Abstract
The serotonin 5-HT2A receptor (5-HT2AR) may play a role in reinstatement of drug-seeking. This study investigated the ability of a selective 5-HT2A receptor (5-HT2AR) antagonist to suppress reinstatement evoked by exposure to cues conditioned to cocaine self-administration. Cocaine self-administration (0.75 mg/kg/0.1 mL/6 s infusion; FR 4) was trained in naïve, free-fed rats to allow interpretation of results independent from changes related to food deprivation stress. Pretreatment with the selective 5-HT2AR antagonist M100907 (volinanserin) failed to reduce rates of operant responding for cocaine infusions. On the other hand, M100907 (0.001 – 0.8 mg/kg, i.p.) significantly suppressed the cue-induced reinstatement of cocaine-seeking behavior following extinction; effective M100907 doses did not alter operant responding for cues previously associated with sucrose self-administration. Importantly, a greater magnitude of active lever presses on the initial extinction session (“high extinction” responders) predicted the maximal susceptibility to M100907-induced suppression of cue-evoked reinstatement. The findings indicate that blockade of the 5-HT2AR attenuates the incentive-motivational effects of cocaine-paired cues, particularly in “high extinction” responders, and suggests that M100907 may afford a therapeutic advance in suppression of cue-evoked craving and/or relapse.
Keywords: Cocaine self-administration, Cue-induced reinstatement, Extinction, M100907, 5-HT2AR antagonist
INTRODUCTION
The 5-hydroxytryptamine (5-HT) system is a prominent target of action for the abused psychostimulant cocaine which binds to and inhibits 5-HT reuptake thereby increasing the availability of 5-HT in the synapse (Koe 1976). Serotonergic manipulations (e.g., 5-HT precursors, neurotoxins, 5-HT agonists and antagonists) interfere with expression of the behavioral effects evoked by acute administration of cocaine (for review, Walsh & Cunningham 1997; Bubar & Cunningham 2006; Muller & Huston 2006) while the neurochemical consequences of repeated cocaine exposure include altered expression of 5-HT transporters and receptor subtypes in both animals (Cunningham, Paris, & Goeders 1992; O’Dell et al., 2006; Banks et al., 2008) and humans (Little et al., 1998; Baumann & Rothman 1998; Patkar et al., 2004). Interestingly, 5-HT may also be involved in the expression of cue-associated behaviors; for example, increased 5-HT efflux was observed in the prefrontal cortex (PFC) of rats (Carey & Damianopoulos 1994; but see, Bradberry & Rubino 2004 in monkeys), upon exposure to the stimuli previously paired with cocaine delivery. Thus, these data suggest that 5-HT plays a contributory role in drug-seeking behavior and may be a factor in relapse and continued use in cocaine dependence (Gawin & Kleber 1986; Wise 1996; O’Leary et al., 2000).
The 5-HT2 receptor (5-HT2R) family (5-HT2AR, 5-HT2BR and 5-HT2CR; Leysen 2004) has been a serotonergic target of interest in understanding the cellular and behavioral effects of cocaine (see reviews, Muller et al., 2006; Bubar et al., 2006). An overall excitatory and inhibitory role for the 5-HT2AR and 5-HT2CR, respectively, has been described in several animal models of cocaine-induced behaviors (see Higgins & Fletcher 2003; Bubar et al., 2006, for reviews). In self-administration studies, a particularly interesting pattern of effects has emerged, although a full dataset of information is not yet available. Acute administration of either selective and non-selective 5-HT2R antagonists failed to alter the reinforcing effects of cocaine in rodent (Fletcher, Grottick, & Higgins 2002; Filip 2005) or non-human primate self-administration studies (Fantegrossi et al., 2002). However, cocaine-primed reinstatement of cocaine-seeking was suppressed by selective 5-HT2AR antagonists, and enhanced by 5-HT2CR antagonists (Fletcher et al., 2002). Given the oppositional outcomes seen following pretreatment with a selective 5-HT2AR vs. 5-HT2CR antagonist, it is perhaps not surprising that non-selective 5-HT2A/2CR antagonists failed to attenuate cocaine-primed drug-seeking (Schenk 2000; Burmeister, Lungren, Kirschner, & Neisewander 2004; Filip 2005). Reinstatement can also be initiated by cocaine-associated cues (e.g., lights; Shaham et al., 2003), and cue-evoked reinstatement was attenuated by non-selective 5-HT2A/2CR antagonists (Burmeister et al., 2004; Filip 2005). Thus, reinstatement induced by either cocaine or cocaine-associated cues may be mediated by actions of 5-HT at the 5-HT2AR. The non-selective nature of the ligands employed thus far complicates the interpretation of the results as the reduction in cue-evoked reinstatement may result from 5-HT2AR blockade, and/or actions at other receptors (e.g., dopamine, α-adrenergic receptors; Baxter, Kennett, Blaney, & Blackburn 1995; Wainscott et al., 1996). Furthermore, because the 5-HT2AR and 5-HT2CR are oppositional in the control of the in vivo effects of cocaine (for review, Bubar et al., 2006), results observed following administration of nonselective 5-HT2A/2CR antagonists are confounded by a concurrent blockade of the 5-HT2CR which would be expected to have oppositional actions to those of blockade of the 5-HT2AR (e.g. Cremers et al., 2004; Bonaccorso et al., 2002). Understanding the specific role of the 5-HT2AR in cue-induced reinstatement is important information needed to fully appreciate the potential means by which to support and extend abstinence during treatment.
Serotonin systems, and the 5-HT2AR in particular, are engaged under stressful circumstances (Chaouloff, Elghozi, Guezennec, & Laude 1985; Tsujii et al., 1988; McBlane & Handley 1994; Popova et al., 2001) and several methodological aspects of cocaine self-administration paradigms necessitate a consideration of suitable protocols to maximize interpretation of studies in which 5-HT2AR ligands are investigated (Bongiovanni & See 2008). The procedures utilized for training of self-administration tasks in rodents typically involve food (or water) deprivation and a history of operant pretraining for a food (or water) reinforcer (Grottick, Fletcher, & Higgins 2000; Fletcher et al., 2002; Burmeister et al., 2004; Fletcher, Chintoh, Sinyard, & Higgins 2004; Filip 2005). Initial operant pretraining for one appetitive reinforcer can influence subsequent behavior maintained by another reinforcer and can result in changes in response rates (Urbain, Poling, Millam, & Thompson 1978; Nader & Thompson 1989). Additionally, food deprivation is a typical feature of self-administration procedures and has been identified as a mild stressor to increase plasma corticosterone (Marinelli, Le Moal, & Piazza 1996; Takao, Nagatani, Kitamura, & Yamawaki 1997; Dallman et al., 1999), disrupt 5-HT function (Chaouloff et al., 1985; Tsujii et al., 1988; McBlane et al., 1994; Popova et al., 2001) and enhance psychostimulant-induced behavioral and neural responses (Carroll 1985; Carr 2007; Stamp, Mashoodh, van Kampen, & Robertson 2008). Thus, the design of self-administration studies without conditions of food deprivation and operant pretraining affords an opportunity to analyze serotonergic regulation of cocaine-seeking and –taking in rodents (Fletcher et al., 2002;, Neisewander & Acosta 2007; Fletcher et al., 2008; but, see Burbassi & Cervo 2008) under conditions that minimize the potential for associated stress-related confounds (Stanford 1996).
The present study investigated the hypothesis that the selective 5-HT2AR antagonist M100907 would suppress cue-evoked reinstatement in both cocaine and sucrose self-administration paradigms. An extensive database indicates that M100907 is the most selective 5-HT2AR antagonist for preclinical studies available (Schreiber, Brocco, & Millan 1994; Kehne et al., 1996; De Paulis 2001; Fletcher et al., 2002; Marek, Martin-Ruiz, Abo, & Artigas 2005; Carli, Baviera, Invernizzi, & Balducci 2006) with selectivity for the 5-HT2AR (Ki= 0.85 nM) over the closely-related 5-HT2CR (Ki=88 nM) and ~150 fold less affinity for other receptors (e.g., dopamine D2 and alpha1 adrenergic receptors; Kehne et al., 1996). In addition to the broad preclinical experience with this investigational compound, the progression of clinical trials with M100907 (volinanserin; National Institutes of Health Clinical Center 2005) suggests that this medication may be available for treatment studies in humans soon. Here, we analyzed the effectiveness of a wide range of doses of M100907 (Hitchcock, Lister, Fischer, & Wettstein 1997; Wettstein, Host, & Hitchcock 1999; Patel, Fernandez-Garcia, Hutson, & Patel 2001; Bonaccorso et al., 2002;. McCreary, Filip, & Cunningham 2003) to alter cue-evoked reinstatement under conditions that minimized potential confounds imposed by food deprivation (or water; Filip 2005) and operant pretraining for an appetitive reinforcer (Grottick et al., 2000; Fletcher et al., 2002; Burmeister et al., 2004; Filip 2005). To analyze the extent to which the ability of M100907 to alter drug-seeking was related to protocols employed during the reinstatement phase, four separate experiments were conducted. Reinstatement was assessed under either an oft-employed within-subjects design (repeated testing; Shaham et al., 2003) or under a between-subjects design in which each rat received a single reinstatement session.
METHODS
Animals
A total of 92 male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) weighing 250–325g at the start of the experiments were used. Upon arrival, rats were handled and given 4–5 days to acclimate to their environment. All animals were individually housed in a climate-controlled vivarium with a 12-h light/dark cycle (lights on: 7 am, lights off: 7 pm) and given access to food (standard rat chow) and water ad libitum throughout the phases of the study (i.e., self-administration, extinction and cue-induced reinstatement). A total of 14 rats were excluded from the study due to weight loss >10% (n=1), failure to demonstrate catheter patency (n=7), failure to meet the acquisition (n=3) or extinction criterion (n=3). All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the Institutional Animal Care and Use Committee at University of Texas Medical Branch.
Surgery
Implantations of intravenous catheters with head mounts were performed under anesthesia with a cocktail containing 8.6 mg/kg of xylazine, 1.5 mg/kg of acepromazine and 43 mg/kg of ketamine in bacteriostatic saline (0.9% NaCl). Small incisions were made in the right posterior neck and the top of the head to expose the right jugular vein and skull, respectively. A subcutaneous burrow was made between the two incisions, and the catheter apparatus was pulled through this burrow. The catheter was composed of Silastic® tubing (Dow Corning, Midland, MI) connected to a bent 22-gauge metal cannula encased within a plastic screw connector (Plastics One, Roanoke, VA) at one end and affixed with a small ball of aquarium sealant ~3 cm from the other end. The distal end of the catheter was inserted into the jugular vein and pushed down until flush with the ball of aquarium sealant, terminating outside the right atrium. The catheter was secured to the vein with sutures (braided silk non absorbable 4.0) on both sides of the aquarium sealant ball. The incision was then sutured (nylon non absorbable 4.0). The metal end of the catheter was secured to the skull using cranioplastic cement and small anchor screws drilled into the skull. Sham surgeries were performed on rats assigned to sucrose self-administration and anesthetized as above. A small incision was made in the right posterior neck to expose the right jugular vein and then sutured.
Catheter patency was maintained by daily flushing with a solution of 0.1 mL bacteriostatic saline containing heparin sodium (10 U/mL, American Pharmaceutical Partners, IL), streptokinase (0.67 mg/mL, Sigma, St. Louis, MO) and ticarcillin disodium (66.67 mg/mL, Research Products International, Mt. Prospect, IL) after each cocaine self-administration session. Proper catheter function was verified periodically throughout the experiment by intravenous administration of 10 mg/kg methohexital sodium (Monarch Pharmaceuticals Inc., Bristol, TN), a dose sufficient to briefly anesthetize the animal only when administered i.v. All rats were allowed at least 5 days of recovery after surgery before initiation of self-administration training.
Apparatus
Standard operant conditioning chambers (Med-Associates, St. Albans, VT) housed in ventilated sound-attenuating cubicles (Med-Associates) with fans to mask outside noise were utilized for the self-administration study. Each operant conditioning chamber was equipped with two retractable response levers, a stimulus light above each response lever, a houselight opposite the levers and a magazine-type pellet dispenser. The cocaine infusions were delivered by a syringe attached to a motor-driven infusion pump (Med Associates) located outside the operant conditioning chamber. The infusion pumps were connected to liquid swivels (Instech, Plymouth Meeting, PA) which were fastened to the catheters via polyethylene 20 tubing encased inside a metal spring leash (Plastics One). Sucrose pellets (45 mg; Bio-Serv, Frenchtown, NJ) were delivered into a pellet receptacle located between the two levers.
Experimental Design
Experiment 1: Effects of M100907 (0.1 – 0.8 mg/kg) during cocaine self-administration and cue-induced reinstatement (within-subjects design)
Cocaine self-administration training consisted of daily 2 hr sessions during which rats were trained to press the active lever to obtain a cocaine infusion (0.75 mg/kg/0.1 mL, i.v.). Animals were not food-restricted or trained on an operant task prior to commencement of self-administration and no cocaine priming infusions were given during the experiment. Scheduled completions on the active lever resulted in simultaneous activation of the house light and stimulus light, followed 1 sec later by either activation of the infusion pump. Rats received a 0.1 mL cocaine infusion delivered over a 6 sec period, after which the pump and stimulus light (conditioned reinforcers paired with delivery of cocaine) were inactivated simultaneously. The house light remained illuminated for a 20 sec timeout period, during which lever presses had no scheduled consequences. Responses on the inactive lever had no scheduled consequences. Animals were initially trained on an FR 1 schedule of cocaine reinforcement until they met a criterion of seven infusions/hr for at least three sessions. After reaching this acquisition criterion and demonstrating stable cocaine self-administration on an FR1 schedule of reinforcement (i.e., < 10% variation in the number of infusions received for three consecutive sessions), animals progressed to an FR 4 schedule of reinforcement. Once the criterion of stable cocaine self-administration on an FR 4 schedule was achieved (i.e., < 10% variation in the number of infusions received for three consecutive sessions), the ability of M100907 to alter responses rates for cocaine self-administration was assessed. Animals (n=10) were administered vehicle or M100907 (0.1, 0.2, 0.4 or 0.8 mg/kg; 30 min, i.p.) prior to self-administration sessions, according to a within-subjects design. The order of M100907 injections was counterbalanced and intervening sessions of cocaine self-administration occurred between each drug challenge to assure retention of baseline responding.
Following the last M100907 test session and three sessions to reestablish stable cocaine self-administration, daily 1 hr extinction sessions were conducted across consecutive sessions until rats met the extinction criterion of response rates less than 15 total responses in the hour for three consecutive sessions. The criterion of ≤ 15 responses/hr as indicative of extinction level responding is commonly employed in self-administration studies (e.g., Leri, Flores, Rodaros, & Stewart 2002) and corresponds to approximately 15% of responding observed during active cocaine self-administration (Shaham et al., 2003). During the extinction sessions, active and inactive lever presses were recorded but had no scheduled consequences (i.e., did not activate the infusion pump or result in presentation of the cocaine-paired stimuli).
Once rats achieved the extinction criterion, the effects of M100907 on cue-induced reinstatement of cocaine-seeking behavior were examined. To initiate reinstatement sessions, one passive presentation of the cocaine-paired cues (pump and stimulus light) was given. During the remaining 1 hr test session, responses on the active lever were reinforced by presentation of the cocaine-paired cues on an FR 1 schedule. Animals were given a minimum of three additional extinction training sessions between the tests to allow extinction baseline response rates to restabilize. The same extinction criterion (≤ 15 active lever responses/session for three consecutive sessions) applied such that rats needed to achieve ≤ 15 active lever responses/session for three consecutive sessions before a cue-induced reinstatement test session was initiated. To assess the effects of M100907 on cue-induced reinstatement of cocaine-seeking behavior, rats were injected with vehicle or M100907 (0.1, 0.2, 0.4 or 0.8 mg/kg; i.p., 30 min) before the start of the reinstatement session according to a within-subjects (n=10) design.
Experiment 2: Effects of M100907 (0.001 – 0.01 mg/kg) on cue-induced reinstatement in a cocaine self-administration paradigm (within-subjects design)
The results of Experiment 1 demonstrated a lack of dose dependency for M100907 to suppress cue-evoked responding. To address the possibility that M100907 exposure during self-administration sessions may explain these observations, a second experiment was conducted in a within-subjects reinstatement design with even lower doses of M100907 than those employed in Experiment 1. Cocaine self-administration training consisted of daily 2 hr sessions during which rats were trained to press the active lever to obtain a cocaine infusion (0.75 mg/kg/0.1 mL, i.v.) on an FR 1 schedule of reinforcement before progressing to a FR 4 schedule. Once the criterion for stable self-administration (< 10% variation in the number of infusions received for three consecutive sessions) was met, rats were subjected to extinction training under identical conditions as in Experiment 1. Once the extinction criterion (≤ 15 active lever responses/session for three consecutive sessions) was met, reinstatement sessions were conducted in a within-subjects design (n=13) under conditions identical to Experiment 1. Prior to reinstatement sessions, rats were injected with vehicle or M100907 (0.001, 0.01, 0.1 mg/kg; i.p., 30 min) under conditions identical to Experiment 1.
Experiment 3: Effects of M100907 on cue-induced reinstatement in a cocaine self-administration paradigm (between-subjects design)
Experiments 1 and 2 indicated a high efficacy of M100907 to suppress cue-evoked reinstatement with little evidence of a classical dose-effect curve. Because reinstatement was assessed in a within-subjects design for the first two experiments, in Experiment 3, we reinvestigated the dose-effect curve for M100907 in groups of rats which were exposed to only one single reinstatement session (between-subjects design). Cocaine self-administration training consisted of daily 3 hr sessions during which rats were trained to press the active lever to obtain a cocaine infusion (0.75 mg/kg/0.1 mL, i.v.) on an FR 1 schedule of reinforcement before progressing to stable responding on an FR 4 schedule. Rats were then assigned to treatment groups (n=7/group) matched for cocaine intake during the self-administration training phase to control for potential differences in cue-induced reinstatement of cocaine-seeking behavior that could be related to prior cocaine intake levels (Deroche, Le, & Piazza 1999; Sutton, Karanian, & Self 2000). All animals were then subjected to extinction training (3 hr/session) until the extinction criterion was met. For assessment of the effects of M100907 on cue-induced reinstatement of cocaine-seeking behavior, rats were injected with vehicle or M100907 (0.0001, 0.001, 0.01, 0.1 mg/kg; i.p., 30 min) before the start of the reinstatement session under conditions identical to Experiment 1.
Experiment 4: Effects of M100907 on cue-induced reinstatement in a sucrose self-administration paradigm (between-subjects design)
Sucrose self-administration (n=20) training consisted of daily 2 hr sessions during which rats were trained to press the active lever to obtain a sucrose pellet. Scheduled completions on the active lever resulted in simultaneous activation of the house light and stimulus light, followed 1 sec later by activation of the sucrose pellet dispenser. Rats received a single sucrose pellet (45 mg). After a 6 sec period, the stimulus light was inactivated and the house light remained illuminated for a 20 sec timeout period, during which lever presses had no scheduled consequences. Responses on the inactive lever had no scheduled consequences. Animals were initially trained on an FR 1 schedule of sucrose reinforcement before progressing to an FR 4 schedule of reinforcement. Upon reaching stability on the FR 4 schedule, animals were assigned to three groups (based on history of pellet intake and levels of operant responding during the self-administration training phase) for testing of cue-induced reinstatement of sucrose-seeking behavior. Following extinction training (identical to conditions described in Experiment 1), the effects of M100907 (0.01 and 0.1 mg/kg; i.p., 30 min) or vehicle on cue-induced reinstatement of sucrose-seeking behavior were examined. These doses were chosen because each dose completely blocked cue-induced reinstatement of cocaine-seeking behavior in the previous experiments.
Statistical analysis
The data from the self-administration, extinction and reinstatement phases were analyzed separately. A one-way ANOVA (SAS for Windows, V8.2, Cary, NC) for repeated measures was used to analyze the dependent measures of the total number of 1) active lever responses/session, 2) inactive lever presses/session, and 3) number of cocaine infusions/session during the self-administration phase and the total number of responses/session on the 1) previously-active, or 2) -inactive lever during the extinction phase. A one-way ANOVA for repeated measures (Experiments 1, 2 and 4) or independent groups (Experiment 3) was used to analyze the effects of pretreatment with M100907 on the total number of 1) responses/session on the previously-active, or 2) -inactive lever, and 3) the latency to respond on the previously-active lever during the reinstatement phase. When appropriate, post hoc comparisons for M100907 pretreatment vs. vehicle group were carried out with Student–Newman–Keuls (SNK) test with the alpha level set at p < 0.05. A paired Student’s t-test was employed to compare responding on the previously-active lever over the last three sessions of extinction with responding observed following vehicle pretreatment during reinstatement sessions.
Responding on the first session of extinction has been noted as a significant predictor of the magnitude of reinstatement upon delivery of cocaine-associated cues (Kruzich et al., 1999). To assess this possibility in Experiments 1–3, individual rats were divided by a median split into “low extinction” and “high extinction” responders based upon the total previously-active lever presses observed on the first extinction session (Experiments 1–3). Differences between the low and high extinction responders related to patterns of behavior displayed during the self-administration and extinction phases were examined with two-way mixed model ANOVA with group (low vs. high extinction responders) as the between-subjects factor and sessions as the within-subjects factor).
The relationship between responding on the previously-active lever during the first extinction session and that seen during reinstatement sessions following M100907 pretreatment was assessed in low and high extinction responders. We analyzed the percentage change from the total mean number of responses on the previously-active lever during the initial extinction session (baseline; “base”) relative to that during reinstatement sessions following M100907 pretreatment (M100), that is, % Δ from base = [(M100-base)/base] x 100. A two-way ANOVA was employed to analyze group (low vs. high extinction) and pretreatment (veh, 0.01, 0.1, 0.2, 0.4, 0.8 mg/kg). Planned comparisons between the low and high extinction responders at M100907 doses which were employed in Experiments 1–3 were conducted with the Student-Newman-Keuls test, this statistical analysis has been supported in a number of statistical texts ((Keppel & Wickens, 2004; Sheskin 2007).
RESULTS
Experiment 1: Effects of M100907 (0.1 – 0.8 mg/kg) during cocaine self-administration and cue-induced reinstatement (within-subjects design)
Acquisition and stable cocaine self-administration
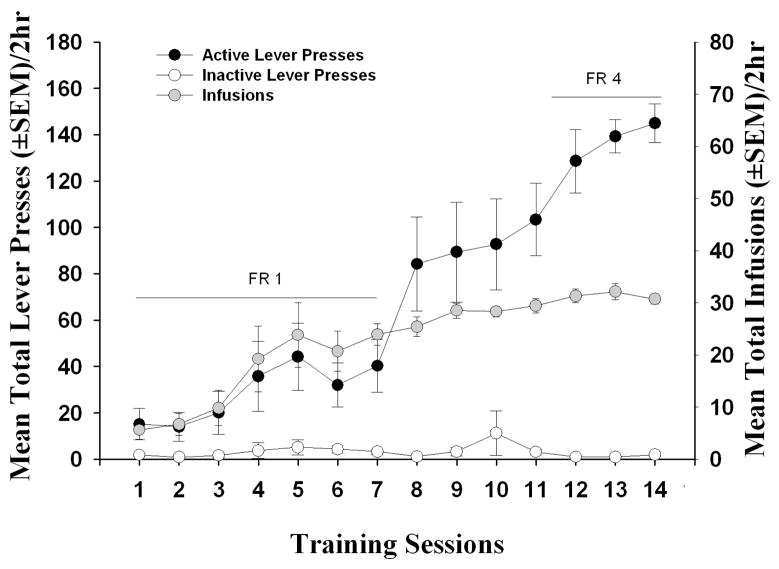
Rats (n = 10) readily acquired cocaine self-administration to stability (i.e., seven infusions/hr on an FR 1 schedule for at least three sessions) and displayed < 10% variation in the number of infusions received (i.e., cocaine intake) by session 7 of the self-administration training phase (Figure 1). Across the last three sessions of stable self-administration on an FR 4 schedule (< 10% variation in the number of infusions received), there was no main effect of session on the number of active [F(2,18) = 1.50, ns] or inactive lever responses [F(2,18) = 3.36, ns] or the number of infusions received [F(2,18) = 1.13, ns]. The average daily cocaine intake over the last three sessions of training was 24.4 ± 0.4 mg/kg.
Figure 1. Acquisition of cocaine self-administration.
Data represent mean responses (± SEM) on the active (black circles) and inactive levers (white circles), and the total number of cocaine infusions (± SEM; gray circles) during the cocaine self-administration training phase under FR1 and FR4 schedules of reinforcement (n = 10). Active lever responses resulted in the delivery of an i.v. cocaine infusion (0.75 mg/kg/0.1 ml) and simultaneous presentation of the cocaine-paired cues.
Effects of M100907 on cocaine self-administration
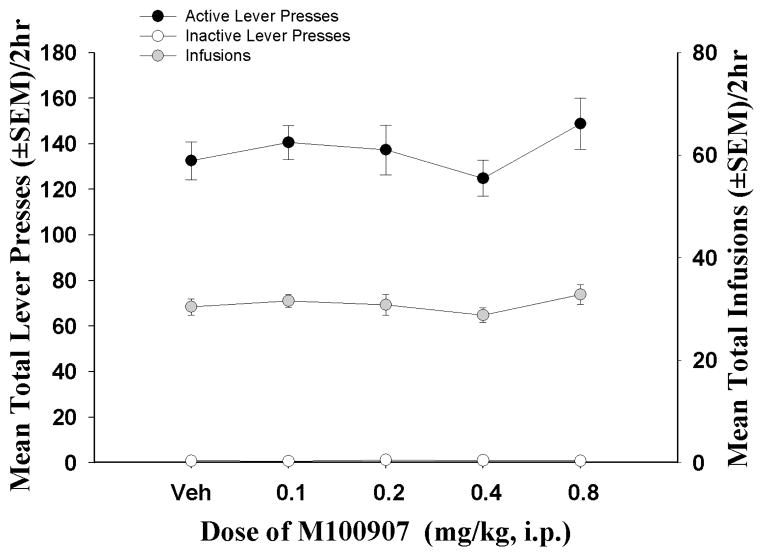
Acute administration of M100907 (0.1 – 0.8 mg/kg) did not alter rates of cocaine self-administration at any of the doses tested for active lever presses [F(4, 36) = 1.71, ns], inactive lever presses [F(4, 36) = 0.90, ns] or the number of infusions taken [F(4, 36) = 1. 64, ns] (n = 10, Figure 2). Pretreatment with M100907 also failed to affect the latency to the first response during the self-administration sessions [F(4, 36) = 0.63, ns] (data not shown).
Figure 2. Effects of M100907 during cocaine self-administration.
Acute pretreatment with M100907 or its vehicle failed to alter cocaine self-administration (0.75 mg/kg/0.1 ml infusion, available according to a FR 4 schedule, n = 10). Data represent mean responses (± SEM) on the active (black circles) and inactive levers (white circles), and the total number of cocaine infusions (± SEM; gray circles) during the cocaine self-administration maintenance phase. Active lever responses resulted in the delivery of an i.v. cocaine infusion (0.75 mg/kg/0.1 ml) and simultaneous presentation of cocaine-paired cues.
Extinction Training
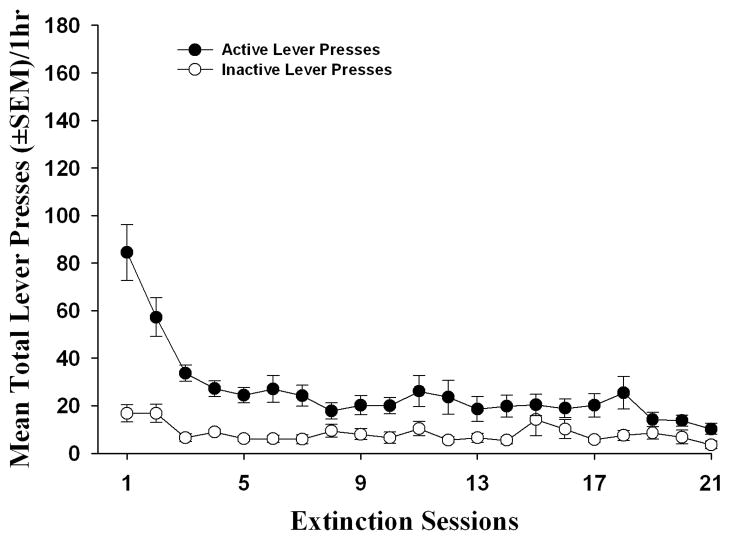
A decrease in presses on the previously-active lever was observed across extinction sessions (Figure 3); the extinction criterion (<15 active lever responses/session for three consecutive sessions) was achieved for all animals in Experiment 1 by session 21 of extinction training (Figure 3). A repeated-measures ANOVA for active lever presses across the extinction sessions for all rats (n = 10) showed a main effect of session [F(20, 180) = 17.37 = p < 0.05]. Although a slight increase in inactive lever responses (16.8 ± 3.6) was noted during the first two extinction sessions relative to the last three sessions of self-administration (0.28 ± 0.2), inactive lever presses decreased across sessions during the extinction period [F(20, 180) = 3.30 = p < 0.05]. “Low extinction” responders exhibited significantly (t(8) = −4.11, p < 0.05) fewer total lever presses (± SEM) on the initial extinction session (55.4 ± 11.5; n=5) vs. the “high extinction” responders (113.4 ± 8.2; n=5). Post-hoc analyses indicated that “low extinction” and “high extinction” responders did not differ in cocaine intake during the first seven [F(1, 8) = 2.04 = ns]) or last six acquisition sessions of the self-administration phase [F(1, 8) = 4.91 = ns] or in the number of sessions required to reach extinction criterion [F(1, 8) = 0.58 = ns] (data not shown).
Figure 3. Extinction of cocaine self-administration.
A decrease in active lever responses was observed across extinction trials following a history of cocaine self-administration. Data represent mean (±SEM) total number of responses on the active (dark circles) and inactive levers (open circles) during daily 1 hr extinction sessions (n = 10). Lever responses during extinction had no programmed consequences.
Effects of M100907 on cue-induced reinstatement
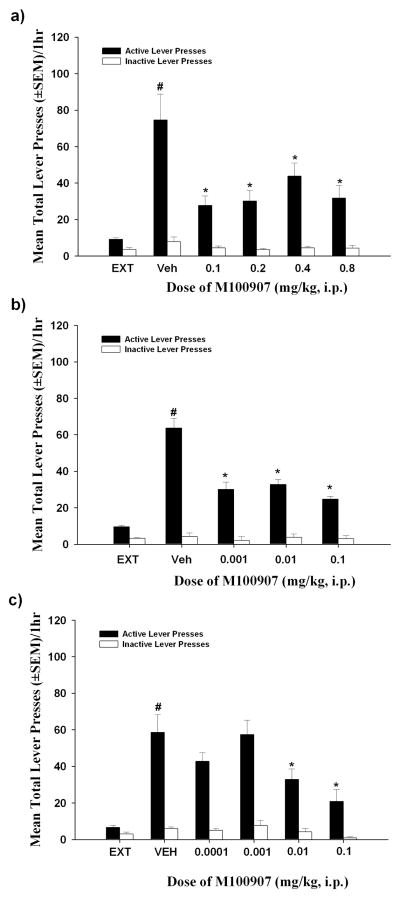
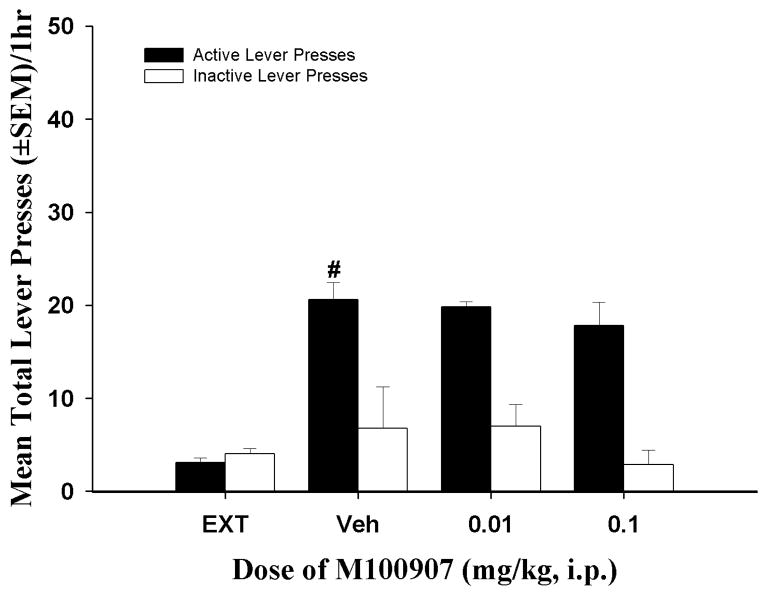
Figure 4a illustrates the effects of M100907 (0.1 – 0.8 mg/kg) pretreatment on reinstatement of responding supported by cues previously associated with cocaine availability. A main effect of pretreatment on active lever presses was observed [F(4, 36) = 10.13; p < 0.05]. Significant decreases in active lever presses relative to vehicle were observed after pretreatment with M100907 at all doses (Figure 4a; p < 0.05). In contrast, there was no main effect of pretreatment on inactive lever responding [F(4, 36) = 1.29; ns] or in the latency to the first lever press response [F(4, 36) = 1.16; ns] (data not shown).
Figure 4. Effects of multiple doses of M100907 on cue-evoked reinstatement of cocaine-seeking behavior.
Lever presses (± SEM) on the active (filled bars) and inactive levers (open bars) on the test day for reinstatement of extinguished cocaine-seeking behavior. Each active lever press resulted in the presentation of the conditioned stimuli in the absence of cocaine delivery. (4a) In a within-subjects design of cue-evoked reinstatement (see Methods, Experiment 1), delivery of cocaine-associated cues significantly increased the number of presses on the previously-active lever [t(9) = 4.50 = p < 0.05] after vehicle (VEH) pretreatment vs. the average number of previously-active lever presses observed on the three preceding extinction sessions (EXT; #p<0.05). Acute pretreatment with M100907 (0.1–0.8 mg/kg) attenuated cocaine-seeking behavior relative to vehicle treatment (n = 10; *p<0.05). A minimum of three extinction sessions between tests assured re-establishment of the extinction criterion prior to each cue-reinstatement test. (4b) In a within-subjects design of cue-evoked reinstatement (see Methods, Experiment 2), delivery of cocaine-associated cues significantly increased the number of presses on the previously-active lever [t(12) = 5.06 = p < 0.05] after vehicle (VEH) pretreatment vs. the average number of previously-active lever presses observed on the three preceding extinction sessions (EXT; #p<0.05). Acute pretreatment with M100907 (0.001–0.1 mg/kg) attenuated cocaine-seeking behavior relative to vehicle pretreatment (n = 13; *p<0.05). A minimum of three extinction sessions between tests assured re-establishment of the extinction criterion prior to each cue-reinstatement test. (4c) In a between-subjects design of cue-evoked reinstatement (see Methods, Experiment 3), delivery of cocaine-associated cues significantly increased the number of presses on the previously-active lever [t(6) = 6.25 = p < 0.05] after vehicle (VEH) pretreatment vs. the average number of previously-active lever presses observed on the three preceding extinction sessions (EXT; #p<0.05). Acute pretreatment with M100907 (0.0001–0.1 mg/kg) attenuated cocaine-seeking behavior relative to vehicle pretreatment (n = 7/dose group; *p<0.05).
Experiment 2: Effects of M100907 (0.001 – 0.01 mg/kg) on cue-induced reinstatement in a cocaine self-administration paradigm (within-subjects design)
Acquisition and stable cocaine self-administration
Rats (n = 13) readily acquired cocaine self-administration in Experiment 2 (data not shown). Across the last three sessions of stable self-administration, there was no main effect of session on the number of active [F(2,24) = 3.19, ns] or inactive operant lever responses [F(2,24) = 1.41, ns] or the number of infusions received [F(2,24) = 1.73, ns]. The average daily cocaine intake over the last three sessions of training was 20.0 ± 0.4 mg/kg.
Extinction Training
The extinction criterion was achieved for all animals (n=13) in Experiment 2 by session 21 of extinction training (data not shown); a main effect of session was observed [F(20, 80) = 9.21 = p < 0.05]. “Low extinction” responders exhibited significantly [t(11) = −3.65, p < 0.05] fewer total lever presses (± SEM) on the initial extinction session (48.2 ± 5.2; n=6) vs. the “high extinction” responders (93.9 ± 10.6; n = 7). Post-hoc analyses indicated that “low extinction” and “high extinction” responders did not differ in cocaine intake during the first seven [F(1, 11) = 3.69 = ns]) or last six acquisition sessions of the self-administration phase [F(1, 11) = 0.06 = ns] or in the number of sessions required to reach extinction criterion [F(1, 11) = 1.45 = ns] (data not shown).
Effects of M100907 on cue-induced reinstatement
Figure 4b illustrates the effects of M100907 (0.001 – 0.1 mg/kg) pretreatment on reinstatement of responding supported by cues previously associated with cocaine availability. A main effect of pretreatment on active lever presses was observed [F(3, 36) = 8.06; p < 0.05]. Significant decreases in active lever presses relative to vehicle were observed after pretreatment with M100907 at all doses (Figure 4b; p < 0.05). In contrast, there was no main effect of pretreatment on inactive lever presses [F(3, 36) = 1.02; ns] or in the latency to the first lever press response [F(3, 36) = 1.20; ns] (data not shown).
Experiment 3: Effects of M100907 on cue-induced reinstatement in a cocaine self-administration paradigm (between-subjects design)
Acquisition and stable cocaine self-administration
Rats (n = 35) readily acquired cocaine self-administration in Experiment 3 (data not shown). Across the last two sessions of stable self-administration on an FR 4 schedule (< 10% variation in the number of infusions received), there was no main effect of session on the number of active [F(1,34) = 1.26, ns] or inactive lever responding [F(1,34) = 0.23, ns] or the number of infusions received [F(1,34) = 3.70, ns]. The rats were then separated into five groups for reinstatement sessions. Cocaine intake did not differ significantly across groups of rats assigned to receive pretreatment with vehicle (42.3 ± 3.1 infusions/session) or M100907 at doses of 0.0001 mg/kg (39.1 ± 3.0 infusions/session), 0.001 mg/kg (32.4 ± 4.5 infusions/session), 0.01 mg/kg (39.5 ± 3.2 infusions/session) or 0.1 mg/kg (37.1 ± 2.8 infusions/session) [F(4, 30) = 1.21; ns].
Extinction Training
The extinction criterion was achieved for all animals (n=35) in Experiment 3 by session 10 of extinction training (data not shown); a main effect of session was observed [F(9, 306) = 100.37 = p < 0.05]. “Low extinction” and “high extinction” responders were divided based on a median split of the total number of previously-active lever presses observed on the initial extinction within each group. Thus, “low extinction” responders (n = 17, 46.9 ± 2.9) (± SEM) exhibited significantly fewer baseline lever presses on the initial extinction session vs. the “high extinction” responders (n = 18, 83.3 ± 5.6) (t(33) = −5.6, p < 0.05). Post-hoc analyses indicated that “low extinction” and “high extinction” responders did not differ in cocaine intake during the first five [F(1, 33) = 0.10 = ns] or last two acquisition sessions [F(1, 33) = 4.05 = ns]) of the self-administration phase or in the number of sessions required to reach extinction criterion [F(1, 33) = 1.74 = ns] (data not shown).
Effects of M100907 on cue-induced reinstatement
Figure 4c illustrates the effects of M100907 (0.0001 – 0.1 mg/kg) pretreatment on reinstatement of responding supported by cues previously associated with cocaine availability. A main effect of pretreatment [F(4, 30) = 6.16; p < 0.05] on active lever responding was observed. Significant decreases in active lever presses relative to vehicle were observed after pretreatment with M100907 at doses of 0.01 and 0.1 mg/kg (p < 0.05; Figure 4c). While the ANOVA revealed a main effect of pretreatment on inactive lever responding [F(4, 30) = 3.57; p < 0.05], post-hoc analysis failed to reveal a difference between vehicle and pretreatment groups. The latency to the first lever press following pretreatment with M100907 was unaltered [F(4, 30) = 0.92; ns] (data not shown).
Individual differences in cue-induced reinstatement in cocaine self-administration
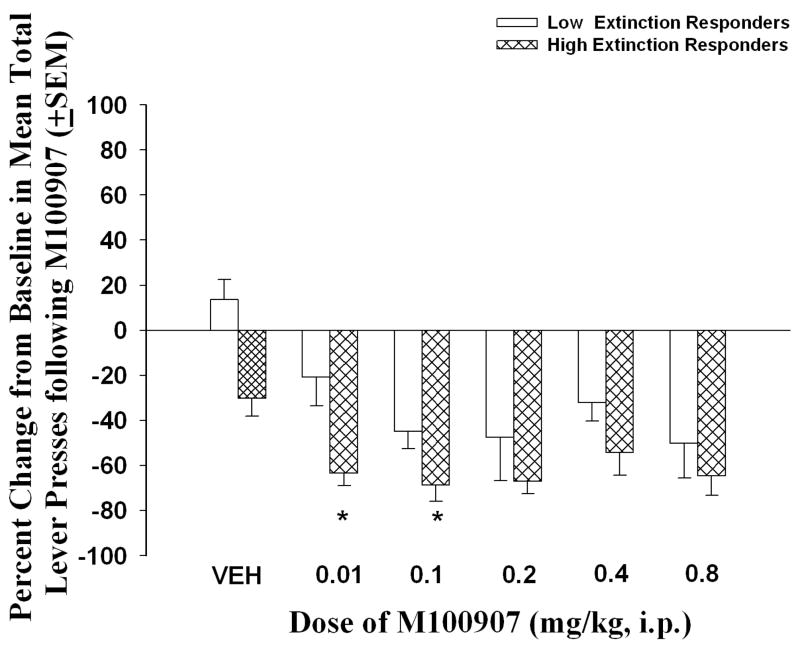
Pretreatment with M100907 suppressed reinstatement in both “low extinction” and “high extinction” responders (Figure 5). The two-way ANOVA indicated a significant main effect of group [F(1, 98) = 18.76; p < 0.05] and dose [F(5, 98) = 10.14; p < 0.05], but no group x dose interaction [F(5, 98) = 0.79; ns]. Planned comparisons between the low and high extinction responders at M100907 doses which were employed in Experiments 1–3 indicated that the “high extinction” responders exhibited greater reduction in reinstatement after M100907 at 0.01 and 0.1 mg/kg than the “low extinction” responders.
Figure 5. Individual differences in cue-induced reinstatement in cocaine self-administration.
Individual responses on the first session of extinction revealed two subsets of rats within each experimental group, which were classified as “low extinction” or “high extinction” responders based on a median split of the number of active lever presses (see Methods). Data represent the mean change in baseline (total lever presses on the initial extinction session) on the reinstatement session represented as a percentage (± SEM). “High extinction” responders exhibited a significantly greater change from baseline after pretreatment with 0.01 and 0.1 mg/kg of M100907 relative to “low extinction” responders (*p<0.05).
Experiment 4: Effects of M100907 on cue-induced reinstatement in a sucrose self-administration paradigm (between-subjects design)
Acquisition and stable sucrose self-administration
Rats (n = 20) readily acquired sucrose self-administration (data not shown). Across the last three sessions of stable self-administration on an FR 4 schedule (< 10% variation in the number of pellets received), there was no main effect of session on the number of active [F(2,38) = 0.38, ns] or inactive operant lever responding [F(2,38) = 1.84, ns] or the number of pellets received [F(2,38) = 0.98, ns]. The average daily sucrose intake over the last six sessions of training was 42.6 ± 5.34 pellets. Sucrose intake did not differ significantly across groups of rats assigned to receive pretreatment with vehicle (43.3 ± 5.9 pellets/session) or M100907 at doses of 0.01 mg/kg (40.0 ± 6.1 pellets/session) or 0.1 mg/kg (42.1 (± 2.8) pellets/session) [F(2, 17) = 0.24; ns].
Extinction Training
The extinction criterion was achieved for all animals (n=20) in Experiment 4 by session 17 of extinction training (data not shown); a main effect of session was observed [F(16, 176) = 28.42 = p < 0.05].
Effects of M100907 on cue-induced reinstatement
M100907 (0.01 or 0.1 mg/kg i.p.) had no effect on active [F(2, 17) = 1.88, ns] or inactive lever presses [F(2, 17) = 0.55, ns] or the latency to first response [F(2,17) = 1.25, ns] in cue-induced reinstatement of sucrose-seeking sessions (Figure 6).
Figure 6. Effects of M100907 on cue-induced reinstatement in a sucrose self-administration paradigm.
Lever presses (± SEM) on the active (filled bars) and inactive levers (open bars) on the test day for reinstatement of extinguished sucrose-seeking behavior. Each active lever press resulted in the presentation of the conditioned stimuli in the absence of sucrose delivery. In a between-subjects design of cue-evoked reinstatement (see Methods, Experiment 4), delivery of sucrose-associated cues significantly increased the number of presses on the previously-active lever [t(7) = −8.40 = p < 0.05] after vehicle (VEH) pretreatment vs. the average number of previously-active lever presses observed on the three preceding extinction sessions (EXT; #p<0.05). However, acute pretreatment with M100907 (0.01 or 0.1 mg/kg) did not alter sucrose-seeking behavior relative to vehicle treatment (n = 6–8; p>0.05).
DISCUSSION
Our results demonstrate for the first time that selective blockade of the 5-HT2AR effectively reduced reinstatement of cocaine-seeking maintained by cocaine-paired cues, identifying a specific role for the 5-HT2AR in reinstatement under self-administration conditions designed to minimize food deprivation stress and associated 5-HT alterations (Chaouloff et al., 1999; Vickers & Dourish 2004; Halford et al., 2007). We show that the response rate during the initial extinction session predicts the maximal susceptibility to M100907-induced suppression of cue-evoked reinstatement, identifying a behavioral phenotype potentially useful in analyses of individual differences in cue-induced cocaine-seeking behavior. The present study also confirms and extends observations to suggest that the reinforcing effects of cocaine are resistant to blockade of the 5-HT2AR (Fletcher et al., 2002) during ongoing self-administration, such that a wide range of M100907 doses was ineffective and neither the rates of responding on a FR task (present results; Fantegrossi et al., 2002) nor the breakpoint on a PR task (Fletcher et al., 2002) were altered. As the reinforcing efficacy of a drug is best taken in the context of both procedures (Depoortere, Li, Lane, & Emmett-Oglesby 1993; Stafford, LeSage, & Glowa 1998; Arnold et al., 1997), the observation that M100907 did not alter rates of responding (present study; Fantegrossi et al., 2002) or the breakpoint for cocaine self-administration (Fletcher et al., 2002) further strengthens the argument that the incentive-motivational effects of cocaine during cocaine self-administration are resistant to pharmacological antagonism of the 5-HT2AR.
Cocaine self-administration was trained in naïve rats with no prior exposure to food deprivation and associated pretraining in an operant task for an appetitive, non-drug reward. Food deprivation is associated with increased 5-HT turnover (Chaouloff et al., 1985; Tsujii et al., 1988; McBlane et al., 1994; Popova et al., 2001), increased cortical 5-HT2AR density and increased functional responses to pharmacological stimulation of the 5-HT2AR (Torda, Culman, Cechova, & Murgas 1988; Chaouloff, Baudrie, & Coupry 1994; Yamada, Watanabe, Nankai, & Toru 1995; Davis, Heal, & Stanford 1995; Stanford 1996; Matuszewich & Yamamoto 2003; Stamatakis & Hetherington 2003). Thus, under conditions that eliminate the possible influence of deprivation-induced changes in 5-HT function, we can confidently state that blockade of the 5-HT2AR does not interfere with the reinforcing effects of cocaine and rule out the possible influence of food deprivation stress as an important independent variable in observed effects (Fletcher et al., 2002; Fantegrossi et al., 2002).
That the attenuation of cocaine-seeking behavior by M100907 is attributable to a selective blockade of the 5-HT2AR is supported by several lines of evidence. Similar low doses of M100907 have been shown to selectively block the in vivo effects of preferential 5-HT2AR agonists (but not 5-HT2CR agonists) in rats, indicating an effective, functional blockade of 5-HT2AR at the chosen doses of M100907 (Hitchcock et al., 1997; Wettstein et al., 1999; Dekeyne, Girardon, & Millan 1999; Vickers et al., 2001; McCreary et al., 2003; Gresch, Barrett, Sanders-Bush, & Smith 2007). Furthermore, analogous doses of M100907 have been shown to block the locomotor stimulant (McMahon & Cunningham 1999; Fletcher et al., 2002) and discriminative stimulus effects of cocaine (McMahon et al., 1999) without evidence of behavioral disruption (i.e., locomotor activity) when administered alone (McMahon & Cunningham 2001; Bankson & Cunningham 2002; Fletcher et al., 2002; McCreary et al., 2003; Szucs, Frankel, McMahon, & Cunningham 2005). In keeping with this observation, the administration of M100907 was not accompanied by delayed initiation of responding or decreased numbers of inactive lever responses (employed as an index of behavioral disruption), during the self-administration period or cue-evoked reinstatement of cocaine-seeking behavior (present results). In addition, the effects of M100907 were specific to cocaine-associated cues as the same doses of M100907 failed to alter operant responding for cues associated with sucrose self-administration.
The control afforded by M100907 over cue-evoked reinstatement, but not over the reinforcing effects of cocaine, is imminently interesting in light of observations to suggest that the neural mechanisms underlying the incentive-motivational aspects of drugs and cues conditioned to drugs are dissociable in experienced subjects (Meil & See 1997; Everitt & Wolf 2002; Grimm & See 2000; Kruzich, Congleton, & See 2001; Kantak et al., 2002; McLaughlin & See 2003; Robinson & Berridge 2003). Elevated dopamine (DA) efflux in the mesoaccumbens pathway is an important mediator of the reinforcing effects of cocaine (Pettit & Justice 1989; Hemby et al., 1997; Hurd & Ponten 2000; Sizemore, Co, & Smith 2000; Weiss et al., 2000) while “mild” increases in DA efflux in the mesocorticolimbic circuit [e.g., prefrontal cortex (PFC), amygdala] has been linked to expression of cue-evoked reinstatement (Neisewander et al., 1996; Tran-Nguyen et al., 1998; Ito, Dalley, Robbins, & Everitt 2002; Phillips et al., 2003; Bradberry et al., 2004; Vanderschuren, Di, & Everitt 2005). The 5-HT2AR regulates DA output in the PFC (Bortolozzi et al., 2005; Pehek et al., 2006), for example, and the selective 5-HT2AR antagonist may block the elevation of DA levels in the PFC or amygdala evoked by cocaine-associated cues (Tran-Nguyen et al., 1998; Di Pietro, Black, & Kantak 2006; Ikegami, Olsen, D’Souza, & Duvauchelle 2007), but not cocaine-evoked accumbal DA efflux during self-administration (Parsons, Koob, & Weiss 1995; Ahmed, Lin, Koob, & Parsons 2003). Alternatively, cocaine-taking bouts are associated with significant elevations of 5-HT efflux (Parsons et al., 1995; Parsons, Koob, & Weiss 1996; Sizemore et al., 2000; Howes et al., 2000) and consequent 5-HT2AR desensitization/downregulation (Eison, Eison, Yocca, & Gianutsos 1989; Leysen, Janssen, & Niemegeers 1989; Premont, Inglese, & Lefkowitz 1995) may underlie the resistance of the reinforcing effects to 5-HT2AR blockade. The abrupt cessation of drug-taking prior to extinction training in the face of dynamic attempts to reestablish the homeostatic 5-HT function may lead to heightened serotonergic sensitivity. This enhanced sensitivity may contribute to a higher susceptibility to reinstatement upon exposure to drug-related stimuli and to the responsiveness to the selective 5-HT2AR antagonist. The observation that the research design employed during the reinstatement phase influences the dose-effect curve for M100907 supports this idea. In fact, supersensitive 5-HT2AR function is reported during withdrawal from repeated, experimenter-delivered cocaine exposure (Darmani, Martin, & Glennon 1992; Levy et al., 1994; Baumann & Rothman 1996; Filip, Bubar, & Cunningham 2004; Carrasco et al., 2006) and in abstinent human cocaine abusers (Handelsman et al., 1998; Patkar et al., 2006; Ghitza et al., 2007), although the cellular mechanisms that underlie this altered sensitivity are unclear (Zeigler, Lipton, Toga, & Ellison 1991; Javaid, Sahni, Pandey, & Davis 1993; Johnson, Fiorella, & Rabin 1993; Neisewander, Lucki, & McGonigle 1994: Carrasco et al., 2006).
Drug-associated stimuli evoke memories for the subjective effects of the drug which are postulated to motivate drug-seeking behavior and subsequent relapse in abstinent individuals (O’Brien, Childress, Ehrman, & Robbins 1998). There is a particular interest in the establishment of objective endophenotypic indicators of cocaine-paired “cue reactivity” to assist in prediction of both relapse and treatment effectiveness (Niaura et al., 1998; Modesto-Lowe & Kranzler 1999; Carter & Tiffany 1999; Ooteman et al., 2006). Individual differences in the intensity of craving in human addicts (Avants, Margolin, Kosten, & Cooney 1995; De La Garza, Newton, & Kalechstein 2005) and the degree of drug- or cue-evoked reinstatement in rats (Kruzich et al., 1999; Sutton et al., 2000; Baker, Tran-Nguyen, Fuchs, & Neisewander 2001; Deroche-Gamonet, Martinez, Le, & Piazza 2003; Homberg, Raaso, Schoffelmeer, & De Vries 2004) have been observed upon presentation of cocaine-paired cues or the drug itself. In the present study, the degree of responding during the first session of extinction predicted the magnitude of reinstatement supported by the presentation of cocaine-associated cues, similar to previous findings (Kruzich et al., 1999). Two subpopulations of rats were identified based upon this differential extinction responsiveness, identifying a behavioral phenotype potentially useful in analyses of individual differences in cue reactivity. Furthermore, high extinction responsiveness was associated with greater susceptibility to suppression of cue-evoked reinstatement after the 5-HT2AR antagonist. These findings support the importance of assessing individual responses to various cocaine-paired stimuli in both preclinical and clinical studies to evaluate the benefit of specific treatment protocols.
The present findings suggest that a selective 5-HT2AR antagonist may be therapeutically useful to curtail withdrawal and craving during the initial period after termination of cocaine use (Coffey, Dansky, Carrigan, & Brady 2000). In practice a medication with efficacy as an abstinence enhancer would be administered to cocaine-dependent patients beginning at termination of cocaine use and continuing for a period of time. Few preclinical studies of chronic medication treatment on cocaine-seeking during withdrawal from cocaine self administration have been conducted (Baker et al., 2001), thus future studies are required to determine whether repeated administration of the 5-HT2AR antagonist M100907 may equally reduce drug-seeking behavior in the rat. Until recently, the paucity of selective 5-HT2AR antagonists available clinically has limited the ability to test this hypothesis in humans, however, M100907 (volinanserin) is in late clinical trials for sleep disorders (www.clinicaltrials.gov) and its launch in the next few years will ultimately provide the opportunity to evaluate this pharmacological approach to treatment in cocaine addiction.
Acknowledgments
The work in this study was supported by grants from the National Institute on Drug Abuse DA 00260, DA 06511 and DA 020087. This work was also partially supported by Nic Dhonnchadha et al., 2008 the intramural research program of the National Institute on Drug Abuse and the National Institute of Diabetes, Digestive and Kidney Diseases.
References
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. Journal of Neurochemistry. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology, Biochemistry and Behavior. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addiction Behavior. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berlin) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Gage HD, Bounds MC, Garg PK, Garg S, et al. Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology. 2008;33:219–225. doi: 10.1038/sj.npp.1301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3, 4-methylenedioxymethamphetamine on locomotor activity: Role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology. 2002;26:40–52. [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Chronic cocaine exposure potentiates prolactin and head shake responses to 5-HT2 receptor stimulation in rats. Neuropharmacology. 1996;35:295–301. doi: 10.1016/0028-3908(95)00166-2. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: Similarities to major depression in humans. Biological Psychiatry. 1998;44:578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- Baxter G, Kennett G, Blaney F, Blackburn T. 5-HT2 receptor subtypes: A family re-united? Trends in Pharmacological Sciences. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Meltzer HY, Li Z, Dai J, Alboszta AR, Ichikawa J. SR46349-B, a 5-HT(2A/2C) receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology. 2002;27:430–441. doi: 10.1016/S0893-133X(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacology Biochemistry and Behavior. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. Journal of Neurochemistry. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Rubino SR. Phasic alterations in dopamine and serotonin release in striatum and prefrontal cortex in response to cocaine predictive cues in behaving rhesus macaques. Neuropsychopharmacology. 2004;29:676–685. doi: 10.1038/sj.npp.1300386. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Current Topics in Medicinal Chemistry. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin (2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berlin) 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN. Conditioned cocaine induced hyperactivity: An association with increased medial prefrontal cortex serotonin. Behavioural Brain Research. 1994;62:177–185. doi: 10.1016/0166-4328(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Carr KD. Chronic food restriction: Enhancing effects on drug reward and striatal cell signaling. Physiology & Behavior. 2007;91:459–472. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van De Kar LD, Sullivan NR, Landry M, Garcia F, Muma NA, et al. Cocaine-mediated supersensitivity of 5-HT2A receptors in hypothalamic paraventricular nucleus is a withdrawal-induced phenomenon. Neuroscience. 2006;143:7–13. doi: 10.1016/j.neuroscience.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug and Alcohol Dependence. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chaouloff F, Baudrie V, Coupry I. Effects of chlorisondamine and restraint on cortical [3H]ketanserin binding, 5-HT2A receptor-mediated head shakes, and behaviours in models of anxiety. Neuropharmacology. 1994;33:449–456. doi: 10.1016/0028-3908(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Elghozi JL, Guezennec Y, Laude D. Effects of conditioned running on plasma, liver and brain tryptophan and on brain 5-hydroxytryptamine metabolism of the rat. British Journal of Pharmacology. 1985;86:33–41. doi: 10.1111/j.1476-5381.1985.tb09432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: A prospective study of mood, sleep and withdrawal symptoms. Drug and Alcohol Dependence. 2000;59:277–286. doi: 10.1016/s0376-8716(99)00126-x. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, et al. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992;11:112–123. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, et al. Starvation: Early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Repeated administration of low doses of cocaine enhances the sensitivity of 5-HT2 receptor function. Pharmacology Biochemistry and Behavior. 1992;41:519–527. doi: 10.1016/0091-3057(92)90367-o. [DOI] [PubMed] [Google Scholar]
- Davis S, Heal DJ, Stanford SC. Long-lasting effects of an acute stress on the neurochemistry and function of 5-hydroxytryptaminergic neurones in the mouse brain. Psychopharmacology (Berlin) 1995;118:267–272. doi: 10.1007/BF02245954. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60-0175: A pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Newton TF, Kalechstein AD. Risperidone diminishes cocaine-induced craving. Psychopharmacology (Berlin) 2005;178:347–350. doi: 10.1007/s00213-004-2010-8. [DOI] [PubMed] [Google Scholar]
- De Paulis T. M-100907 (Aventis) Current Opinions in Investigative Drugs. 2001;2:123–132. [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacology Biochemistry and Behavior. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le MM, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. European Journal of Neuroscience. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Martinez A, Le MM, Piazza PV. Relationships between individual sensitivity to CS- and cocaine-induced reinstatement in the rat. Psychopharmacology (Berlin) 2003;168:201–207. doi: 10.1007/s00213-002-1306-9. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. European Journal of Neuroscience. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Eison AS, Eison MS, Yocca FD, Gianutsos G. Effects of imipramine and serotonin-2 agonists and antagonists on serotonin-2 and beta-adrenergic receptors following noradrenergic or serotonergic denervation. Life Sciences. 1989;44:1419–1427. doi: 10.1016/0024-3205(89)90400-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: A neural systems perspective. Journal of Neuroscience. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: Serotonergic involvement. Psychopharmacology (Berlin) 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacological Reports. 2005;57:35–46. [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: Acute and chronic pharmacological analyses. Journal of Pharmacology and Experimental Therapeutics. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives of General Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Rothman RB, Gorelick DA, Henningfield JE, Baumann MH. Serotonergic responsiveness in human cocaine users. Drug and Alcohol Dependence. 2007;86:207–213. doi: 10.1016/j.drugalcdep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin) 2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. Journal of Pharmacology and Experimental Therapeutics. 2007;320:662–669. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. Journal of Pharmacology and Experimental Therapeutics. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: Effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Kahn RS, Sturiano C, Rinaldi PJ, Gabriel S, Schmeidler JP, et al. Hostility is associated with a heightened prolactin response to meta-chlorophenylpiperazine in abstinent cocaine addicts. Psychiatry Research. 1998;80:1–12. doi: 10.1016/s0165-1781(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berlin) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Serotonin and drug reward: Focus on 5-HT(2C) receptors. European Journal of Pharmacology. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Lister S, Fischer TR, Wettstein JG. Disruption of latent inhibition in the rat by the 5-HT2 agonist DOI: Effects of MDL 100,907, clozapine, risperidone and haloperidol. Behavioural Brain Research. 1997;88:43–49. doi: 10.1016/s0166-4328(97)02315-2. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Raaso HS, Schoffelmeer AN, De Vries TJ. Individual differences in sensitivity to factors provoking reinstatement of cocaine-seeking behavior. Behavioural Brain Research. 2004;152:157–161. doi: 10.1016/j.bbr.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: Relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berlin) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Ponten M. Cocaine self-administration behavior can be reduced or potentiated by the addition of specific dopamine concentrations in the nucleus accumbens and amygdala using in vivo microdialysis. Behavioural Brain Research. 2000;116:177–186. doi: 10.1016/s0166-4328(00)00271-0. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, D’Souza MS, Duvauchelle CL. Experience-dependent effects of cocaine self-administration/conditioning on prefrontal and accumbens dopamine responses. Behavioral Neuroscience. 2007;121:389–400. doi: 10.1037/0735-7044.121.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. Journal of Neuroscience. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid JI, Sahni SK, Pandey SC, Davis JM. Repeated cocaine administration does not affect 5-HT receptor subtypes (5-HT1A, 5-HT2) in several rat brain regions. European Journal of Pharmacology. 1993;238:425–429. doi: 10.1016/0014-2999(93)90880-q. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Fiorella D, Rabin RA. Effects of chronic cocaine administration on the serotonergic system in the rat brain. Pharmacology Biochemistry and Behavior. 1993;46:289–293. doi: 10.1016/0091-3057(93)90355-w. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. Journal of Neuroscience. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, et al. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. Journal of Pharmacology and Experimental Therapeutics. 1996;277:968–981. [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: A researcher’s handbook. Englewood Cliffs, NJ: Prentice Hall; 2004. [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. Journal of Pharmacology and Experimental Therapeutics. 1976;199:649–661. [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behavioral Neuroscience. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grimm JW, Rustay NR, Parks CD, See RE. Predicting relapse to cocaine-seeking behavior: A multiple regression approach. Behavioural Pharmacology. 1999;10:513–521. doi: 10.1097/00008877-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. Journal of Neuroscience. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AD, Rittenhouse PA, Li Q, Yracheta J, Kunimoto K, Van De Kar LD. Influence of repeated cocaine exposure on the endocrine and behavioral responses to stress in rats. Psychopharmacology (Berlin) 1994;113:547–554. doi: 10.1007/BF02245238. [DOI] [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Current Drug Targets CNS and Neurological Disorders. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Janssen PFM, Niemegeers CJE. Rapid desensitization and down regulation of 5-HT2 receptors by DOM treatment. European Journal of Pharmacology. 1989;163:145–149. doi: 10.1016/0014-2999(89)90409-3. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. American Journal of Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Research. 1996;724:251–255. doi: 10.1016/0006-8993(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia in male rats. Psychopharmacology (Berlin) 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- McBlane JW, Handley SL. Effects of two stressors on behaviour in the elevated X- maze: Preliminary investigation of their interaction with 8-OH-DPAT. Psychopharmacology (Berlin) 1994;116:173–182. doi: 10.1007/BF02245060. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Filip M, Cunningham KA. Discriminative stimulus properties of (+/−)-fenfluramine: The role of 5-HT2 receptor subtypes. Behavioral Neuroscience. 2003;117:212–221. doi: 10.1037/0735-7044.117.2.212. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berlin) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Cunningham KA. Antagonism of the locomotor stimulant and discriminative stimulus effects of cocaine in rats by the 5-HT2A receptor antagonist. MDL. 1999;100:907. Society for Neuroscience Abstract, 254, 561. [Google Scholar]
- McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. Journal of Pharmacology and Experimental Therapeutics. 2001;297:357–363. [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behavioural Brain Research. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Kranzler HR. Using cue reactivity to evaluate medications for treatment of cocaine dependence: A critical review. Addiction. 1999;94:1639–1651. doi: 10.1046/j.1360-0443.1999.941116393.x. [DOI] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends in Pharmacological Sciences. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nader MA, Thompson T. Interaction of reinforcement history with methadone on responding maintained under a fixed-interval schedule. Pharmacology Biochemistry and Behavior. 1989;32:643–649. doi: 10.1016/0091-3057(89)90011-7. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Clinical Center. An investigation of the antidepressant efficacy of the 5-HT2A antagonist, M100907, in combination with Citalopram in treatment resistant depression. 2005 Retrieved March 21, 2006, from http://clinicaltrials.gov/ct/show/NCT00070694.
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behavioural Pharmacology. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Lucki I, McGonigle P. Time-dependent changes in sensitivity to apomorphine and monoamine receptors following withdrawal from continuous cocaine administration in rats. Synapse. 1994;16:1–10. doi: 10.1002/syn.890160102. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. Serials Solutions [Context Link] [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Manzardo AM, Polis I, Stouffer DG, Parsons LH. Biphasic alterations in serotonin-1B (5-HT1B) receptor function during abstinence from extended cocaine self-administration. Journal of Neurochemistry. 2006;99:1363–1376. doi: 10.1111/j.1471-4159.2006.04163.x. [DOI] [PubMed] [Google Scholar]
- O’Leary TA, Rohsenow DJ, Martin R, Colby SM, Eaton CA, Monti PM. The relationship between anxiety levels and outcome of cocaine abuse treatment. American Journal of Drug and Alcohol Abuse. 2000;26:179–194. doi: 10.1081/ada-100100599. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MWJ, Vserheul R, Schippers GM, Brink W. Measuring craving: An attempt to connect subjective craving with cue reactivity. Alcoholism: Clinical and Experimental Research. 2006;30:57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. Journal of Pharmacology and Experimental Therapeutics. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behavioural Brain Research. 1996;73:225–228. doi: 10.1016/0166-4328(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Fernandez-Garcia E, Hutson PH, Patel S. An in vivo binding assay to determine central alpha(1)-adrenoceptor occupancy using [(3)H]prazosin. Brain Research Brain Research Protocols. 2001;8:191–198. doi: 10.1016/s1385-299x(01)00110-6. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Berrettini WH, Mannelli P, Gopalakrishnan R, Hoehe MR, Bilal L, et al. Relationship between serotonin transporter gene polymorphisms and platelet serotonin transporter sites among African-American cocaine-dependent individuals and healthy volunteers. Psychiatric Genetics. 2004;14:25–32. doi: 10.1097/00041444-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Hill KP, Gopalakrishnan R, Berrettini WH. Relationship of disinhibition and aggression to blunted prolactin response to meta-chlorophenylpiperazine in cocaine-dependent patients. Psychopharmacology (Berlin) 2006;185:123–132. doi: 10.1007/s00213-005-0261-7. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacology Biochemistry and Behavior. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Popova NK, Ivanova LN, Amstislavskaya TG, Melidi NN, Naumenko KS, Maslova LN, et al. Brain serotonin metabolism during water deprivation and hydration in rats. Neuroscience and Behavioral Physiology. 2001;31:327–332. doi: 10.1023/a:1010346904526. [DOI] [PubMed] [Google Scholar]
- Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. The Federation of American Societies for Experimental Biology. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schenk S. Effects of the serotonin 5-HT(2) antagonist, ritanserin, and the serotonin 5-HT(1A) antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacology Biochemistry and Behavior. 2000;67:363–369. doi: 10.1016/s0091-3057(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effects of DOI by MDL 100,907 and the “atypical” antipsychotics, clozapine and risperidone. European Journal of Pharmacology. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berlin) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. New York: Chapman & Hall/CRC; 2007. [Google Scholar]
- Sizemore GM, Co C, Smith JE. Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology (Berlin) 2000;150:391–398. doi: 10.1007/s002130000456. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacology (Berlin) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Hetherington MM. Neuroimaging in eating disorders. Nutritional Neuroscience. 2003;6:325–334. doi: 10.1080/10284150310001640338. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Research. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Stanford SC. Stress: A major variable in the psychopharmacologic response. Pharmacology Biochemistry and Behavior. 1996;54:211–217. doi: 10.1016/0091-3057(95)02107-8. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology. 2000;22:626–641. doi: 10.1016/S0893-133X(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Szucs RP, Frankel PS, McMahon LR, Cunningham KA. Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behavioral Neuroscience. 2005;119:1173–1183. doi: 10.1037/0735-7044.119.5.1173. [DOI] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Yamawaki S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. European Journal of Pharmacology. 1997;333:123–128. doi: 10.1016/s0014-2999(97)01126-6. [DOI] [PubMed] [Google Scholar]
- Torda T, Culman J, Cechova E, Murgas K. 3-H-ketanserin (serotonin type 2) binding in the rat frontal cortex: Effect of immobilization stress. Endocrinologic Experimentalis. 1988;22:99–105. [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, et al. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Nakai Y, Fukata J, Nakaishi S, Takahashi H, Usui T, et al. Monoamine metabolism and its responses to food deprivation in the brain of Zucker rats. Physiology & Behavior. 1988;44:495–500. doi: 10.1016/0031-9384(88)90311-3. [DOI] [PubMed] [Google Scholar]
- Urbain C, Poling A, Millam J, Thompson T. d-amphetamine and fixed-interval performance: Effects of operant history. Journal of the Experimental Analysis of Behavior. 1978;29:385–392. doi: 10.1901/jeab.1978.29-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di CP, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. Journal of Neuroscience. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT. Serotonin receptor ligands and the treatment of obesity. Current Opinion in Investigational Drugs. 2004;5:377–388. [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, et al. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacology Biochemistry and Behavior. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL. Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: Evidence for species differences. Journal of Pharmacology and Experimental Therapeutics. 1996;276:720–727. [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berlin) 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proceedings of the National Academy of Sciences USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Progress in Neuropsychopharmacology and Biological Psychiatry. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Current Opinion in Neurobiology. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Yamada S, Watanabe A, Nankai M, Toru M. Acute immobilization stress reduces (±)DOI-induced 5-HT2A receptor-mediated head shakes in rats. Psychopharmacology (Berlin) 1995;119:9–14. doi: 10.1007/BF02246047. [DOI] [PubMed] [Google Scholar]
- Zeigler S, Lipton J, Toga A, Ellison G. Continuous cocaine administration produces persisting changes in brain neurochemistry and behavior. Brain Research. 1991;552:27–35. doi: 10.1016/0006-8993(91)90655-f. [DOI] [PubMed] [Google Scholar]