discrepancy between serovars originating from clinical or animal sources and those from the marine environment has been observed in previous studies of the presence of salmonellae in coastal water (1, 3, 28) and seafood products (9, 27). Assuming that the main source of Salmonella contamination in the marine environment is of human or animal origin, the different population structures may be attributed to the different rates of growth and survival of these serovars in the marine environment. Salmonella serovar Typhimurium is the clinically important serovar found most often in this study and in most of the previous studies of Salmonella contamination in marine environments (1, 3, 16, 27), which attests to its capacity of adaptation and survival in this environment, as has been suggested by other authors (1, 4). This resistance of Salmonella serovar Typhimurium has also been demonstrated in experimental studies (5). Most Salmonella survival studies have been conducted under controlled and stable conditions, and little is known about the survival of most Salmonella serovars in constantly changing and unpredictable marine ecosystems.
Salmonella serovar Senftenberg was detected most frequently in this study (42% of the isolates); serovar Typhimurium was a distant second (15% of the isolates). Recent studies indicate that the presence of serovar Senftenberg is increasing in marine environments. It was one of the predominant serovars in the coastal waters of Portugal (3), in crustaceans from India (8), in seafood imported into the United States especially from tropical countries (9), and in environmental samples in France and Brazil (1, 17). The Spanish Boletín Epidemiológico Semanal (20, 21) reports that the number of Salmonella serovar Senftenberg isolates from food and animal feed increased from 23 in 1997 to 89 in 1998, and during that period, serovar Senftenberg went from being the seventh to the third most frequently isolated serovar. In Galicia, this serovar was detected for the first time in the rias of Galicia in 1998 (13) and was associated simultaneously with molluscs and mussel processing plants (12). In fact, Salmonella serovar Senftenberg is the only serovar that has been detected in Galician mussel processing plants during the last 5 years and has persisted in all the factories studied. The sites of Boiro and Cambados on the Ria of Arousa are near mussel processing plants and account for 65% of the serovar Senftenberg isolates. Considering this persistent and localized contamination of serovar Senftenberg and the lack of correlation with any environmental parameter, there appears to be a strong link to mollusc processing and its prevalence in the marine environment. This relationship is further supported by the seasonality of the Salmonella serovar Senftenberg isolates in the marine environment and of mussel processing activity. Due to the variations in yields of mussel meat, processing begins in June and extends to the end of the year. The rest of the year the factories are closed or have little activity. The isolates of Salmonella serovar Senftenberg during this study have shown clear seasonality, with 90% of the isolates being detected between July and December. Our laboratory is currently examining isolates from both sources using molecular subtyping methods to support this hypothesis.
Detection of Salmonella serovar Typhimurium did not correlate with any of the environmental parameters studied, and in contrast with other serotypes, it was isolated most frequently in the summer. The distribution of this serovar in the Ria of Arousa also differed from those of other serovars in that it was localized to specific sites with heavy tourism during the summer (O Grove, Cambados, and Arousa Island) (Fig. 2E). Salmonella serovar Agona was detected only in the Ria of Arousa, and almost all the isolates appeared to be associated with a single contamination event in November 2000 that affected the whole Cambados area. The remaining serovars were distributed on the south shores of the rias around the mouths of the rivers. These isolates correlated with rainfall, wind, and season, and their prevalence appeared to be associated with severe storms that frequently bring high southerly winds and heavy rainfall into Galicia during the fall season. This seasonal pattern in Salmonella prevalence has been observed in previous studies where a relationship was detected with storm-generated flows, torrential rains, or the monsoon season (1, 6, 8, 11, 14, 26). The western coast of Spain has a stable and short summer (July and August) with low rainfall, followed by an abrupt change in the autumn (September, October, and November) due to frequent cyclonic storms from the Atlantic Ocean characterized by strong winds and rains. Westerly or southerly winds predominate during autumn and winter and pile surface water up against the coast, reducing the estuarine circulation (15) and retaining storm-generated flows and the possible contaminants mobilized by them. The combination of rainfall and strong winds not only introduces contamination but also provokes stratification, with the freshwater staying in the surface layer as it is lighter than the seawater. This phenomenon also may explain the significant difference in the incidences of Salmonella in the marine bottom and in the water column found in this study.
Interpretation of data from the studies of microbial contamination of coastal water varies with the type of samples analyzed. Seawater samples provide a snapshot, whereas the bivalve mollusc analysis provides information over a longer period of time. The use of bivalves provides data on the situation that existed on the days before sample collection. Nevertheless, it is not possible to determine the exact day of the contamination and establish a direct relationship with its origin. This became apparent in the statistical analysis that was applied to determine the relationship between the environmental parameters and the Salmonella contamination. When the coefficient of correlation, which compares the monthly average of the environmental factors and the monthly number of Salmonella isolates, was used, the relationships between the isolates were clear and direct. Nevertheless, when multiple logistic regression analysis was utilized, using the environmental data from the same day as the sample collection, such a clear relationship could not be observed. Thus, it was not possible to determine the exact day the contamination took place because the molluscs analyzed could have been contaminated on various days before. The relationship of the environmental conditions with the number of days before sample collection was investigated. The data from the days before the sample collection best fitted the model, and in some cases the summary of the data of various days before the collection day better explained the variance in isolation frequency. This result indicates not only that certain environmental conditions need to exist but also that they need to persist for several days. The results obtained utilizing the resulting models of the multiple logistic regression analysis do not completely justify the tendencies obtained by the use of the correlation coefficients, suggesting the possibility that the relationships between the environmental parameters and the presence of Salmonella isolates are not linear and that there may exist more-complicated relationships of a functional character that describe the relationships between both variables that have been considered in this study. This information would be of considerable value in predicting contamination events and in being able to take a proactive approach in controlling the harvest of molluscan shellfish.
Acknowledgments
We are grateful to A. DePaola for careful review of the manuscript and helpful comments. We also thank Xosé Luis Otero Cepeda, Teresa Seoane Pillado, and Carmen María Cadarso Suárez (Departamento de Estadística e Investigación Operativa, Universidad de Santiago de Compostela) for assistance in statistical analysis of data, the staff of the Unidad de Control de Moluscos Laboratory (Beatriz Castromán, Javier Pena, Begoña Piñeiro, Irene Vazquez, Alfonso Naveiro, Susana Vazquez, and Luis Treviño) for technical assistance, and Victoria Carrera for her help in the elaboration of the manuscript.
REFERENCES
- 1.Baudart, J., K. Lemarchand, A. Brisabois, and P. Lebaron. 2000. Diversity of Salmonella strains isolated from the aquatic environment as determined by serotyping and amplification of the ribosomal DNA spacer regions. Appl. Environ. Microbiol. 66:1544-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, C., and A. Kyriakides. 2002. Salmonella: a practical approach to the organism and its control in foods. Blackwell Science Ltd., Oxford, United Kingdom.
- 3.Catalao Dionisio, L. P., M. Joao, V. Soares Ferreiro, M. Leonor Fidalgo, M. E. García Rosado, and J. J. Borrego. 2000. Ocurrence of Salmonella spp in estuarine and coastal waters of Portugal. Antonie Leeuwenhoek 78:99-106. [DOI] [PubMed] [Google Scholar]
- 4.Dupray, E., and A. Derrien. 1995. Influence of the previous stay of Escherichia coli and Salmonella spp. in waste water on their survival in seawater. Water Res. 29:1005-1011. [Google Scholar]
- 5.Galdiero, E., G. Donnarumma, L. de Martino, A. Marcatili, G. Cipollaro de l'Ero, and A. Merone. 1994. Effect of low-nutrient seawater on morphology, chemical composition, and virulence of Salmonella typhimurium. Arch. Microbiol. 162:41-47. [DOI] [PubMed] [Google Scholar]
- 6.Goyal, S. M., C. P. Gerba, and J. L. Melnick. 1977. Occurrence and distribution of bacterial indicators and pathogens in canal communities along the Texas coast. Appl. Environ. Microbiol. 34:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackney, C. R., and M. E. Potter. 1994. Animal-associated and terrestrial bacteria pathogens, p. 172-209. In C. R. Hackney and M. D. Pierson (ed.), Environmental indicators and shellfish safety. Chapman & Hall, New York, N.Y.
- 8.Hatha, A. A. M., and P. Lakshmanaperumalsamy. 1997. Prevalence of Salmonella in fish and crustaceans from markets in Coimbatore, South India. Food Microbiol. 14:111-116. [Google Scholar]
- 9.Heinitz, M. L., R. D. Ruble, D. E. Wagner, and S. R. Tatini. 2000. Incidence of Salmonella in fish and seafood. J. Food Prot. 63:579-592. [DOI] [PubMed] [Google Scholar]
- 10.International Organization for Standardization. 1993. Microbiology: general guidance on methods for the detection of Salmonella, 3rd ed. International Standard ISO 6579. International Organization for Standardization, Geneva, Switzerland.
- 11.Kaper, J. B., G. S. Sayler, M. M. Baldini, and R. R. Colwell. 1977. Ambient-temperature primary nonselective enrichment for isolation of Salmonella spp. from an estuarine environment. Appl. Environ. Microbiol. 33:829-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Urtaza, J., J. Peiteado, A. Lozano-Leon, and O. García-Martín. 2004. Detection of Salmonella Senftenberg associated with high saline environments in mussel processing facilities. J. Food Prot. 256-263 67:. [DOI] [PubMed]
- 13.Martínez-Urtaza, J., M. Saco, G. Hernández-Córdova, A. Lozano, O. García-Martín, and J. Espinosa. 2003. Identification of Salmonella serovars isolated from live molluscan shellfish and their significance in the marine environment. J. Food Prot. 66:226-232. [DOI] [PubMed] [Google Scholar]
- 14.O'Shea, M. L., and R. Field. 1991. Detection and disinfection of pathogens in storm-generated flows. Can. J. Microbiol. 38:267-276. [DOI] [PubMed] [Google Scholar]
- 15.OSPAR Commission. 2000. Quality status report 2000: region IV—Bay of Biscay and Iberian coast. OSPAR Commission, London, United Kingdom.
- 16.Polo, F., M. J. Figueras, I. Inza, J. Sala, J. M. Fleisher, and J. Guarro. 1999. Prevalence of Salmonella serotypes in enviromental waters and their relationships with indicator organisms. Antonie Leeuwenhoek 75:285-292. [DOI] [PubMed] [Google Scholar]
- 17.Tavechio, A. T., A. C. Ghilardi, J. T. Peresi, T. O. Fuzihara, E. K. Yonamine, M. Jakabi, and S. A. Fernandes. 2002. Salmonella serotypes isolated from nonhuman sources in Sao Paulo, Brazil, from 1996 through 2000. J. Food Prot. 65:1041-1044. [DOI] [PubMed] [Google Scholar]
- 18.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, and A. Echeita. 1997. Análisis de las cepas de Salmonella sp aisladas de muestras clínicas de origen humano en España. Año 1996. Bol. Epidemiol. Semanal 5:21-23. [Google Scholar]
- 19.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, P. Cerdán, R. Gutiérrez, and A. Echeita. 2000. Análisis de las cepas de Salmonella spp aisladas de muestras de origen no humano en España en el año 1999. Bol. Epidemiol. Semanal 8:133-140. [Google Scholar]
- 20.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, R. Gutiérrez, P. Cerdán, and A. Echeita. 1998. Análisis de las cepas de Salmonella sp aisladas de muestras de origen no humano en España en el año 1997. Bol. Epidemiol. Semanal 6:137-143. [Google Scholar]
- 21.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, R. Gutiérrez, P. Cerdán, and A. Echeita. 1999. Análisis de las cepas de Salmonella sp aisladas de muestras de origen no humano en España en el año 1998. Bol. Epidemiol. Semanal 7:93-98. [Google Scholar]
- 22.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, R. Gutiérrez, P. Cerdán, and A. Echeita. 1998. Análisis de las cepas de Salmonella sp aisladas de muestras clínicas de origen humano en España en el año 1997. Bol. Epidemiol. Semanal 6:129-132. [Google Scholar]
- 23.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, R. Gutiérrez, P. Cerdán, and A. Echeita. 1999. Análisis de las cepas de Salmonella spp aisladas de muestras clínicas de origen humano en España en el año 1998. Bol. Epidemiol. Semanal 7:45-48. [Google Scholar]
- 24.Usera, M. A., A. Aladueña, R. Díez, M. de la Fuente, R. Gutiérrez, P. Cerdán, and A. Echeita. 2000. Análisis de las cepas de Salmonella spp aisladas demuestras clínicas de origen humano en España en el año 1999. Bol. Epidemiol. Semanal 8:45-48. [Google Scholar]
- 25.Usera, M. A., A. Aladueña, R. Díez, P. Cerdán, R. Gutiérrez, and A. Echeita. 1997. Análisis de los serotipos de Salmonella sp aisladas de muestras no humanas en 1996 en España. Bol. Epidemiol. Semanal 5:69-76. [Google Scholar]
- 26.Venkateswaran, K., T. Takai, I. M. Navarro, H. Nakano, H. Hashimoto, and R. J. Siebeling. 1989. Ecology of Vibrio cholerae non-O1 and Salmonella spp. and role of zooplankton in their seasonal distribution in Fukuyama coastal waters, Japan. Appl. Environ. Microbiol. 55:1591-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willson, I. G., and J. E. Moore. 1996. Presence of Salmonella spp. and Campylobacter spp. in shellfish. Epidemiol. Infect. 116:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yam, W. C., C. Y. Chan, S. W. Ho Bella, T. Y. Tam, C. Kueh, and T. Lee. 2000. Abundance of clinical enteric bacterial pathogens in coastal waters and shellfish. Water Res. 34:51-56. [Google Scholar]