Abstract
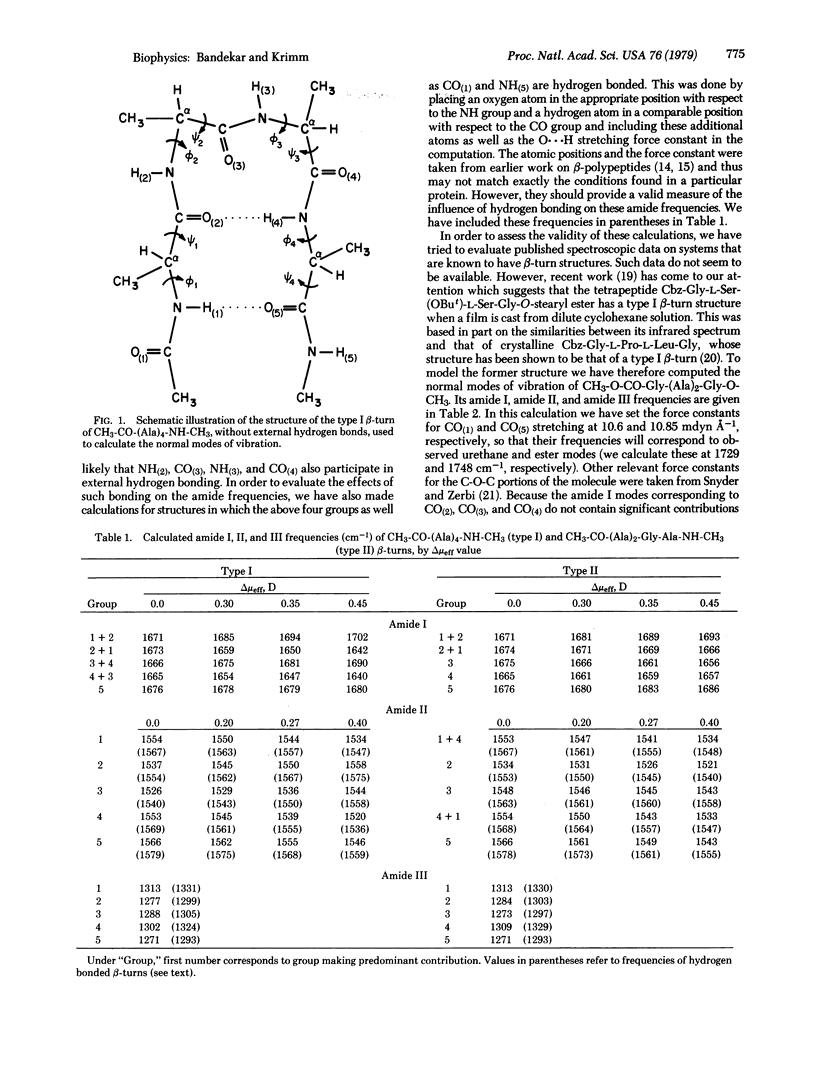
Normal vibration calculations have been done for a type I β-turn of CH3-CO-(Ala)4-NH-CH3 and a type II β-turn of CH3-CO-(Ala)2-Gly-Ala-NH-CH3. The force field was the one we refined for β-sheet and α-helical structures. A calculation was also done for CH3-O-CO-Gly-(Ala)2-Gly-O-CH3, which is an appropriate model for two tetrapeptides for which infrared data are available. The agreement between observed and calculated frequencies in this case is good, thus supporting the conclusions drawn from the above β-turn calculations. The most important result of the calculations is the prediction of bands near 1690 cm-1, a region heretofore associated only with the antiparallel-chain pleated sheet structure. This means that bands observed in proteins near 1690 cm-1 should be associated with the presence of β-turns as well as of β-sheets. We also find that a band near 1665 cm-1 is characteristic of type II turns.
Keywords: normal coordinate calculations, infrared and Raman spectra, chain folding
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Hsu S. L., Moore W. H., Krimm S. Vibrational spectrum of the unordered polypeptide chain: a Raman study of feather keratin. Biopolymers. 1976 Aug;15(8):1513–1528. doi: 10.1002/bip.1976.360150807. [DOI] [PubMed] [Google Scholar]
- Krimm S., Abe Y. Intermolecular interaction effects in the amide I vibrations of polypeptides. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2788–2792. doi: 10.1073/pnas.69.10.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz I. D. Protein folding. J Am Chem Soc. 1972 May 31;94(11):4009–4012. doi: 10.1021/ja00766a060. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Chain reversals in proteins. Biochim Biophys Acta. 1973 Apr 20;303(2):211–229. doi: 10.1016/0005-2795(73)90350-4. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. H., Krimm S. Transition dipole coupling in Amide I modes of betapolypeptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4933–4935. doi: 10.1073/pnas.72.12.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. H., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins. I. Polyglycine I. Biopolymers. 1976 Dec;15(12NA-NA-770103-770104):2439–2464. doi: 10.1002/bip.1976.360151210. [DOI] [PubMed] [Google Scholar]
- Moore W. H., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins. II. beta-poly(L-alanine) and beta-poly(L-anaylglycine). Biopolymers. 1976 Dec;15(12NA-NA-770103-770104):2465–2483. doi: 10.1002/bip.1976.360151211. [DOI] [PubMed] [Google Scholar]
- Peticolas W. L. Application of Raman spectroscopy to biological macromolecules. Biochimie. 1975;57(4):417–428. doi: 10.1016/s0300-9084(75)80328-2. [DOI] [PubMed] [Google Scholar]
- Rabolt J. F., Moore W. H., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins. 3. alpha-Poly(L-alanine). Macromolecules. 1977 Sep-Oct;10(5):1065–1074. doi: 10.1021/ma60059a034. [DOI] [PubMed] [Google Scholar]
- Shields J. E., McDowell S. T. Conformation of small peptides. I. Secondary structure in a tetrapeptide. J Am Chem Soc. 1967 May 10;89(10):2499–2500. doi: 10.1021/ja00986a055. [DOI] [PubMed] [Google Scholar]
- Shields J. E., McDowell S. T., Pavlos J., Gray G. R. Conformation of small peptides. II. Synthesis and infrared studies of small peptides. J Am Chem Soc. 1968 Jun 19;90(13):3549–3556. doi: 10.1021/ja01015a046. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Gaber B. P. Laser Raman scattering as a probe of protein structure. Annu Rev Biochem. 1977;46:553–572. doi: 10.1146/annurev.bi.46.070177.003005. [DOI] [PubMed] [Google Scholar]
- Ueki T., Ashida T., Kakudo M., Sasada Y., Katsube Y. Structure of p-bromocarbobenzoxy-glycyl-prolyl-leucyl-glycine. Acta Crystallogr B. 1969 Sep 15;25(9):1840–1849. doi: 10.1107/s056774086900481x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Long M. M., Ohnishi T., Jacobs M. Circular dichroism and absorption of the polytetrapeptide of elastin: a polymer model for the beta-turn. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1427–1433. doi: 10.1016/s0006-291x(74)80442-0. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Onishi T. Studies on the conformations and interactions of elastin. Proton magnetic resonance of the repeating tetramer. Biopolymers. 1974 Jun;13(6):1223–1242. doi: 10.1002/bip.1974.360130614. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]


