Abstract
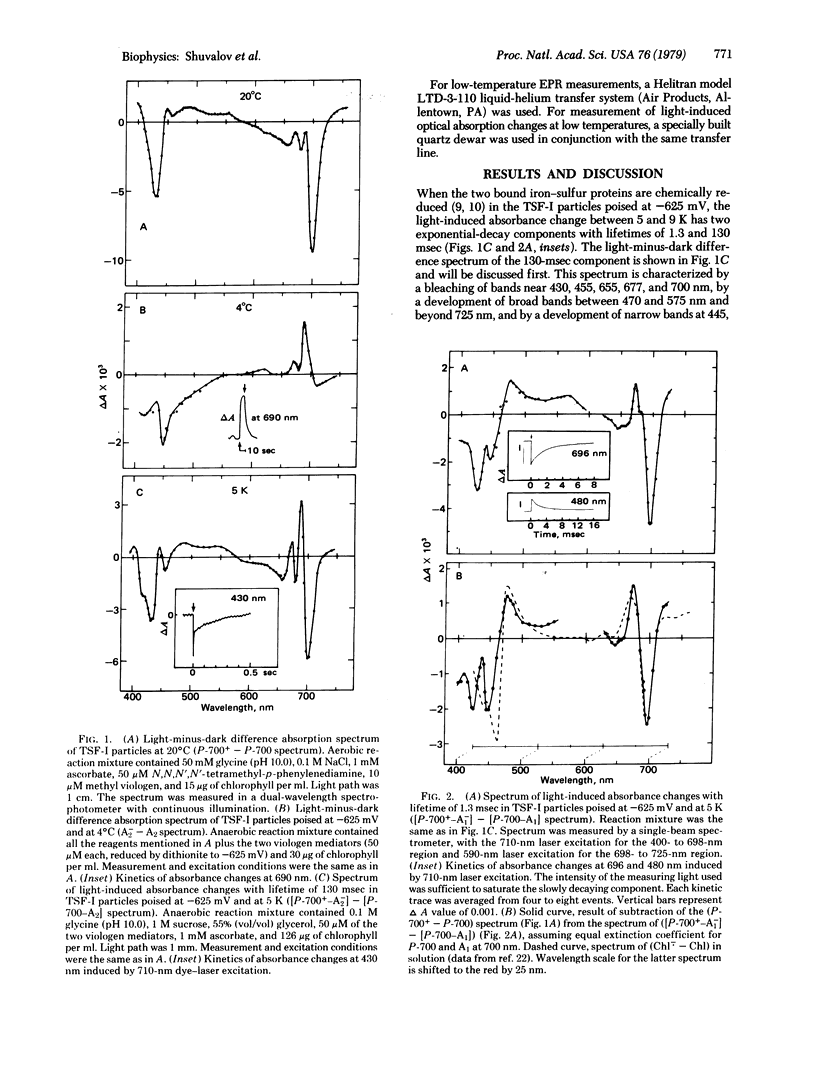
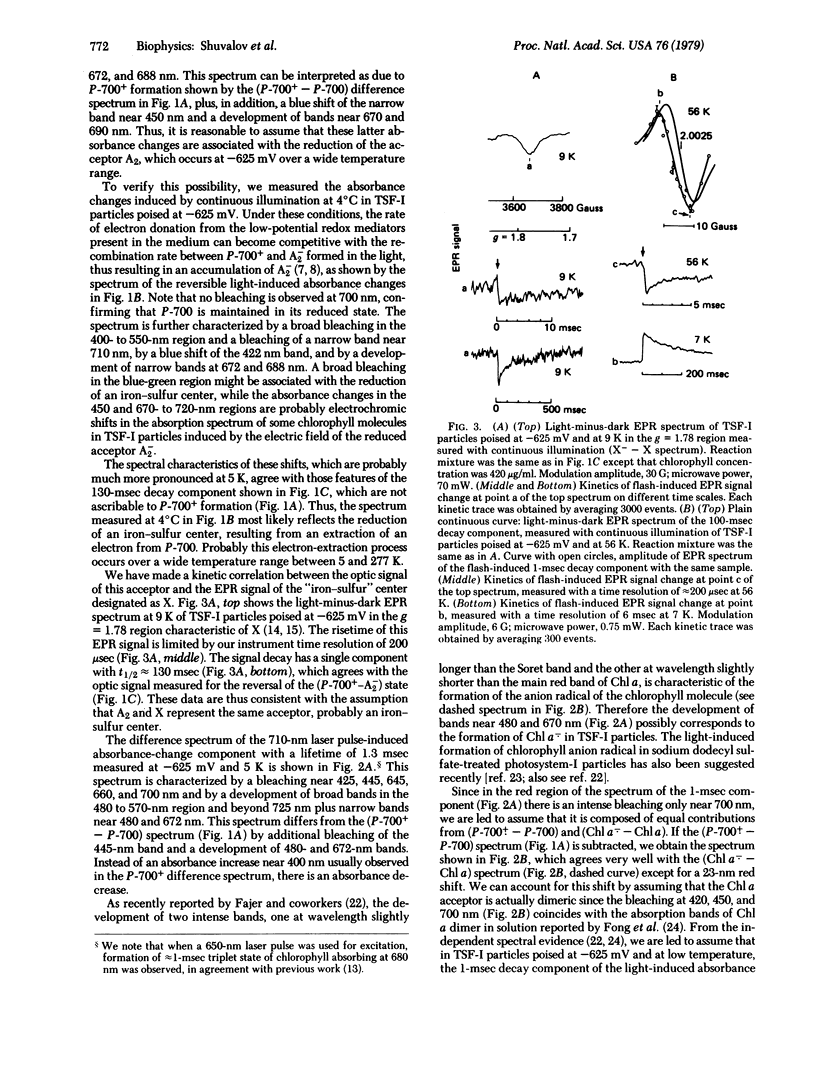
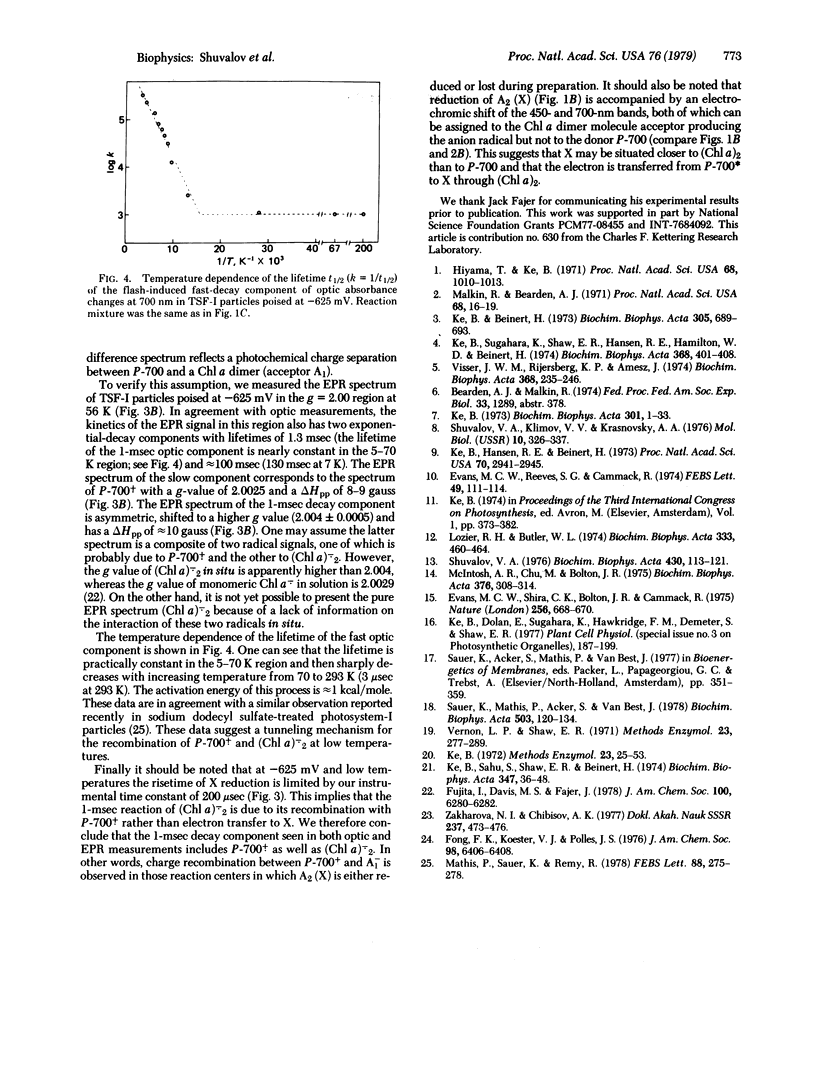
Triton-fractionated photosystem-I particles poised at -625 mV, where the two bound iron-sulfur proteins are reduced, have been studied by optical and electron paramagnetic resonance spectroscopies from 293 to 5 K. At 5-9 K, these particles exhibit two decay components with lifetimes of 1.3 and 130 msec in the laser pulse-induced absorption and electron paramagnetic resonance signal changes. Spectral properties of the 130-msec decay component reflect the charge separation between P-700 and some iron-sulfur center having a broad optical absorbance in the 400- to 550-nm region and a previously reported electron paramagnetic resonance signal with g = 1.78, 1.88, and 2.08. Spectral properties of the 1-msec decay component indicate photoinduced charge separation between P-700 and a chlorophyll a dimer having absorption bands at 420, 450, and 700 nm. It is assumed that these two acceptors participate in the electron transfer from P-700* to the bound iron-sulfur proteins.
Keywords: electron transfer, chlorophyll photoreduction
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon K. E., Sugahara K., Shaw E. R., Hansen R. E., Hamilton W. D., Beinert H. Kinetics of appearance and disappearance of light-induced EPR signals of P700+ and iron-sulfur proteins(s) at low temperature. Biochim Biophys Acta. 1974 Dec 19;368(3):401–408. doi: 10.1016/0005-2728(74)90185-6. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. A new photosynthetic pigment, "P430": its possible role as the priary electron acceptor of photosystem I. Proc Natl Acad Sci U S A. 1971 May;68(5):1010–1013. doi: 10.1073/pnas.68.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B., Beinert H. Evidence for the identity of P430 of Photosystem I and chloroplast-bound iron-sulfur protein. Biochim Biophys Acta. 1973 Jun 28;305(3):689–693. doi: 10.1016/0005-2728(73)90094-7. [DOI] [PubMed] [Google Scholar]
- Ke B. Flash kinetic spectrophotometry. Methods Enzymol. 1972;24:25–53. doi: 10.1016/0076-6879(72)24053-8. [DOI] [PubMed] [Google Scholar]
- Ke B., Hansen R. E., Beinert H. Oxidation-reduction potentials of bound iron-sulfur proteins of photosystem I. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2941–2945. doi: 10.1073/pnas.70.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B., Sahu S., Elwood S., Beinert H. Further characterization of a photosystem-II particle isolated from spinach chloroplasts by triton treatment: the reaction-center components. Biochim Biophys Acta. 1974 Apr 23;347(1):36–48. doi: 10.1016/0005-2728(74)90198-4. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A. R., Chu M., Bolton J. R. Flash photolysis electron spin resonance studies of the electron acceptor species at low temperatures in photosystem I of spinach subchloroplast particles. Biochim Biophys Acta. 1975 Feb 17;376(2):308–314. doi: 10.1016/0005-2728(75)90023-7. [DOI] [PubMed] [Google Scholar]
- Sauer K., Mathis P., Acker S., van Best J. A. Electron acceptors associated with P-700 in Triton solubilized photosystem I particles from spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):120–134. doi: 10.1016/0005-2728(78)90166-4. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Klimov V. V., Krasnovskii A. A. Issledovanie pervichnykh fotoprotsessov v legkikh fragmentakh khloroplastov. Mol Biol (Mosk) 1976 Mar-Apr;10(2):326–339. [PubMed] [Google Scholar]
- Shuvalov V. A. The study of the primary photoprocesses in photosystem I of chloroplasts. Recombination luminescence, chlorophyll triplet state and triplet-triplet annihilation. Biochim Biophys Acta. 1976 Apr 9;430(1):113–121. doi: 10.1016/0005-2728(76)90227-9. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Rijgersberg K. P., Amesz J. Light-induced reactions of ferredoxin and P700 at low temperatures. Biochim Biophys Acta. 1974 Nov 19;368(2):235–246. doi: 10.1016/0005-2728(74)90152-2. [DOI] [PubMed] [Google Scholar]



