Abstract
High-density lipoprotein (HDL) levels are inversely associated with coronary heart disease due to HDL's ability to transport excess cholesterol in arterial macrophages to the liver for excretion [i.e., reverse cholesterol transport (RCT)]. However, recent advances highlight additional atheroprotective roles for HDL beyond bulk cholesterol removal from cells through RCT. By promoting cellular free cholesterol (FC) efflux, HDL and its apolipoproteins (apoA-I and apoE) decrease plasma membrane FC and lipid raft content in immune and hematopoietic stem cells, decreasing inflammatory and cell proliferation signaling pathways. HDL and apoA-I also dampen inflammatory signaling pathways independent of cellular FC efflux. In addition, HDL lipid and protein cargo provide protection against parasitic and bacterial infection, endothelial damage, and oxidant toxicity. Here, current knowledge is reviewed regarding the role of HDL and its apolipoproteins in regulating cellular cholesterol homeostasis, highlighting recent advances on novel functions and mechanisms by which HDLs regulate inflammation and hematopoiesis.
Keywords: high-density lipoprotein, ABC transporter, hematopoietic stem cell, cholesterol, lipid raft
INTRODUCTION
High-density lipoproteins (HDLs) are the smallest and most dense plasma lipoproteins, containing a core of nonpolar polar lipid consisting of cholesteryl ester (CE) and triglyceride, surrounded by a surface monolayer of phospholipid (PL), free cholesterol (FC), and apolipoproteins (29). Much work has been focused on understanding the metabolism and function of HDL because of the well-documented inverse association between plasma HDL cholesterol concentrations and risk of developing atherosclerotic cardiovascular disease (37). This association is primarily thought to be due to HDL's ability to mediate reverse cholesterol transport (RCT), a process in which cholesterol from peripheral tissues, especially from macrophages in aortic plaques, is transported to the liver for elimination via biliary secretion and elimination in the feces (32). Growing insight into new roles for HDL in controlling inflammation and hematopoiesis is rapidly changing the perception that HDL particles are mere carriers of excess cholesterol in RCT. A molecular understanding of the pleiotropic functions of HDL is just beginning to unfold.
HDL contains a more complex proteome than previously appreciated. The most abundant apolipoprotein in HDL is apoA-I, which comprises 60% to 70% of the total HDL protein mass. In addition, as revealed by a recent study (109), HDL particles not only contain classical apolipoproteins, such as apoA-I, apoAII, apoE, and apoCs that participate in lipid metabolism, but also minor atypical apolipoproteins such as apoL1 and apoM that function in host defense and transport bioactive lipids, respectively. HDL also contains proteins associated with the acute phase response and complement regulation. In addition to its diverse protein cargo and its usual surface (PL, FC) and core (CE, TG) lipids, HDL also contains minor lipids, such as ceramide, sphingolipid/sphingomyelin species (116), and sphingosine-1-phosphate (S1P) (60) that function as lipid mediators in signaling pathways and in the regulation of innate immunity. Of note, although some HDL functions have been mapped to particular constituents of the particle, other functions are less well understood and require additional investigation. Recent emerging data suggest a novel and important role for apoA-I and HDL in the regulation of T lymphocyte proliferation and hematopoietic stem cell proliferation. This review focuses on recent data regarding HDL in these emerging areas of interest.
HDL AND RCT
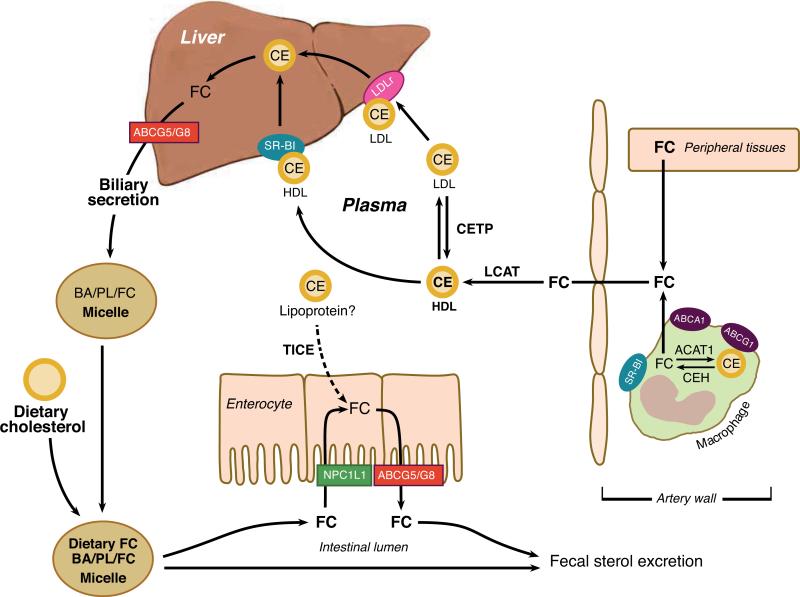
The major cardiovascular-protective role for HDL has been attributed to RCT (47). Because cholesterol cannot be degraded in the body and is water insoluble, excess cholesterol in peripheral tissues must be transported by lipoprotein particles to the liver for excretion. The initial step of RCT involves efflux of cellular FC and PL to lipid-free or lipid-poor apoA-I or to spherical HDL particles (Figure 1). Two main mechanisms are involved in cholesterol efflux. The first is passive diffusional efflux of FC from the plasma membrane through the aqueous medium to the HDL particles (121). This process is driven by the chemical gradient of FC between the plasma membrane and the acceptor HDL. The second efflux mechanism is a transporter-mediated active lipid efflux involving members of the ATP-binding cassette (ABC) transporter family, ABCA1 and ABCG1. Cholesterol efflux to lipid-free or lipid-poor apoA-I is mediated by ABCA1, resulting in the formation of nascent, discoidal particles of HDL about 7–12 nm in size, composed of a bilayer of PL and FC with apoA-I surrounding the perimeter (4, 73). Unlike aqueous diffusion of FC, ABCA1 hydrolyzes ATP to provide energy for FC efflux. Mutations that inactivate the human ABCA1 gene result in Tangier disease, a rare disorder characterized by extremely low (<5% of normal) plasma HDL cholesterol concentrations and accumulation of cholesterol in macrophages (11, 12, 19, 93), highlighting the essential role of this transporter in HDL formation and macrophage RCT. FC in nascent HDLs is esterified to fatty acid by the plasma enzyme lecithin:cholesterol acyltransferase (LCAT), generating CE that is sparing (3 mol%) soluble in the nascent HDL surface PL. Thus, LCAT-generated CE forms a separate core phase in the nascent HDL, converting it to a spherical HDL particle typical of that in plasma. The esterification of FC by LCAT decreases FC concentration in HDL, producing a cholesterol chemical gradient between the HDL particle surface and the cell surface membrane, which promotes passive aqueous diffusion of cellular FC.
Figure 1.
Reverse cholesterol transport (RCT). Excess cellular free cholesterol (FC) can be esterified by acyl CoA:cholesterol acyltransferase (ACAT), forming a cholesteryl ester (CE) cytosolic lipid droplet. CE can be mobilized into FC by cholesterol ester hydrolase (CEH) for transport to the plasma membrane. Excess FC in peripheral tissues, including arterial macrophages, is transported out of cells to high-density lipoproteins (nascent HDL and plasma HDL) via passive diffusion or by a transporter-mediated active lipid efflux involving ABCA1, ABCG1, and/or SR-BI. LCAT converts FC to CE in HDL particles. HDL FC and CE are taken up in the liver by the hepatic scavenger receptor (SR-BI) or transferred to apoB-containing lipoproteins by cholesterol ester transfer protein (CETP) with subsequent uptake by hepatic low-density lipoprotein (LDL) receptors (LDLr). Hepatic FC can be directly secreted into the bile via ABCG5/G8 or converted to bile acids (BA) before secretion into bile. Cholesterol in secreted bile mixes with dietary cholesterol and is available for absorption into intestinal enterocytes by Niemann-Pick C1-like 1 (NPC1L1) protein. Absorbed cholesterol can be transported back into the intestinal lumen by ABCG5/G8. A poorly defined trans-intestinal transport and excretion (TICE) pathway bypasses the liver and delivers excess plasma cholesterol by an unknown lipoprotein into the intestinal lumen. The final step in RCT is the ultimate excretion of cholesterol in the feces. The net result of these RCT pathways is decreased accumulation of excess cellular cholesterol.
Another active transporter, ABCG1, plays a critical role in transporting FC from cells to mature HDL particles (46, 114). Uptake of HDL cholesterol (FC and CE) by the liver is mediated directly by hepatic scavenger receptor BI (SR-BI) (130) or indirectly by hepatic low-density lipoprotein receptors (LDLr) after CEs are transferred from HDL to apoB-containing lipoproteins, very-low-density lipoprotein (VLDL), and LDL by cholesterol ester transfer protein (CETP) in humans (mice do not express CETP) (Figure 1).
Although SR-BI accelerates FC efflux to mature HDL in vitro (122), in vivo studies of RCT indicate that ABCA1 and ABCG1, but not SR-BI, promote macrophage RCT (115). Thus, the three major players in macrophage RCT have distinct functions. ABCA1 is mainly responsible for initial efflux of FC to lipid-free apoA-I, forming nascent HDL. ABCG1 then mediates additional FC efflux from cells to nascent and spherical HDL particles. SR-BI is responsible for hepatic removal of cholesterol from plasma HDL. Once taken in by the liver, the excess cholesterol can be directly secreted into bile or converted to bile acids before secretion into bile for ultimate excretion into the feces (Figure 1).
Another recently described pathway for RCT bypasses the liver and delivers the excess cholesterol to the intestine for trans-intestinal transport and excretion into feces (111) (Figure 1). The net result of these RCT pathways is to decrease the accumulation of excess cellular cholesterol. Although RCT has been mainly associated with removal of cholesterol from atherosclerotic foam cells, it functions normally to provide orderly processing of cholesterol from apoptotic and necrotic cells. More recently, a broader role for HDL has been implicated in controlling plasma membrane cholesterol that is related to inflammation and cellular proliferation, which is discussed in the following sections.
HOMEOSTATIC REGULATION OF INTRACELLULAR CELLULAR STEROL CONTENT AND INFLAMMATION
Regulation of Intracellular Cholesterol Levels by Liver X Receptors and Sterol Response Element-Binding Proteins
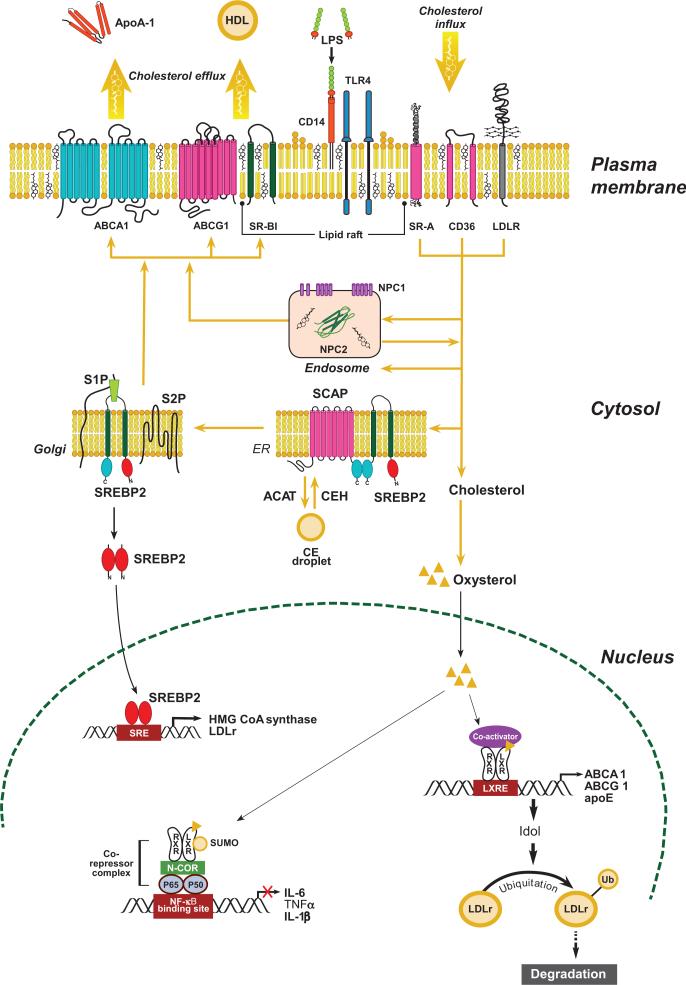
Cellular cholesterol content is normally tightly regulated, reflecting a balance between cholesterol uptake, efflux, endogenous synthesis, and storage (Figure 2). Liver X receptors (LXRs) and sterol response element-binding proteins (SREBPs) are master transcription factors that regulate cellular cholesterol content.
Figure 2.
Regulation of intracellular cholesterol homeostasis. Cellular cholesterol content is normally tightly regulated by balancing uptake and endogenous synthesis with efflux and storage. Cholesterol in lipoproteins is internalized via scavenger receptors (CD36, SR-A) or low-density lipoprotein receptors (LDLr) and delivered to endosomes where cholesteryl ester (CE) is hydrolyzed to free cholesterol (FC). Niemann-Pick C1 (NPC1) together with NPC2 regulates FC movement out of the endosomes for delivery to the endoplasmic reticulum (ER) or the plasma membrane. Excess FC in the ER can be esterified to CE by acyl CoA:cholesterol acyltransferase (ACAT), forming a cytosolic lipid droplet. CE can be mobilized from the lipid droplet by cholesterol ester hydrolase (CEH) for transport to the plasma membrane. When cellular cholesterol level is low, sterol regulatory element-binding protein 2 (SREBP2) is escorted by SREBP cleavage-activating protein (SCAP) from the ER to the Golgi, where it undergoes cleavage by site 1 and site 2 proteases (S1P and S2P). The proteolytic processing of SREBP2 releases the N-terminus, which is a basic helix-loop-helix leucine zipper transcription factor that travels to the nucleus to activate transcription of genes involved in cholesterol biosynthesis and cholesterol uptake (i.e., LDLr). A minor amount of FC can be oxidized into oxysterol, an activating ligand for liver X receptors (LXRs), resulting in increased transcription of cholesterol efflux genes (i.e., ABCA1, ABCG1, and apoE) and Idol (inducible degrader of LDLr), which mediates degradation of the LDLr. FC transported to the plasma membrane can be effluxed to lipid-free apolipoproteins (i.e., apoA-I and apoE) by ABCA1 or to high-density lipoprotein (HDL) particles by passive diffusion or transporters (ABCG1 or SR-BI). Modified from (31).
LXRα and LXRβ are members of the nuclear receptor family of transcription factors that regulate pathways of cellular cholesterol efflux (14). Both LXRs activate gene transcription by binding to response elements located in the promoter region of target genes as a heterodimer with the retinoid X receptor. The natural ligands for LXRs are oxysterols as well as intermediates in the cholesterol biosynthetic pathway (42, 52). LXRs regulate gene expression in a tissue-specific manner to enhance whole-body RCT. In response to cholesterol loading, LXR activation in peripheral cells, such as macrophages, induces expression of ABCA1 and ABCG1, phospholipid transfer protein, and apoE (9), enhancing removal of excess cellular cholesterol.
In the liver, activation of LXR up-regulates the expression of CYP7A1, the rate-limiting enzyme in the conversion of FC to bile acids, and ABCG5 and ABCG8 transporters that promote hepatic FC secretion into bile (79, 88), both of which enhance secretion of excess hepatic FC into bile. In the intestine, LXR activation up-regulates ABCG5 and ABCG8, which are located on the basolateral side of the intestinal epithelial cell, enhancing movement of absorbed FC back into the intestinal lumen.
The net effect of the concerted activation of LXR-responsive genes in different tissues is to promote the directional movement of excess cholesterol in peripheral tissues to the liver, bile, and intestinal lumen for excretion in the feces (Figure 1). LXRs also can suppress cholesterol uptake through induction of LDLr degradation via an inducible degrader of the LDLr (Idol)-dependent ubiquitination of the receptor (Figure 2). Idol is an E3 ubiquitin ligase that triggers ubiquitination of the LDLr, resulting in its degradation (129).
Acting as cholesterol sensors, SREBPs are membrane-bound transcription factors that regulate the expression of genes harboring a sterol responsive element (SRE) in their promoter region that are involved in cholesterol and fatty acid biosynthesis (36). SREBPs initially reside in the endoplasmic reticulum. When intracellular cholesterol levels decrease, SREBPs, escorted by SREBP cleavage-activating protein, travel to the Golgi apparatus, where the N-terminus is cleaved by two proteases, S1P and S2P (36). The released N-terminus of SREBPs is a basic helix-loop-helix leucine zipper transcription factor that travels to the nucleus to bind and activate a family of genes involved in lipid biosynthesis (Figure 2). Of the three major SREBP isoforms (SREBP-1a, SREBP-1c, SREBP-2), SREBP2 favors cholesterol biogenesis and its responsive genes, including the rate-limiting enzyme HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase in cholesterol biosynthesis (36).
Coordinate regulation of LXR and SREBPs by oxysterol and cholesterol, respectively, tightly maintains cellular sterol homeostasis. When cellular cholesterol accumulates, SREBP2 remains in the endoplasmic reticulum, the proteolytic activation of SREBP2 stops, and expression of SREBP target genes declines. The net result of these steps is to reduce endogenous cholesterol biosynthesis and expression of the LDLr. An intronic sequence of SREBP2 also encodes a microRNA (miR-33) that binds to the 3′ untranslated region of ABCA1 and ABCG1, down-regulating their mRNA and protein expression (65, 86, 87). Thus, under conditions of cellular cholesterol accumulation, SREBP2 and miR-33 expression are decreased, resulting in increased expression of ABCA1 and ABCG1 to export excess cholesterol. Therefore, SREBP2 and LXR work in concert to reduce excess cellular cholesterol by controlling uptake (i.e., down-regulation of LDLr expression), synthesis, and efflux.
Regulation of Inflammation by LXRs
LXRs have also emerged as important regulators of inflammatory gene expression and innate immunity. LXR activation represses stimulation of Toll-like receptor 4 (TLR4), TNF-α, and interleukin (IL)-1β, reducing expression of classical inflammatory genes (16, 44, 71). LXRs also positively regulate expression of arginase, a gene that may have anti-inflammatory effects through antagonism of nitric oxide signaling (56), suggesting that activation of LXRs may drive the macrophage phenotype switch from proinflammatory M1 macrophages to anti-inflammatory M2 macrophages. Interestingly, activation of TLR3 and TLR4 by bacterial and viral components inhibits LXR signaling via the transcription factor IRF3 (17). In addition, activation of LXRs, especially LXRα, protects mice from bacterial infection (i.e., Listeria monocytogenes, Escherichia coli, and Salmonella typhimurium) due to induced expression of macrophage antiapoptotic genes, including AIM/Spα (43, 110).
The inhibition of proinflammatory gene expression by LXRs is mediated through a mechanism termed transrepression. Nuclear factor-κB (NF-κB) and activator protein (AP)-1 are key inflammatory transcription factors, and binding sites for these proteins are present on promoters of many immediate inflammatory response genes. On inactive promoters, factors such as NF-κB are bound to corepressor complexes that contain the nuclear receptor corepressor, maintaining basal repression. Ligand-bound LXRs are conjugated to small ubiquitin-like modifier (SUMO)2/3 through interaction with histone deacetylase-4 E3, and the SUMOylated LXR complex targets NF-κB binding sites in inflammatory promoters and blocks the clearance of the nuclear receptor corepressor complex, repressing inflammatory gene expression (Figure 2) (35).
Compartmental Sterol Accumulation and Inflammation
Emerging evidence suggests that sterol accumulation in different intracellular compartments triggers activation of distinct inflammatory pathways. FC accumulation resulting from deletion of ABCA1 and ABCG1 increases lipid raft content of the plasma membrane, enhancing myeloid differentiation primary response protein 88 (MyD88)-dependent TLR4 activation (see details in the next section) (128, 131). FC accumulation in the endoplasmic reticulum activates stress pathways and induces cytotoxicity, cytokine expression, and apoptosis (23, 30, 54). Excess FC accumulation in late endosomes also can activate the p38 mitogen-activated protein kinase (MAPK) pathways through TLR3 without specific agonist stimulation, indicating that FC overloading alone is sufficient to activate TLR (100). Cholesterol crystals in phagolysosomes activate the caspase-1-activating NLRP3 inflammasome, a cytokine-activating protein complex of the IL-1β family, resulting in cleavage and secretion of the IL-1 family of cytokines (28, 85). In vivo studies revealed that cholesterol crystals deposited in arteries act as an endogenous danger signal, activating the NLRP3 inflammasome and worsening atherosclerosis (28). The mechanism by which crystalized cholesterol activates the inflammasome still remains unclear. Danger signals induced by phagolysosomal membrane disruption or the leakage of cathepsins into the cytoplasm may be involved in inflammasome activation (28, 85). Moreover, a recent report indicates that FC accumulation in macrophages without formation of crystals activates the NLRP3 inflammasome, although the underlying mechanism is unknown (124). Taken together, these data suggest that maintenance of intracellular sterol homeostasis is critical for normal macrophage survival and function, with excess FC accumulation in different cellular compartments activating multiple inflammatory signaling pathways and exaggerating inflammatory responses.
HDL AND APOA-I AS MODULATORS OF CELLULAR MEMBRANE CHOLESTEROL, LIPID RAFTS, AND INFLAMMATION
HDL and Membrane Lipid Rafts
Lipid rafts consist of an ordered membrane microdomain, which is enriched in FC, glycosphingolipid, and glycosylphosphatidylinositol-anchored proteins, and are platforms for signal transduction (31, 81, 98). Mounting evidence suggests an association between the presence of lipid rafts in the plasma membrane and the proinflammatory activation of macrophages by TLR4 agonists, such as lipopolysaccharide (LPS) (45, 72, 106). Indeed, HDL can directly interact with lipid rafts and efflux cholesterol from these microdomains (57, 82). In contrast, lipid efflux to apoA-I does not occur in lipid rafts (57), suggesting that apoA-I may remove cholesterol from other specialized domains of the plasma membrane. Interestingly, the expression of ABCA1, which is likely enriched in nonraft domains (39, 57), results in a significant redistribution of FC and sphingomyelin from lipid rafts to nonrafts through its ATPase-related functions, and apoA-I preferentially associates with nonraft membranes in ABCA1-expressing cells (51). These results suggest that ABCA1 actively disrupts raft microdomains, shifting FC distribution from highly orderly lipid rafts to disordered nonraft regions. FC in nonraft membrane compartments may be more readily available to acceptors, thus facilitating cholesterol-apoA-I interaction and lipid efflux.
HDL, Lipid Rafts, and Inflammation
Because lipid rafts play a critical role in TLR4 signal transduction in macrophages, could HDL, apoA-I, and ABC transporters modulate macrophage inflammation via regulation of lipid rafts in immune cells? Francone et al. (33) reported that LPS-induced septic shock was exacerbated in Abca1–/–/Ldlr–/– mice compared with Ldlr–/– mice, suggesting a potential relationship between ABCA1 function and inflammation. However, macrophages from Abca1–/–/Ldlr–/– mice were enriched 80-fold in CE, and plasma HDL concentrations in these mice were extremely low. Thus, it was not clear whether the exacerbated proinflammatory response to LPS of macrophages from Abca1–/–/Ldlr–/– mice was due to the massive cellular CE accumulation and/or the absence of plasma HDL or to some other alteration of macrophages. Using macrophage-specific ABCA1-knockout (Abca1–M/–M) mice generated using a Cre-loxP strategy, we found that ABCA1-deficient versus wild-type macrophages had >95% less ABCA1 protein, failed to efflux lipid to apolipoprotein A-I, and had a significant increase in FC and membrane lipid rafts, without induction of endoplasmic reticulum stress (131). Abca1-deficient macrophages exhibited enhanced expression of proinflammatory cytokines in response to MyD88-dependent TLR (TLR2, TLR4, TLR7, and TLR9) but not MyD88-independent TLR3 activation (131, 132). In vivo LPS injection confirmed the proinflammatory response in Abca1–M/–M compared with wild-type mice (131). Furthermore, acute cholesterol depletion of macrophages with methyl-β-cyclodextrin normalized FC content between the two genotypes and their response to LPS; cholesterol repletion of macrophages resulted in increased cellular FC accumulation and enhanced cellular response to LPS, indicating that lipid rafts play a central role in this enhanced proinflammation in ABCA1-deficient macrophages (131). Lipid rafts from ABCA1-deficient macrophages contained significantly more FC than did wild-type macrophages, resulting in significantly more TLR4, as well as more of the predominantly intracellular TLR9, distributed in lipid rafts in response to specific agonists (132), which can partially explain the enhanced inflammatory response of ABCA1-deficient macrophages to TLR stimulation.
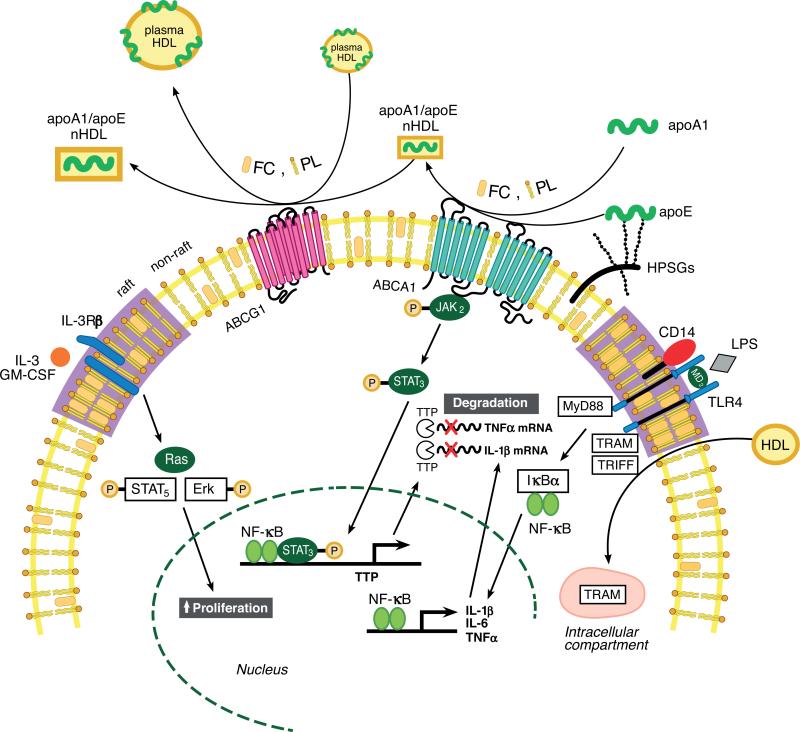
Collectively, these results suggest that the anti-inflammatory activity of ABCA1 is secondary to its ability to modulate the cholesterol content of membrane lipid rafts. Macrophage ABCA1 expression may protect against atherosclerosis by facilitating the net removal of excess lipid from macrophages, reducing lipid rafts on the cell surface, and as a consequence, dampening proinflammatory MyD88-dependent signaling pathways (Figure 3). These anti-inflammatory effects are not specific to ABCA1, as ABCG1, which promotes cholesterol efflux to HDL, also dampens inflammatory responses in mice (Figure 3). When both ABCA1 and ABCG1 are deficient, the greatest proinflammatory response to TLR stimulation is due to increased lipid raft content in macrophages (128). Thus, in normal macrophages, cholesterol efflux to apoA-I via ABCA1 or to HDL via ABCG1 efficiently maintains cellular cholesterol homeostasis and “normal” content of plasma membrane lipid rafts (Figure 3). Failure to efflux cholesterol from the cells due to a defect in ABC transporters disrupts cholesterol homeostasis and consequently leads to hyperinflammation in macrophages.
Figure 3.
Summary of roles of high-density lipoprotein (HDL), apolipoproteins, and ABC transporters in regulating inflammatory response and hematopoietic stem cell proliferation. Lipid-poor apoA-I or cell surface proteoglycan-bound apolipoprotein (apo)E stimulates free cholesterol (FC) and phospholipid (PL) efflux from macrophages or hematopoietic stem cells via ATP-binding cassette transporter (ABC) A1 to form nascent HDL (nHDL) particles. nHDL or plasma HDL can efflux additional FC and PL from the cells via ABCG1, forming FC-enriched nHDL and plasma HDL, thereby reducing cellular cholesterol levels. The reduction in membrane FC content mediated by ABCA1 and ABCG1 efflux results in reduced membrane lipid raft content. This, in turn, reduces inflammatory and proliferative pathways by reducing Toll-like receptor (TLR) 4 receptor and the common beta subunit of the interleukin (IL)-3/granulocyte macrophage colony-stimulating factor (GM-CSF) receptor (IL-3Rβ) in lipid rafts on the macrophage and hematopoietic stem cell surface, respectively. HDL also inhibits lipopolysaccharide (LPS)-stimulated type I interferon response in macrophages by inducing sequestration of TLR4 adapter TRIF-related adapter molecule (TRAM) in an intracellular compartment in a sterol-independent manner. Interaction of apoA-I with ABCA1 activates Janus kinase 2/signal transducer and activator of transcription 3 ( JAK2/STAT3) and increases transcription of tristetraprolin (TTP), resulting in destabilization of microRNAs of proinflammatory genes. Modified from (61, 64).
In addition to murine macrophages, apoA-I and HDL down-regulate lipid raft content in human monocytes and neutrophils and cause a dose-dependent reduction in the activation of blood monocytes and neutrophils (62, 63). The anti-inflammatory effects of apoA-I and HDL are thought to be mediated by ABCA1 and SR-BI (62, 63), respectively, suggesting a broader role for HDL in regulating lipid raft content and immune cell function.
Studies have also shown that apoA-I/ABCA1 interaction activates the Janus kinase 2/signal transducer and activator of transcription 3 ( JAK2/STAT3) pathway, which is anti-inflammatory in macrophages (Figure 3) (102). The interaction of apoA-I and its mimetic peptides with ABCA1-expressing cells rapidly activates JAK2, and this enhances the direct interaction of apoA-I with ABCA1 required for lipid removal from the cells (103). Interestingly, ABCA1 contains two STAT3 docking sites. The apoA-I/ABCA1-mediated activation of JAK2 then activates downstream STAT3, which is independent of the lipid transport function of ABCA1. In another study, apoA-I-mediated activation of JAK2/STAT3 resulted in increased transcription of tristetraprolin (TTP), a zinc finger protein that binds to the adenylate-uridylate-rich elements in the 3′ untranslated region of mRNA of several proinflammatory cytokines, resulting in destabilization of their mRNA and increased turnover (123). As a result, the activation of STAT3 suppressed induction of inflammatory cytokines by LPS. These findings highlight a pathway in which apoA-I interaction with ABCA1 activates an anti-inflammatory pathway that is independent of FC efflux and plasma membrane lipid rafts.
By contrast, a novel pathway was recently described in which apoA-I acts through a MyD88-dependent pathway to couple cholesterol trafficking to inflammation (99). Lipid-free apoA-I, but not HDL, elicited a proinflammatory response (induction of cytokines) in macrophages dependent on MyD88/TLR2/4 expression. Rigorous control experiments (i.e., polymyxin or protease treatment of apoA-I) were conducted to rule out possible LPS contamination in the apoA-I. In vitro FC efflux from MyD88–/– macrophages to apoA-I was defective compared with wild-type macrophages, and in vivo RCT from MyD88–/– versus wild-type macrophages was significantly reduced. These findings identify apoA-I as an endogenous stimulus of innate immunity that couples cholesterol trafficking to inflammation through MyD88.
HDL also suppresses the type I interferon (IFN) response in macrophages challenged with LPS (Figure 3) (101). LPS activation of TLR4 can act through the canonical MyD88 pathway to activate NF-κB and downstream type II IFN response genes (i.e., TNF-α and IL-1β), as discussed above. TLR4 activation can also signal through an alternative pathway that involves the adapter proteins TRIF (Toll/interleukin-1 receptor domain-containing adapter inducing IFN-β) and TRAM (TRIF-related adapter molecule) to activate IFN regulatory factor (IRF3), which increases IFN-β expression and its type I IFN-inducible genes. This pathway appears independent of cellular cholesterol content and expression of ABCA1 and ABCG1 or binding of LPS to HDL. Mechanistically, this pathway is poorly understood but appears to involve the sequestration of the adapter TRAM in an endosomal pool that it is unavailable for cell surface signals through TLR4. In vivo evidence for this pathway was obtained by injecting wild-type and apoA-I–/– mice with gram-negative bacteria and monitoring plasma cytokine response. Although 10-day mortality and plasma levels of TNF-α were similar between the two genotypes, IFN-β concentrations were sixfold higher in apoA-I–/– mice, which had an 80% reduction in plasma HDL cholesterol. These data suggest that HDL also dampens inflammation in a sterol-independent manner.
Of interest, whole-body ABCA1-deficient mice have a severe plasma HDL deficiency (<5% normal) and an abnormal lung phenotype, with foamy macrophage formation (6). In ABCG1-deficient mice, in which plasma HDL concentrations are normal, the abnormal lung phenotype was more profound, with severe pulmonary lipidosis, massive deposition of cholesterol in alveolar macrophages and type 2 cells, and progressive disease with chronic inflammation (5, 46). These results suggest a unique role for ABCG1 in sterol homeostasis and regulation of pulmonary inflammatory response.
HDL PROTEIN/LIPID CARGO AND INFLAMMATION
HDL Proteome
The HDL proteome has been investigated by numerous laboratories over the past five years (1, 21, 38, 41, 53, 89, 109). These studies vary extensively in techniques of HDL isolation and mass spectrometry. However, one common theme is the diversity of proteins associated with HDL. To date, the largest and most comprehensive mass spectrometry–based study of the HDL proteome was performed by Vaisar and colleagues (109). In this study, 48 proteins were identified in the HDL3 proteome, 22 of which were linked to cholesterol and lipid metabolism. Unexpectedly, 23 of the 48 proteins were acute-phase-response proteins; six were regulators of complement activation, eight were protease inhibitors, and eight were heparin-binding proteins.
Several studies have now provided convincing evidence that human HDL contains proteins involved in the acute phase response, proteolysis, complement activation, and coagulation, suggesting a key role for HDL cargo in inflammation (1, 89, 109). The HDL proteome is different in patients with coronary artery disease and acute coronary syndrome compared with controls (1, 109) and among distinct HDL subfractions (21). Interestingly, HDL in atherosclerotic plaques contains a subset of proteins found in plasma HDL of people with coronary artery disease, suggesting that the HDL proteome could be a biomarker for risk of coronary artery disease (109). However, HDL proteomic analysis is still in its infancy, and general conclusions about the HDL proteome as a biomarker for disease are limited by the relatively few published studies, the difference in isolation and quantification techniques, and the variation in HDL cargo identified among studies to date. Due to the complex nature of the HDL proteome, we limit our review in the next subsections to several examples of individual proteins with documented roles in oxidation, inflammation, and innate immunity.
The Antioxidative Enzymes PON and PAF-AH
HDLs have antioxidant properties that protect against oxidative damage by reducing reactive oxygen species. This beneficial effect has been attributed to HDL-associated antioxidative enzymes, such as paraoxonase 1 (PON1) and platelet-activating factor acetylhydrolase (PAF-AH). PON1 is an HDL-associated calcium-dependent enzyme that catalyzes the hydrolysis of lipid peroxides and prevents their accumulation in LDLs (67). PAF-AH is a calcium-independent extracellular phospholipase A2 that protects against LDL oxidation by hydrolyzing oxidized phospholipids and blocks inflammation induced by oxidized LDL (105).
PON1-deficient mice had greater plasma accumulation of oxidized PL and LDL-immunoglobulin complexes, HDLs that did not prevent LDL oxidation in vitro, and more atherosclerosis when crossed into the apoE knockout background and fed an atherogenic diet (92, 96, 97). The antiatherogenic properties of PON1 appear related to reduced macrophage oxidative stress, decreased leukocyte adhesion to vascular endothelium (107), and inhibited macrophage differentiation (90). PON1 can also enhance cholesterol efflux from macrophages by forming lysoPC, which stimulates HDL binding (91). In a large, prospective EPIC (European Prospective Investigation into Cancer and Nutrition)–Norfolk study, PON1 activity was inversely associated with risk of coronary artery disease risk but not independent of HDL, raising doubts as to whether PON1 activity is independently related to risk of coronary artery disease in humans (10).
The anti-inflammatory and antiatherosclerotic roles of PAF-AH are mainly documented in animal studies. Inactivation of PAF by PAF-AH (a) reduces vascular leakage and inflammation (105), (b) reduces macrophage homing and adhesion to the endothelium (104), (c) reduces lipoprotein oxidation and arterial macrophage and smooth muscle cell accumulation (83), (d ) facilitates HDL-associated cholesterol efflux from macrophages (70), and (e) promotes dendritic cell mobilization (2). Collectively, these studies suggest that HDL-associated PAF-AH may reduce atherosclerosis primarily by reducing inflammation.
ApoL1
ApoL1 is an apolipoprotein found in the HDL proteome that is involved in innate immunity against parasitic infection (27, 80, 112). Humans are normally resistant to infection by the parasite Trypanosome brucei brucei (T. b. brucei ), which causes African sleeping sickness. Resistance is attributed to the ability of apoL1 to lyse trypanosomes. ApoL1 contains a membrane pore–forming domain functionally similar to that of bacterial colicins, flanked by a membrane-addressing domain (78). HDL particles containing apoL1 are taken up by trypanosomes by a receptor that recognizes haptoglobin-related protein complexed to HDL. Once internalized by the parasite, HDL particles traffic to the lysosome, where the acidic pH results in a conformation change in the membrane-addressing domain of apoL1 that causes its dissociation from HDL. ApoL1 then binds to the lysosomal membrane, forming pores that allow continuous chloride influx, osmotic swelling of the lysosome, and eventual death of the parasite (80). Several trypanosome subspecies, T. b. rhodesiense and T. b. gambiense, have evolved to evade this mechanism of killing by apoL1 and thus can infect humans and cause sleeping sickness in Africa. The infectivity of T. b. rhodesiense is due to serum resistance–associated protein (SRA) that interacts with the C-terminal helix of apoL1 in lysosomes, preventing apoL1 from forming pores and killing trypanosomes (112). ApoL1 also kills Leishmania metacyclic promastigotes (94), suggesting a general role for apoL1 in killing intracellular parasites. Finally, apoL1 contains a Bcl-2 homology domain 3 and can induce autophagic cell death when overexpressed (113), highlighting another potential apoL1-dependent mechanism to eliminate parasitic-infected cells.
ApoL1 is an example of a protein that evolved as an important component of innate immunity to protect ancestral Africans against sleeping sickness, but in modern Western society it has become a potent mediator of chronic kidney disease in African Americans. A recent seminal study showed that two apoL1 variants (G1 and G2) are associated with a 7- to 10-fold increased relative risk for nondiabetic African Americans to develop focal segmental glomerulosclerosis and hypertension-attributed end-stage kidney disease (34). The G1 risk variant consists of two nonsynonymous coding variants (S342G and I384M) in the last exon of APOL1 that are in perfect linkage disequilibrium, whereas the G2 risk variant contains a six base pair deletion close to G1 that results in the deletion of N388 and Y389. G1 and G2 risk variant haplotypes have been subjected to strong positive selection in Africa but not in Europe or Asia (34). Plasma samples from individuals with wild-type apoL1 can lyse T. b. brucei, which do not express SRA, but not T. b. rhodesiense, which do express SRA. However, plasma from African Americans harboring apoL1 G1 and G2 apoL1 risk variants were able to lyse SRA-expressing T. b. rhodesiense.
Interestingly, the G1 and G2 sequence variants are located in the SRA-binding domain of apoL1, suggesting that the risk variants may have lost their ability to bind SRA and thus allowing these apoL1 risk variants to lyse trypanosomes. However, although the G2 variant lost its ability to bind to SRA, the G1 variant did not, suggesting a more complex mechanistic explanation for the ability of G1 and G2 variants to kill SRA-expressing trypanosomes. Although it is tempting to hypothesize that similar mechanisms of G1 and G2 risk variant cellular toxicity may damage kidney cells, resulting in end-stage renal disease, this idea is likely too simplistic. Protection of apoL1 risk variants against T. b. rhodesiense is dominant, requiring only one risk allele, whereas susceptibility to end-stage renal disease is recessive, requiring inheritance of two risk alleles (34).
ApoM and Sphingosine 1-Phosphate
ApoM is another recently discovered cargo protein of HDL that appears to play a novel role in inflammation (120). ApoM is a member of the lipocalin gene family and lacks a signal peptidase cleavage site, such that the mature protein retains its signal peptide. Other apolipoproteins bind to HDL by association of their amphipathic alpha helixes with the PL surface; however, only apoM binds to the HDL PL surface via its retained hydrophobic signal peptide. ApoM is involved in the formation and size distribution of nascent HDL particles (59, 119), but a recent paper reported that apoM is a specific transporter of plasma sphingosine 1-phosphate (S1P) (18). The hydrophobic chain of S1P points toward the interior binding calyx of apoM, and the phosphate group interacts with several arginine residues, suggesting that binding of S1P to apoM is specific (18). ApoM-containing HDLs bind S1P, whereas HDLs devoid of apoM contain no detectable S1P. Transgenic overexpression of apoM increases plasma S1P, whereas targeted deletion of apoM reduces plasma S1P (18). Human HDL containing apoM-S1P induces S1P1 receptor internalization, downstream MAPK and Akt activation, endothelial cell migration, and formation of endothelial adherent junctions, whereas apoM-negative HDL does not. Plasma S1P may activate lumenal endothelial S1P1 receptors to maintain cell spreading and cell-cell junctions. Alternatively, differences in S1P1 signaling at the lumenal versus ablumenal surface of endothelial cells may permit detection of S1P in plasma entering the subendothelial space, resulting in dynamic signaling that senses and closes intercellular gaps caused by leakage-inducing agents (15). Thus, apoM-containing HDL with its S1P lipid mediator cargo provides a partial molecular explanation for some of the vascular-protective roles of HDL.
OTHER ESTABLISHED ANTI-INFLAMMATORY ROLES OF HDL
There are other well-established anti-inflammatory roles of HDL that are not clearly linked to a specific HDL protein, lipid cargo, or its capacity to efflux cellular cholesterol. Examples include HDL's role in neutralizing bacterial endotoxin and maintaining endothelial cell integrity.
HDL particles bind the majority of LPS in plasma, decreasing the availability of LPS to bind to TLRs for initiation of inflamma-tory signaling (50). Infusion of rHDL dampens the inflammatory reaction to injected LPS in human subjects and experimental animals (64, 76). This protective effect of HDL likely occurs because of its ability to absorb LPS in its PL surface, thus preventing LPS from engaging with TLRs. The preference of HDL to bind LPS compared with other lipoproteins, such as VLDL and LDL, may be due to the ability of HDL to remodel dynamically in plasma more than in apoB-containing lipoproteins.
HDLs also help maintain endothelial cell integrity through several distinct pathways. One pathway involves S1P signaling and is relatively specific for HDL because apoM, the S1P transporter, preferentially binds HDL (see above). HDL-associated S1P induces PI3K-Akt activation and endothelial nitric oxide synthase (eNOS) stimulation, with subsequent inhibition of NF-κB signaling and adhesion molecule expression (48, 49). HDLs also bind to SR-BI on endothelial cells, resulting in stimulation (phosphorylation) of eNOS enzymatic activity and nitric oxide production, which also attenuates NF-κB activation and decreases adhesion molecule expression (58, 125). HDL binding to SR-BI may also preserve the integrity of eNOS signaling in caveolae through the maintenance of caveolae FC by uptake from HDL (108). Inhibitory actions of HDL on TNF-α-induced adhesion molecule expression and NF-κB activation were partially suppressed by either SR-BI siRNA or S1P receptor antisense oligonucleotides and were completely abolished by their combination (48), suggesting that HDL inhibits expression of adhesion molecules through a mechanism involving both SR-BI and S1P receptors.
Functionally, HDL inhibits expression of several key adhesion molecules on endothelial cells activated in vitro by cytokines or acute-phase reactive proteins (13, 20, 77). In vivo infusion of rHDL, apoA-I, and PL vesicles inhibited early pro-oxidant and proinflammatory changes induced by a periarterial collar in normocholesterolemic rabbits (68). These studies suggest that the beneficial effects of HDL on endothelial function are multifactorial, involving S1P signaling, sterol trafficking through caveolae, and SR-BI-mediated signaling.
HDL, CELLULAR PROLIFERATION, AND HEMATOPOIESIS
LXRs in Lymphocyte Proliferation
In addition to their importance in innate immune cells, LXRs also inhibit lymphocyte proliferation and play an important role in adaptive immune responses (8). An important characteristic of adaptive immunity is the capacity of antigen-specific lymphocytes to undergo rapid and extensive proliferation in response to antigenic challenge. Bensinger et al. (8) found that intracellular availability of sterols is dynamically regulated during lymphocyte cell activation, with induction of the SREBP pathway and down-regulation of LXR-responsive gene expression. Activation of LXRβ by physiologic or pharmacologic ligands diminishes the proliferative capacity of B and T cells. Conversely, genetic loss of LXRβ rescued cells from the inhibitory effect of an LXR agonist and potentiated mitogenand antigen-driven lymphocyte expansion. The inhibitory effect of LXR is completely blocked if lymphocytes receive an excess of mevalonate, the precursor for cholesterol and oxysterols. Furthermore, the ability of LXRs to impact cell proliferation has a functional consequence for lymphoid homeostasis and antigen-driven immune responses in vivo. Unexpectedly, the effects of LXRs on cell proliferation were not due to transrepression of inflammatory signaling pathways or induction of apoptosis, but rather to regulation of G1 to S phase transition. In addition, the sterol transporters ABCA1 and ABCG1, both LXR targets, are rapidly down-regulated upon T cell receptor crosslinking. The ability of LXR to inhibit proliferation is markedly reduced in lymphocytes from Abcg1-null mice, but not from Abca1-null mice, suggesting that ABCG1 plays a critical role in LXR-regulated lymphocyte proliferation.
The mechanism by which LXR activity is regulated during T cell activation does not involve alteration in ligand production but instead is due to induction of the enzyme SULT2B1 (sulfotransferase family, cytosolic, 2B, member 1), which transfers sulfate groups to oxysterols, inactivating them as LXR ligands and facilitating their export out of the cell. This leads to cellular depletion of LXR ligands and suppresses LXR-responsive gene expression. Thus, up-regulation of SULT2B1 during cell proliferation beneficially alters cellular cholesterol metabolism to support new membrane synthesis and cell division. In summary, this work outlines a previously unrecognized role for LXRβ and sterol signaling in the regulation of lymphocyte proliferation.
ABCG1 and CD4 T Lymphocyte Proliferation
Although ABCG1 plays an important role in LXR-regulated lymphocyte proliferation, the underlying mechanism was unclear until recently. Cholesterol is a key structural component of the cell membrane and is essential for cell growth and proliferation. Using Abcg1-deficient mice, Armstrong et al. (3) discovered that ABCG1 negatively regulates thymocyte and peripheral CD4 T cell signaling and proliferation by regulating cholesterol homeostasis and membrane lipid raft formation. ABCG1 deficiency in T cells does not affect IL-2 production and signaling, apoptosis, or cell survival. Compared to wild-type T cells, ABCG1-deficient thymocytes and CD4 T cells have significantly increased cholesterol ester accumulation and enhanced membrane lipid raft formation, presumably due to defective cholesterol efflux. Interestingly, increased plasma membrane lipid raft content in ABCG1-deficient T cells resulted in greater phosphorylation of zeta-chain-associated protein kinase (ZAP)70 and extracellular signal-related kinase (Erk)1/2 in the basal, resting condition. ZAP70 phosphorylation is a very early signaling event after T cell receptor (TCR) stimulation, which branches into many signaling pathways, with the MAPK pathway being the major signal pathway for cellular proliferation. TCR stimulation of T cells lacking ABCG1 expression promotes sustained ERK1/2 phosphorylation, resulting in hyperproliferation. The hyperproliferative phenotype of T cells can also be produced by acute cholesterol overloading. Collectively, this work suggests that ABCG1 acts as an important negative regulator of lymphocyte proliferation through the maintenance of cellular cholesterol homeostasis and that excess cholesterol accumulation in T lymphocytes alters their surface TCR signaling, resulting in hyperproliferation in developing and peripheral T cells. Thus, disruption of cellular cholesterol homeostasis in leukocytes affects not only innate immunity but also adaptive immunity.
HDL AND HEMATOPOIETIC STEM CELL PROLIFERATION
ABCA1, ABCG1, and Hematopoiesis
Studies in Abca1–/–Abcg1–/– mice suggest that deletion of cholesterol efflux transporters results in leukocytosis, which is associated with exaggerated atherogenesis (75, 127). Yvan-Charvet et al. (126) found that chow-fed Abca1–/–Abcg1–/– mice developed increased myeloid cells (Gr-1high CD11bhigh), monocytosis, neutrophilia, and eosinophilia with normal numbers of blood and bone marrow T and B lymphocytes. When these mice were fed a high-fat diet, peripheral leukocytes and monocytes increased further, with equivalent increases in inflammatory Ly6Chigh and patrolling ly6Clow monocyte subsets, suggesting an important role of ABCA1 and ABCG1 in maintenance of myeloid cell homeostasis.
Interestingly, leukocytosis in Abca1–/–Abcg1–/– mice was not mediated by TLR/MyD88-dependent inflammatory response but rather by increased proliferation of hematopoietic stem cells (126). ABCA1 and ABCG1 are both highly expressed in hematopoietic stem and multipotential progenitor cells (HSPCs). ABCA1 and ABCG1 deletion significantly increased the number of HSPCs [Lin-Sca+cKit+(LSK) population], granulocyte-monocyte progenitor cells, and common myeloid progenitor cells, reflecting a greater cell cycling in the progenitor cells. Furthermore, the increased proliferation of Abca1–/–Abcg1–/– LSK cells was associated with increased cholesterol-rich lipid rafts, activation of phosphor-Erk, Ras protein, and IL-3 receptor β subunit in the plasma membrane. Treatment of Abca1–/–Abcg1–/– LSK cells with cyclodextrin to disrupt lipid rafts or with a farnesyl transferase inhibitor to prevent the anchorage of Ras in the plasma membrane markedly reduced proliferation. Taken together, these data suggest that increased membrane cholesterol content secondary to ABC transporter deficiency increases lipid rafts in stem cell membranes, resulting in increased surface localization of IL-3/granulocyte macrophage colony-stimulating factor (GM-CSF) receptors, which in turn, leads to increased downstream Ras/Erk signaling and cell proliferation in response to IL-3 and GM-CSF (Figure 3).
HDL can reverse the hyperproliferation of HSPCs in vitro and in vivo. HDL, but not lipid-poor apoA-I, markedly suppresses proliferation of IL-3 and GM-CSF-induced hyperproliferation of Abca1–/–Abcg1–/– bone marrow cells in a dose-dependent manner, suggesting that HDL can suppress HSPC proliferation by promoting cholesterol efflux even in the absence of ABC transporters, perhaps via passive aqueous cholesterol diffusion or other unknown mechanisms. Transplantation of bone marrow from wild-type or Abca1–/–Abcg1–/– mice into wild-type or apoA-I transgenic recipients further confirmed the suppressive role of HDL on myeloid progenitor proliferation; there was a stark decrease in the frequency, number, and cycling activity of the LSK population in apoA-I transgenic recipients of Abca1–/–Abcg1–/– BM, but not in wild-type, recipients. Expression of the apoA-I transgene in recipient mice was associated with significantly decreased cell surface expression of the IL-3 receptor β subunit on Abca1–/–Abcg1–/– bone marrow or LSK cells and complete suppression of myeloid cell infiltration in different organs. In addition, diet-induced atherosclerosis in apoA-I transgenic/Ldlr+/– recipient mice receiving Abca1–/–Abcg1–/– bone marrow was significantly lower compared to Ldlr+/– recipient mice not expressing the apoA-I transgene. Overall, this study highlights a novel role for HDL in the regulation of hematopoietic stem cell homeostasis and provides evidence of a hitherto unappreciated mechanism by which it confers cardiovascular protection.
ApoE and Hematopoiesis
The atheroprotective role of apoE is well accepted and has been attributed to its function in the clearance of atherogenic apoB lipoproteins as well as local effects at the artery wall (7, 55). Recently, apoE was reported also to regulate hematopoietic stem cell proliferation, providing a new role for its atheroprotection (61). ApoE–/– mice fed a chow or Western-type diet developed monocytosis and neutrophilia associated with proliferation and expansion of bone marrow HSPCs. In contrast, apoA-I–/– mice and subjects with low plasma HDL due to genetic mutations did not display monocytosis or expansion of HSPCs, suggesting that apoE played a specific role in suppressing stem cell proliferation independent of reductions in plasma HDL. Relative to lineage-committed progenitor populations, HSPCs had the highest expression of apoE but no detectable expression of apoA-I.
A subset of total cellular apoE bound to cell surface proteoglycans is responsible for controlling HSPC proliferation. The cell surface proteoglycan-bound apoE requires ABCA1 and ABCG1 expression to exert an antiproliferative effect, most likely by stimulating FC and/or PL efflux via the transporters (Figure 3). Furthermore, apoE–/– HSPCs had increased membrane lipid rafts and increased ERK1/2 and STAT5 activation, which are classical cell survival and proliferation pathways. Removal of cellular cholesterol by cyclodextrin or recombinant HDL (rHDL) decreased proliferation of apoE–/– progenitors in response to IL-3/GM-CSF. Moreover, rHDL (80 mg/kg) infusion into apoE–/– mice fed a Western diet completely reversed expansion of HSPC and myeloid progenitors, with concomitant reduction in membrane lipid raft content and dampening of p-ERK1/2 and p-STAT5 signaling. When Western diet–fed apoE–/– mice received both low-dose rHDL (40 mg/kg) and an LXR agonist to activate ABCA1 and ABCG1, myeloid proliferation was reduced far more effectively than with either treatment alone. Taken together, these findings suggest a key role for cell surface proteoglycan-bound apoE in controlling HSPC proliferation, myeloid cell expansion, monocytosis, and accumulation of monocytes/macrophages in atherosclerotic lesions.
Thus, bone marrow hematopoietic stem cells possess a cell-autonomous cholesterol efflux mechanism consisting of the cholesterol transporters ABCA1 and ABCG1 and acceptor protein apoE. The close proximity of cell surface apoE to ABC transporters can modulate plasma membrane FC and lipid raft content to fine-tune cellular responses to proliferation signals (i.e., IL-3 and GM-CSF) (Figure 3). This process can function in the absence of exogenous HDL or apoA-I due to the critical role of cell surface–localized apoE or can be facilitated by an increase in FC acceptors (i.e., HDL and apoA-I) or up-regulation of ABCA1 and ABCG1 (i.e., LXR activation).
ApoA-I Affects T Lymphocyte Balance
Although apoA-I is not expressed in hematopoietic stem cells and apoA-I deficient mice do not have monocytosis or expansion of HSPCs (126), apoA-I–/–LDLr–/– mice consuming an atherogenic diet had enlarged peripheral lymph nodes (LNs) and spleens compared with their LDLr–/– mouse counterparts (117), suggesting that apoA-I plays a role in regulating peripheral lymphocyte proliferation during hypercholesterolemia. ApoA-I deficiency increased cholesterol enrichment in LNs and led to expansion of all classes of LN cells, with a significant increase in T cell proliferation and activation. In addition, apoA-I–/–LDLr–/– mice displayed an autoimmune-like pheno-type, including increased plasma antibodies to double-stranded DNA, β2-glycoprotein I, and oxidized LDL. Treatment of mice with lipid-free apoA-I reversed the autoimmune phenotype and lowered the number of LN immune cells. Interestingly, apoA-I treatment proportionally increased T regulatory cells (Treg) and decreased the percentage of effector/effector memory T cells (Teff), suggesting that apoA-I may control T cell homeostasis in peripheral lymphoid tissues by regulating the Treg/Teff balance (118). How apoA-I modulates Treg/Teff balance remains unknown but likely involves cellular FC and lipid raft homeostasis and downstream signaling events.
Inflammasome Activation and Thymopoiesis
The collapse of thymic microenvironment with advancing age and resultant inability of the thymus to produce naïve T cells contributes to lower immune surveillance in the elderly. Thus, delaying thymic aging is thought to promote lifespan (25). The early collapse of the thymic microenvironment is characterized by loss of thymic lymphopoiesis-supporting thymic epithelial cells, an increase in fibroblasts, and the emergence of lipid-laden cells (25).
Little was known about the mechanisms underlying age-related thymic involution and diminished ability of the thymus to produce naïve T cells with progressive aging. Recently, Youm et al. (124) reported that age-related increases in FC and ceramide act as danger signals, inducing NLRP3 inflammasome and downstream caspase-1 activation, which promotes age-related thymic involution. They showed that age-related inflammasome activation reduced thymic epithelial cells and T cell progenitors in thymus and bone marrow. These changes may adversely impact thymic function, since age-related reduction of these cells is the major mechanism that compromised naïve T cell export from thymus (26, 40). Furthermore, genetic elimination of Nlrp3 and Asc, a critical adapter required for the inflamma-some assembly, provides substantial protection from age-related thymic involution together with an increase in thymic epithelial cells, T cell progenitors, and maintenance of T cell receptor diversity, enhancing thymic lymphopoiesis. This study suggests that pharmacological Nlrp3 inflammasome blockers that specifically target the thymus may delay immunosenescence, maintain a diverse T cell repertoire, and enhance immune reconstitution in the elderly. In addition, since FC and ceramide accumulation activate the Nlrp3 inflammasome, an alternative treatment with HDL and/or LXR agonists, similar to that described to decrease hematopoietic stem cell proliferation, may also be an attractive therapy to delay thymic aging.
HDL AND APOLIPOPROTEIN THERAPY
With growing evidence that HDL is atheroprotective and anti-inflammatory, there is intense interest in developing treatments to elevate HDL and reduce disease risk. The simple notion that elevating HDL levels is sufficient to reduce risk of coronary heart disease (CHD) has been challenged by recent data that indicate HDL quality is more important than quantity. Khera et al. (47) found that serum efflux capacity was significantly associated with decreased risk of CHD. Another study found that serum pre-β1 HDL concentration in a population of individuals with HDL cholesterol concentrations at the 25th and 75th percentile was positively associated with ABCA1-mediated FC efflux, suggesting that pre-β1 concentration was a better determinant of cholesterol efflux potential than HDL cholesterol (22). In support of this concept, infusion of apoA-IMilano containing rHDL led to significant regression of coronary atherosclerosis in human patients (69), and infusion of rHDL recently was shown to reduce lipid and inflammation in human atherosclerotic plaque (95). The apoA-I mimetic peptides exhibited anti-inflammatory properties similar to those of rHDL in rabbits (24) and protected mice from diet-induced atherosclerosis (66, 74). Results from recent studies described herein provide a mechanistic underpinning for treatments involving infusion of HDL, apolipoproteins, and/or amphipathic peptides to reduce CHD and inflammation. In addition, specific LXR agonists that stimulate RCT without the untoward side effect of increasing hepatic lipogenesis (84) may also prove beneficial in the treatment of CHD and inflammation.
CONCLUSIONS
The ability of HDL and apolipoproteins (i.e., apoA-I and apoE) to promote cholesterol efflux results in regulation of cholesterol homeostasis and lipid raft content in immune cells and hematopoietic stem cells, modulating signaling pathways that control inflammation and cellular proliferation. There is now mounting evidence that HDL and apoA-I can also blunt inflammatory signaling pathways independent of changes in cellular cholesterol and lipid raft content. In addition, the HDL lipid and protein cargo provides multifaceted protection against oxidation, parasitic infection, endotoxemia, and endothelial damage, apparently independent of HDL's ability to efflux cholesterol. Many of HDL's beneficial and novel functions in curbing inflammation and proliferation have been elucidated using animal models, so their relevance to human physiology remains to be proven. However, increasing evidence that rHDL reduces inflammation and hematopoietic cell proliferation suggests that rHDL (or a combination of rHDL and LXR treatment) specifically targeting immune cells and their progenitors may be a promising therapeutic strategy for cardiovascular disease and other inflammation-related diseases.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants P01HL049373 and R01 HL094525. We gratefully acknowledge Karen Klein (Research Support Core, Wake Forest School of Medicine) for editing the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Alwaili K, Bailey D, Awan Z, Bailey SD, Ruel I, et al. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim. Biophys. Acta. 2011;1721:405–15. doi: 10.1016/j.bbalip.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–74. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J. Immunol. 2010;184:173–83. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001;42:1717–26. [PubMed] [Google Scholar]
- 5.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J. Immunol. 2008;180:3560–68. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 6.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L980–89. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, et al. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J. Clin. Invest. 1995;96:2170–79. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–77. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 10.Birjmohun RS, Vergeer M, Stroes ES, Sandhu MS, Ricketts SL, et al. Both paraoxonase-1 genotype and activity do not predict the risk of future coronary artery disease; the EPIC-Norfolk Prospective Population Study. PLoS One. 2009;4:e6809. doi: 10.1371/journal.pone.0006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999;22:347–51. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 12.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999;22:336–45. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 13.Calabresi L, Franceschini G, Sirtori CR, De Palma A, Saresella M, et al. Inhibition of VCAM-1 expression in endothelial cells by reconstituted high density lipoproteins. Biochem. Biophys. Res. Commun. 1997;238:61–65. doi: 10.1006/bbrc.1997.7236. [DOI] [PubMed] [Google Scholar]
- 14.Calkin AC, Tontonoz P. Liver X receptor signaling pathways and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:1513–18. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 2009;119:1871–79. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J. Biol. Chem. 2003;278:10443–49. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 17.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, et al. Crosstalk between LXR and Toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 2003;12:805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 18.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 2011;108:9613–18. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clee SM, Kastelein JJ, van DM, Marcil M, Roomp K, et al. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 2000;106:1263–70. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 1995;15:1987–94. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 21.Davidson WS, Silva RAG, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters. Arterioscler. Thromb. Vasc. Biol. 2009;29:870–76. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Bartolo BA, Nicholls SJ, Bao S, Rye KA, Heather AK, et al. The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis. 2011;217:395–400. doi: 10.1016/j.atherosclerosis.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr. Opin. Lipidol. 2010;22:521–28. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: Is it ever too old to become young again? Nat. Rev. Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 27.Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas—identification, cloning, characterization, and plasma distribution of apolipoprotein L. J. Biol. Chem. 1997;272:25576–82. doi: 10.1074/jbc.272.41.25576. [DOI] [PubMed] [Google Scholar]
- 28.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberg S. High density lipoprotein metabolism. J. Lipid Res. 1984;25:1017–58. [PubMed] [Google Scholar]
- 30.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 31.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 2011;187:1529–35. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36:211–28. [PubMed] [Google Scholar]
- 33.Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, et al. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 2005;25:1198–205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- 34.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–45. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 38.Heller M, Schlappritzi E, Stalder D, Nuoffer JM, Haeberli A. Compositional protein analysis of high density lipoproteins in hypercholesterolemia by shotgun LC-MS/MS and probabilistic peptide scoring. Mol. Cell. Proteomics. 2007;6:1059–72. doi: 10.1074/mcp.M600326-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Hinrichs JW, Klappe K, Hummel I, Kok JW. ATP-binding cassette transporters are enriched in non-caveolar detergent-insoluble glycosphingolipid-enriched membrane domains (DIGs) in human multidrug-resistant cancer cells. J. Biol. Chem. 2004;279:5734–38. doi: 10.1074/jbc.M306857200. [DOI] [PubMed] [Google Scholar]
- 40.Holland AM, van den Brink MR. Rejuvenation of the aging T cell compartment. Curr. Opin. Immunol. 2009;21:454–59. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hortin GL, Shen RF, Martin BM, Remaley AT. Diverse range of small peptides associated with high-density lipoprotein. Biochem. Biophys. Res. Commun. 2006;340:909–15. doi: 10.1016/j.bbrc.2005.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–31. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 43.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 44.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003;9:213–19. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 45.Kay JG, Murray RZ, Pagan JK, Stow JL. Cytokine secretion via cholesterol-rich lipid raft-associated SNAREs at the phagocytic cup. J. Biol. Chem. 2006;281:11949–54. doi: 10.1074/jbc.M600857200. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–31. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 2006;281:37457–67. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 49.Kimura T, Tomura H, Mogi C, Kuwabara A, Ishiwara M, et al. Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell Signal. 2006;18:841–50. doi: 10.1016/j.cellsig.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Kitchens RL, Thompson PA, Munford RS, O'Keefe GE. Acute inflammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J. Lipid Res. 2003;44:2339–48. doi: 10.1194/jlr.M300228-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 2006;281:36091–101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- 52.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272:3137–40. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 53.Levels J, Geurts P, Karlsson H, Maree R, Ljunggren S, et al. High-density lipoprotein proteome dynamics in human endotoxemia. Proteome Sci. 2011;9:34. doi: 10.1186/1477-5956-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Schwabe RF, Devries-Seimon T, Yao PM, Gerbod-Giannone MC, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and MAP kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 2005;280:21763–72. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 55.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–37. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 56.Marathe C, Bradley MN, Hong C, Lopez F, Ruiz de Galarreta CM, et al. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J. Biol. Chem. 2006;281:32197–206. doi: 10.1074/jbc.M605237200. [DOI] [PubMed] [Google Scholar]
- 57.Mendez AJ, Lin G, Wade DP, Lawn RM, Oram JF. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 2001;276:3158–66. doi: 10.1074/jbc.M007717200. [DOI] [PubMed] [Google Scholar]
- 58.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006;98:1352–64. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 59.Mulya A, Seo J, Brown AL, Gebre AK, Boudyguina E, et al. Apolipoprotein M expression increases the size of nascent pre beta HDL formed by ATP binding cassette transporter A1. J. Lipid Res. 2010;51:514–24. doi: 10.1194/jlr.M002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murata N, Sato K, Kon J, Tomura H, Yanagita M, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 2000;352(Pt. 3):809–15. [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 2011;121:4138–49. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008;28:2071–77. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 63.Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1333–41. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 64.Murphy AJ, Westerterp M, Yvan-Charvet L, Tall AR. Anti-atherogenic mechanisms of high density lipoprotein: effects on myeloid cells. Biochim. Biophys. Acta. 2012;1821:513–21. doi: 10.1016/j.bbalip.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–69. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, et al. Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290–92. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 67.Ng CJ, Shih DM, Hama SY, Villa N, Navab M, Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic. Biol. Med. 2005;38:153–63. doi: 10.1016/j.freeradbiomed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 68.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–50. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 69.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 70.Noto H, Hara M, Karasawa K, Iso O, Satoh H, et al. Human plasma platelet-activating factor acetylhydrolase binds to all the murine lipoproteins, conferring protection against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:829–35. doi: 10.1161/01.ATV.0000067701.09398.18. [DOI] [PubMed] [Google Scholar]
- 71.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–21. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 2006;43:607–12. doi: 10.1016/j.molimm.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 2001;42:1173–79. [PubMed] [Google Scholar]
- 74.Ou J, Wang J, Xu H, Ou Z, Sorci-Thomas MG, et al. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ. Res. 2005;97:1190–97. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2008;28:258–64. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 76.Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, et al. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp. Med. 1996;184:1601–8. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SH, Park JH, Kang JS, Kang YH. Involvement of transcription factors in plasma HDL protection against TNF-alpha-induced vascular cell adhesion molecule-1 expression. Int. J. Biochem. Cell Biol. 2003;35:168–82. doi: 10.1016/s1357-2725(02)00173-5. [DOI] [PubMed] [Google Scholar]
- 78.Pays E, Vanhollebeke B, Vanhamme L, Paturiaux-Hanocq F, Nolan DP, Perez-Morga D. The trypanolytic factor of human serum. Nat. Rev. Microbiol. 2006;4:477–86. doi: 10.1038/nrmicro1428. [DOI] [PubMed] [Google Scholar]
- 79.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 80.Perez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science. 2005;309:469–72. doi: 10.1126/science.1114566. [DOI] [PubMed] [Google Scholar]
- 81.Pike LJ. Lipid rafts: bringing order to chaos. J. Lipid Res. 2003;44:655–67. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Puff N, Lamaziere A, Seigneuret M, Trugnan G, Angelova MI. HDLs induce raft domain vanishing in heterogeneous giant vesicles. Chem. Phys. Lipids. 2005;133:195–202. doi: 10.1016/j.chemphyslip.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Quarck R, De Geest B, Stengel D, Mertens A, Lox M, et al. Adenovirus-mediated gene transfer of human platelet-activating factor-acetylhydrolase prevents injury-induced neointima formation and reduces spontaneous atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;103:2495–500. doi: 10.1161/01.cir.103.20.2495. [DOI] [PubMed] [Google Scholar]
- 84.Quinet EM, Basso MD, Halpern AR, Yates DW, Steffan RJ, et al. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J. Lipid Res. 2009;50:2358–70. doi: 10.1194/jlr.M900037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–7. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rayner KJ, Suárez Y, Daválos A, Parathath S, Fitzgerald ML, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–73. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002;277:18793–800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 89.Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JCM. Proteomic analysis of high-density lipoprotein. Proteomics. 2006;6:721–30. doi: 10.1002/pmic.200500191. [DOI] [PubMed] [Google Scholar]
- 90.Rosenblat M, Volkova N, Ward J, Aviram M. Paraoxonase 1 (PON1) inhibits monocyte-to-macrophage differentiation. Atherosclerosis. 2011;219:49–56. doi: 10.1016/j.atherosclerosis.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 91.Rozenberg O, Howell A, Aviram M. Pomegranate juice sugar fraction reduces macrophage oxidative state, whereas white grape juice sugar fraction increases it. Atherosclerosis. 2006;188:68–76. doi: 10.1016/j.atherosclerosis.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 92.Rozenberg O, Rosenblat M, Coleman R, Shih DM, Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic. Biol. Med. 2003;34:774–84. doi: 10.1016/s0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- 93.Rust S, Rosier M, Funke H, Real J, Amoura Z, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999;22:352–55. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 94.Samanovic M, Molina-Portela MP, Chessler AD, Burleigh BA, Raper J. Trypanosome lytic factor, an antimicrobial high-density lipoprotein, ameliorates Leishmania infection. PLoS Pathog. 2009;5:e1000276. doi: 10.1371/journal.ppat.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 2008;103:1084–91. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 96.Shih DM, Gu L, Xia YR, Navab M, Li WF, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–87. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 97.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 2000;275:17527–35. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 98.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 99.Smoak KA, Aloor JJ, Madenspacher J, Merrick BA, Collins JB, et al. Myeloid differentiation primary response protein 88 couples reverse cholesterol transport to inflammation. Cell Metab. 2010;11:493–502. doi: 10.1016/j.cmet.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, et al. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ. Res. 2009;104:455–65. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki M, Pritchard DK, Becker L, Hoofnagle AN, Tanimura N, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–27. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 2009;284:32336–43. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J. Lipid Res. 2006;47:107–14. doi: 10.1194/jlr.M500240-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Theilmeier G, De Geest B, Van Veldhoven PP, Stengel D, Michiels C, et al. HDL-associated PAF-AH reduces endothelial adhesiveness in apoE–/– mice. FASEB J. 2000;14:2032–39. doi: 10.1096/fj.99-1029com. [DOI] [PubMed] [Google Scholar]
- 105.Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374:549–53. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- 106.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 2002;115:2603–11. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 107.Tward A, Xia YR, Wang XP, Shi YS, Park C, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–90. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 108.Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J. Biol. Chem. 2000;275:11278–83. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 109.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 2007;117:746–56. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc. Natl. Acad. Sci. USA. 2004;101:17813–18. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Velde AE, Brufau G, Groen AK. Transintestinal cholesterol efflux. Curr. Opin. Lipidol. 2010;21:167–71. doi: 10.1097/MOL.0b013e3283395e45. [DOI] [PubMed] [Google Scholar]
- 112.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature. 2003;422:83–87. doi: 10.1038/nature01461. [DOI] [PubMed] [Google Scholar]
- 113.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J. Biol. Chem. 2008;283:21540–49. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 2004;101:9774–79. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–24. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009;50:574–85. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 117.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, et al. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler. Thromb. Vasc. Biol. 2009;29:843–49. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, et al. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr–/–, ApoA-I–/– mice. J. Biol. Chem. 2010;285:36158–69. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wolfrum C, Poy MN, Stoffel M. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat. Med. 2005;11:418–22. doi: 10.1038/nm1211. [DOI] [PubMed] [Google Scholar]
- 120.Xu N, Dahlback B. A novel human apolipoprotein (apoM). J. Biol. Chem. 1999;274:31286–90. doi: 10.1074/jbc.274.44.31286. [DOI] [PubMed] [Google Scholar]
- 121.Yancey PG, Bortnick AE, Kellner-Weibel G, Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2003;23:712–19. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 122.Yancey PG, Llera-Moya M, Swarnakar S, Monzo P, Klein SM, et al. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000;275:36596–604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- 123.Yin K, Deng X, Mo ZC, Zhao GJ, Jiang J, et al. Tristetraprolin-dependent post-transcriptional regulation of inflammatory cytokine mRNA expression by apolipoprotein A-I. J. Biol. Chem. 2011;286:13834–45. doi: 10.1074/jbc.M110.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Youm Y-H, Kanneganti T-D, Vandanmagsar B, Zhu X, Ravussin A, et al. The NLRP3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–57. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 126.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–93. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–8. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–4. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2005;115:2870–74. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283:22930–41. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51:3196–206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]