Abstract
Women disproportionately suffer from many deep tissue pain conditions. Experimental studies show that women have lower pain thresholds, higher pain ratings and less tolerance to a range of painful stimuli. Most clinical and epidemiological reports suggest female gonadal hormones modulate pain for some, but not all, conditions. Similarly, animal studies support greater nociceptive sensitivity in females in many deep tissue pain models. Gonadal hormones modulate responses in primary afferents, dorsal horn neurons and supraspinal sites, but the direction of modulation is variable. This review will examine sex differences in deep tissue pain in humans and animals focusing on the role of gonadal hormones (mainly estradiol) as an underlying component of the modulation of pain sensitivity.
Keywords: Pain, Human, Animal, Viscera, Muscle, Estrogen, Testosterone, Primary afferents, Dorsal horn neuron, Brain imaging
1. Introduction
Chronic pain affects more people than heart disease, diabetes and cancer combined (Institute of Medicine report: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (2011)) and visceral pain is the number one reason patients seek medical attention (International Association for the Study of Pain: Global Year Against Visceral Pain, http://www.iasp-pain.org/Content/NavigationMenu/GlobalYearAgainstPain/GlobalYearAgainstVisceralPain/PressRelease/default.htm). Furthermore, sex matters and it is generally accepted there is a sex difference in pain and analgesia (International Association for the Study of Pain: Global Year Against Pain in Women, http://www.iasp-pain.org/Content/NavigationMenu/GlobalYearAgainstPain/RealWomenRealPain/default.htm), although the magnitude of sex differences may be small and the direction can differ depending upon many factors including type of test, peripheral organ, and genetics (including species and strain in animals). In the patient population there are significantly more pain conditions/syndromes that are more prevalent in women than men (Berkley, 1997; Greenspan and Traub, 2013.). It is therefore imperative to understand what drives sex differences in deep tissue pain in order to better optimize treatment, perhaps on a sex directed basis. As such, how gonadal hormones modulate deep tissue pain is an important question.
Many excellent and informative reviews have been published on the general topic of sex differences in pain in the last 15 years (Aloisi, 2003; Berkley, 1997; Craft, 2007; Fillingim et al., 2009; Gintzler and Liu, 2012; Greenspan et al., 2007; Greenspan and Traub, 2013.; Heitkemper and Jarrett, 2008; Holdcroft and Berkley, 2006.; Hurley and Adams, 2008; Martin, 2009b; Mogil and Bailey, 2010; Racine et al., 2012a; Racine et al., 2012b; Riley et al., 1998; Unruh, 1996) and this review will not duplicate those efforts. In addition, we will not attempt to review deep tissue pain in general. Rather, the state of understanding of sex differences and the role of gonadal hormones in deep tissue pain conditions (visceral, somatic) will be examined although contrasts to superficial somatic pain will be addressed.
2. Human studies supporting a sex difference and hormonal modulation of deep tissue pain
Many chronic pain conditions involving deep tissue such as irritable bowel syndrome (IBS), fibromyalgia (FM), temporomandibular joint disorder (TMD), painful bladder syndrome/interstitial cystitis (PBS/IC), and chronic fatigue syndrome (CFS) have greater female prevalence or symptom severity (see (Berkley, 1997; Fillingim et al., 2009; Greenspan and Traub, 2013.; Unruh, 1996) for review). For example, women with IBS had greater abdominal pain vs. males and lower rectal discomfort thresholds than healthy women or men with IBS (Chang et al., 2006; Tang et al., 2012). Likewise, pain in TMD patients was greater in women compared to men (Schmid-Schwap et al., 2013). These conditions involve different peripheral organs but symptoms may coexist in the same patient (Aaron et al., 2000; Chang et al., 2003a; Kurland et al., 2006; Tietjen et al., 2010; Veale et al., 1991). Although genetic predisposition and previous psychological or physical (e.g., inflammation) challenges may contribute to the occurrence of deep tissue pain, in many cases no obvious pathophysiological signs in the peripheral tissue/nerve could be detected at the time of medical evaluation, pointing to dysregulation of central nervous system function as a potential cause of excessive pain.
2.1. Sex differences in deep tissue pain
Temporal summation (the progressive increase in response to a constant, repetitive stimulus) measures increases in central processing of nociceptive stimuli. In a recent review of experimental thermal and mechanical pain studies involving healthy volunteers, more than half of the studies reported increased temporal summation in females compared with males (Racine et al., 2012b). Another review that included patient populations and healthy volunteers reported greater temporal summation in women (Fillingim et al., 2009). Greater temporal summation of pain was observed in female TMD, lower back pain and IBS patients compared with healthy volunteers or male patients (George et al., 2007; Sarlani et al., 2007; Zhou et al., 2011). These studies indicate that central sensitization is more readily evoked in women and is further augmented in women suffering from deep tissue pain.
In contrast to temporal summation measuring pain facilitation, central pain modulation (CPM, formerly called DNIC (diffuse noxious inhibitory controls)) examines the ability of the central nervous system to inhibit pain. CPM measures the ability of a noxious conditioning stimulus to inhibit the response to a noxious test stimulus. Reduced CPM is associated with greater risk of chronic pain and comorbid pain conditions (see (Lewis et al., 2012; Staud, 2012; Yarnitsky, 2010) for review).
In healthy volunteers repetitive thermal stimulation evoked temporal summation. CPM inhibited the summation in healthy men, but had no effect in healthy women (Staud et al., 2003). In contrast, a different study reported temporal summation was not reduced by a conditioning stimulus in either sex, but CPM did reduce mean pain ratings and peak pain (Tousignant-Laflamme et al., 2008). In another study, distracting and painful conditioning stimuli significantly reduced heat pain intensity and unpleasantness ratings for both sexes, with significantly larger distraction effects on intensity ratings for men than women (Quiton and Greenspan, 2007). In a model of induced muscle pain two bilateral injections of hypertonic saline into the trapezius muscle induced greater pain ratings and lower pressure pain thresholds following the second injection in women compared to men. Correspondingly, the pressure pain threshold increased in men, but not women, in an area of referred pain (Ge et al., 2004; Ge et al., 2006). These data were interpreted to suggest men had greater inhibitory control mechanisms. In a review of 13 studies, approximately half showed less pain inhibition in women compared to men, the remaining studies reporting no sex difference (reviewed in (Fillingim et al., 2009)). These data suggest CPM may be less effective in women although how the experiments were conducted clearly affects the results.
Clinically, several studies have reported significantly less pain inhibition from CPM in women with fibromyalgia compared to healthy controls (Kosek and Hansson, 1997; Normand et al., 2011) although distraction improved the efficacy of CPM in this patient population (Staud et al., 2003). Female patients with IBS or TMD failed to show pain inhibition during CPM compared to healthy subjects and showed a different pattern of brain activation revealed by fMRI (Heymen et al., 2010; King et al., 2009; Wilder-Smith et al., 2004).
2.2. Hormonal regulation of deep tissue pain
A meta-analysis on studies of experimental pain reactivity in healthy women of reproductive age indicated women in the follicular phase had higher pain thresholds than later phases (Riley et al., 1999). A more recent review reported that increased reactivity to pain occurs during the perimenstrual phase or around ovulation (Martin, 2009a), suggesting lower levels of female gonadal hormones or rising /declining of these hormones is correlated to higher pain perception. In addition, greater CPM was observed in ovulatory phase than during the other times of the menstrual cycle (Rezaii et al., 2012; Tousignant-Laflamme and Marchand, 2009), suggesting pain perception and the intrinsic pain inhibitory system are both regulated by female gonadal hormones in healthy premenopausal women.
Several studies in chronic pain patients point to a negative correlation between female gonadal hormone levels and deep tissue pain severity. For example, a population-based questionnaire indicated IBS symptom severity increased after menopause and post-menopausal women had greater abdominal pain/discomfort symptoms compared to pre-menopausal women or men (Olafsdottir et al., 2012). However, another study reported abdominal pain severity decreased in women over age 50 (Palsson et al., 2003). In pre-menopausal women abdominal pain and rectal sensitivity increased during menses in IBS patients, but not healthy women (Houghton et al., 2002). A recent review concluded there is not enough data to make a firm conclusion regarding menopause and IBS symptomatology, although this same review concluded IBS symptoms were heightened around menses (Adeyemo et al., 2010). Estrogen withdrawal from oral contraceptive regimens contributed to pelvic pain and fluctuation in estrogen levels contributed to menstrual migraine (Bitzer, 2013; Mathew et al., 2013). Similarly, TMD pain ratings increased towards the end of the menstrual cycle and peaked during menstruation when the body had the lowest estrogen and progesterone level. A second peak of pain rating occurred around the time of ovulation when there was a rapid surge in estrogen level. The latter was not seen in women using oral contraceptives (LeResche et al., 2003; Slade et al., 2011).
The modulation of fibromyalgia pain by gonadal hormones is less certain. Several studies report FM did not fluctuate during the menstrual cycle (Alonso et al., 2004; Okifuji and Turk, 2006; Samborski et al., 2005), although another study reported menstrual cycle effects in about 50% of patients (Pamuk and Cakir, 2005). In postmenopausal women with fibromyalgia, hormone replacement did not affect pain ratings (Stening et al., 2011). In general, there was an increase in deep tissue pain symptoms around the time of menses and early menopause, at times of declining or low ovarian hormones, suggesting that estrogen and progesterone withdrawal may contribute either directly or indirectly to pain hypersensitivity.
2.3. Brain imaging of deep tissue pain
Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) measures differences/changes in brain area activation at rest and in response to tasks. It is well acknowledged that the limbic system including cingulate cortex, amygdala, hippocampal formation and other limbic related brain regions such as the insula cortex (IC) and prefrontal cortex are involved in emotion, cognition, anticipation of tasks, attention responses and arousal status of the body. Brain imaging studies indicate the anterior cingulate cortex (ACC), IC, the medial prefrontal cortex (MPFC) and other brain areas are activated by noxious somatic and visceral stimuli (Benson et al., 2012; Craig, 2002; Hayes and Northoff, 2012; Petrovic and Ingvar, 2002; Rainville et al., 1997; Tolle et al., 1999; Vogt et al., 1996).
Sex differences in brain region activation in response to thermal and electrical stimuli have been reported by several groups, with women showing more activation in MPFC (Straube et al., 2009) (Paulson et al., 1998), IC, thalamus (Paulson et al., 1998) and ACC (Derbyshire et al., 2002), but greater deactivation in anterior insula and dorsolateral prefrontal cortex (Moulton et al., 2006). Reduced activity in limbic brain areas during intense pain may reflect transient interruption of regional basal brain activity (Raichle et al., 2001; Vogt et al., 1996). On the other hand, deactivation in the MPFC and ACC have been reported when anticipating painful stimuli (Hsieh et al., 1999; Simpson, Jr. et al., 2001), possibly reflecting a cognitive coping mechanism (Petrovic and Ingvar, 2002).
There is also a sex difference in brain region activation in healthy human subjects in response to deep tissue stimuli. High intensity rectal distention activated the insula and ACC in both men and women, but left thalamus and ventral striatum only in men. Lower intensity distention and expectation of distention activated the insula to a greater extent in men while women had greater deactivation of the amygdala and midcingulate cortex (Berman et al., 2006). Likewise, in women anticipation of esophageal distention produced greater inactivation in the right amygdala and left parahippocampal gyrus and greater activation in the cuneus, precuneus, and supplementary motor area (Kano et al., 2013). However, rectal pain threshold was negatively correlated with distension-induced activation in the ACC, insula, thalamus and somatosensory cortex II in women, but positively correlated with activation of the insula in men, though there was no sex difference in threshold or pain ratings to rectal distention in healthy volunteers. Interestingly, pain ratings were negatively correlated with insula activation in women, but positively correlated with ACC activation in men (Benson et al., 2012). In addition, sex differences in the regional activation of the µ-opioid system in response to persistent pain induced by injecting hypertonic saline into the masseter muscle were detected. Healthy men showed larger magnitude of µ-opioid system activation in the anterior thalamus, ventral basal ganglia, and amygdala than women during the follicular phase (Zubieta et al., 2002). Taken together, these data suggest there is a sex difference in the brain areas involved in pain perception and modulation.
Patients with deep tissue pain show changes in regional brain activation. In the anterior insular cortex, clinical pain is preferentially processed more rostrally compared with acute experimental pain in healthy subjects (Schweinhardt et al., 2006). The shift in spatial representation under chronic pain conditions may underlie changes in the brain circuit involved in response to painful stimuli, partially explaining the affective/emotional changes in chronic pain patients. In female FM patients, greater activation of the middle ACC subregion has been demonstrated in response to somatic pressure when compared with their age-matched healthy controls (Chang et al., 2003b). In a male predominant study, patients with IBS had expanded activation of right prefrontal cortex, increased activation of rostral ACC and posterior cingulate cortices and reduced activation of brain stem and other brain areas in response to rectal distention (Naliboff et al., 2001). Similarly, female IBS patients showed increased activation of the middle ACC as compared with healthy controls in response to rectal distention (Chang et al., 2003b). Female IBS patients that were hypersensitive to colorectal distention also had greater activation of the insula and reduced deactivation in the pregenual ACC compared to normosensitive IBS patients and healthy controls (Larsson et al., 2012). In vulvodynia patients, noxious stimulation of the thumb evoked greater activation with the insula, dorsal midcingulate, posterior cingulate, and thalamus compared to healthy controls, suggesting central pathology in this disorder (Hampson et al., 2013).
A comparison between male and female IBS patients indicated there is a sex difference in brain areas activated by a visceral stimulus as well as anticipation of a visceral stimulus. Female patients showed greater activation in the ventromedial prefrontal cortex, right ACC and left amygdala in response to the visceral stimulus, whereas male patients showed greater activation of the right dorsolateral prefrontal cortex, insula, and dorsal pons/PAG, suggesting male and female patients with IBS differ in activation of brain areas involved in cognition, memory and emotional aspects of the pain experience, and the descending pain modulatory system (Berman et al., 2000; Labus et al., 2008; Naliboff et al., 2003).
Changes in functional connectivity of the ACC and other brain areas have also been observed under deep tissue pain conditions. Healthy females showed more periaqueductal grey (PAG) - mid-cingulate cortex connectivity while males showed more PAG connectivity with the left medial orbital prefrontal cortex, right insula and prefrontal cortex (Kong et al., 2010). In female FM patients the ACC and thalamus showed reduced connectivity to the amygdala, hippocampus, brainstem and orbitofrontal cortex compared with healthy controls (Jensen et al., 2012). In female TMD patients, increased connectivity between the left anterior insular cortex and pregenual ACC was observed at rest and during pressure pain stimuli. More importantly, the functional connectivity between IC-ACC was negatively correlated with pain rating in patients (Ichesco et al., 2012), suggesting this pain inhibitory circuit is more active under deep tissue pain conditions. Female TMD patients also showed a decrease in gray matter volume in the left ACC, in the right posterior cingulate gyrus, the right anterior insular cortex and other brain areas (Gerstner et al., 2011). Therefore, changes in ACC connectivity might reflect a gain/loss of ACC function, which might be dependent on the subregions of ACC investigated, the origin, time course, severity of deep tissue pain as well as menstrual phases and psychological status at the time of testing. Unfortunately, since many deep tissue pain syndromes are more prevalent in women, most of the above mentioned brain imaging studies only included female subjects and therefore sex difference in brain region activation data are unavailable.
2.4. Summary
Brain imaging studies of experimental and clinical deep tissue pain in humans reveal complex brain networks are involved in sensory, cognitive, emotional aspects of pain. Overall, greater ACC activation in females and insular activation in males have been observed under several chronic pain conditions. Increased ACC-IC connectivity under these conditions may reflect an effort of the body to recruit pain inhibitory mechanisms to suppress pain. Sexually dimorphic brain area activation could contribute to sex difference in pain response, and thereby lay the basis for the development of sexually dimorphic treatment under different conditions. Nevertheless, detailed information about sex differences in neuroanatomical/neurochemical organization in the peripheral and central nervous system is not readily available from these studies, demanding the use of animal models for deep tissue pain research.
3. Animal studies supporting a sex difference and hormonal modulation of deep tissue pain
Clinical and experimental human studies suggest greater pain in women and there is evidence that gonadal hormones contribute to fluctuations in nociceptive processing, especially deep tissue pain in women. A sex difference in processing of nociceptive stimuli and gonadal hormone modulation of deep tissue pain is supported by animal studies with two caveats. First, the direction of the sex difference, are males or females more sensitive, is not straightforward. The direction of sensitivity depends on many factors including, but not limited to test stimuli and measured endpoint, acute or persistent/chronic pain model, hormonal status, species and strain (genetic background). For example, more or less female sensitivity compared to males was reported for different strains of rats and mice in nociceptive and antinociceptive tests (Kest et al., 1999; Mogil et al., 2000). Second, while there is strong evidence that gonadal hormones modulate nociception, the direction and magnitude of effect is controversial.
In several deep tissue pain models, females showed greater sensitivity to noxious stimuli compared to males and gonadal hormones likely contribute to the sex difference. In many of these cases nociceptive sensitivity was greater when estradiol (E2) levels were higher compared to lower (e.g., proestrus vs. met/diestrus, ovariectomized plus E2 replacement (Ovx+E2) vs. ovariectomized). In other cases however, sensitivity was greater when the relative levels of E2 were lower. How then can E2 contribute to sex differences in pain? There are several possible explanations. In some models focusing on lower abdominal/pelvic pain, low plasma E2 levels are associated with increased nociceptive sensitivity which may result from effects of E2 on peripheral tissue. A reduction in the plasma estrogen concentration following ovariectomy or menopause could result in vaginal atrophy increasing pelvic pain and estrogen replacement could alleviate the pain (Pessina et al., 2006; Ting et al., 2004). Alternatively, it may not be the absolute level of E2 that is important, but changes in concentration (i.e., fluctuating levels during the estrous or menstrual cycle). Estrogen or progesterone withdrawal may be more significant in modulating nociceptive sensitivity than maintaining a high level of either hormone (Devall and Lovick, 2010; Heitkemper and Chang, 2009; Ji et al., 2003; Martin et al., 2007; Martin, 2008; Puri et al., 2011; Robbins et al., 2010). Finally, the site where hormones produce their effect, peripheral tissue or the central nervous system (CNS), likely influences any conclusions. E2 has anti-inflammatory effects in the periphery that could lead to anti-nociceptive effect, offsetting pro-nociceptive effects on CNS functioning.
3.1. Sex differences and gonadal hormone modulation of visceral pain (Table 1)
Table 1.
Sex differences and hormonal modulation of visceral stimuli a
| Ref | Species/prep | Test (stim→response) | Result | Additional results and Notes |
|---|---|---|---|---|
| Sex difference | ||||
| Holdcroft (Holdcroft et al., 2000) | Rat/ M,F | CRD→VMR | F>M; | Greater female sensitivity to CRD; increased during high E2. |
| Ji (Ji et al., 2012) | Rat/ M,F | CRD→VMR; normal, inflamed colon | F>M. inflame F>M | Greater female sensitivity to CRD ± inflammation. NMDA antagonist more potent in males, greater NMDA receptor activity, expression in membrane in females. |
| Bourdu (Bourdu et al., 2005) | Rat/ M,F | CRD→VMR; colitis | F>M | Greater female sensitivity to CRD ± inflammation. |
| Wang (Wang et al., 2009) | Rat/ M,F | CRD→ VMR, imaging, pain behavior | VMR: M=F Pain scores F>M | No SD in the VMR, but females had higher pain scores. There was a SD in brain activity to CRD. |
| Rosztoczy (Rosztoczy et al., 2003) | Rat/ M,F | CRD→VMR | basal M=F; mat.sep F>M; mat.sep+ stress F>M | No SD in basal visceral sensitivity. Maternal separation ± adult stress increased visceral sensitivity that was greater in females. |
| Kamp (Kamp et al., 2003) | Mice/different strains/ M,F | CRD→VMR | F>M | Greater female sensitivity to CRD in some strains. |
| Larauche (Larauche et al., 2012) | Rat/ M,F | CRD→intracolonic pressure | basal M=F; acute stress ↓M,F; repeated stress ↑F. | No SD in basal visceral sensitivity, greater effect of chronic stress in females. Intracolonic pressure differs from abdominal muscle VMR. |
| Ness (Ness et al., 2001) | Rat/ M,F | UBD→VMR | F,p>M | Greater female sensitivity to UBD |
| Affaitati (Affaitati et al., 2011) | Rat/ M,F | Ureteral stone→ crises; | F>M | Greater ureteral stone pain in females; ER, AR antagonists ↓ pain in females only. |
| Aloisi (Aloisi et al., 2010) | Rat/ M,F; supraphys E2 or T | Ureteral stone→ crises | F>M | Greater ureteral stone pain in females; Supraphys E2 was antinociceptive in females only (similar to pregnancy?); testosterone had no effect. |
| Larsson (Larsson et al., 2003) | Mice/ M,F | CRD→ VMR | F=M | Duration of VMR was half duration of distention. Unusual response. |
| Hormone modulation | ||||
| Ji (Ji et al., 2008) | Rat/ F, cycle | CRD→VMR | proestrus>met/diestrus | E2 is pronociceptive. |
| Ji (Ji et al., 2003) | Rat/ OVx,+E2 | CRD→VMR | E2>OVx | E2 is pronociceptive. |
| Ji (Ji et al., 2005) | Rat/ F,OVx,+E2,+prog | CRD→VMR; normal, inflamed colon | E2=F>OVx; E2>E2+P. inflame: E2>OVx>F. | E2 is pronociceptive, progesterone blunted effect of E2. |
| Tang (Tang et al., 2008) | Rat/ OVx,+E2 | CRD→VMR | E2>OVx. +APV: E2>OVx. | E2 is pronociceptive. E2 increased GluN1, pGluN1 expression, E2 decreased potency of NMDAr antagonist. |
| Ji (Ji et al., 2011) | Rat/ OVx,+E2,+PPT | CRD→VMR | E2=PPT>OVx | ERα activation is pronociceptive, mediated effect of E2 on VMR. A MEK inhibitor attenuated the effect of PPT. |
| Fan (Fan et al., 2009) | Rat/ F,OVx,+E2,+prog | CRD→VMR; colitis | E2>OVx=E2+prog | E2 is pronociceptive in colitis model, progesterone blunted effect of E2. |
| Lu (Lu et al., 2007) | Rat/ OVx,+E2 | CRD→VMR; normal, inflamed colon | E2>OVx | E2 is pronociceptive. E2 increased pCREB in spinal cord. |
| Lu (Lu et al., 2009) | Rat/ F,OVx,+E2; ER antagonists | CRD→VMR; normal, inflamed colon | E2,E2+prog,E2+ICI>OVx | E2 is pronociceptive via GPER, not ERα/β. |
| Bradesi (Bradesi et al., 2003) | Rat/ F,OVx,+E2 | CRD→VMR | E2>OVx stress: E2>OVx. | E2 is pronociceptive in stress model. NK-1 antagonist was antinociceptive and E2 dependent. |
| Ball (Ball et al., 2010) | Rat/ F, cycle | UBD→VMR; normal, inflamed bladder | Inflam ↑VMR, met,p>di; nal ↑VMR, e>met>di>p | E2 is pronociceptive; influences opioid receptor in normal and inflamed bladder. |
| Robbins (Robbins et al., 2010) Peng (Peng et al., 2008) | Rat/ OVx,+E2 | UBD→VMR; | E2>OVx* | E2 withdrawal (changing concentration) is pronociceptive. Constant E2 had no effect. |
| Rat/ F, cycle | Uterine capsaicin evokes PnUR | p>met | E2 is pronociceptive; increases cross organ sensitization. NMDA receptor dependent. | |
| Peng (Peng et al., 2010) | Rat/ OVx,+E2 | colonic mustard oil evokes PnUR | E2>OVx | Cross organ sensitization is facilitated by E2. E2 ↑pAKT→↑pGluN2B→↑cross organ sensitization |
| Lin (Lin et al., 2006) | Rat/F,OVx,+E2 | Repetitive Stimulation to potentiate PnUR | E2>OVx | E2 potentiates PnUR, is NMDA dependent. |
| Cason (Cason et al., 2003) | Rat/ F, cycle | VD→ escapes (Vaginal Hyperalgesia); endometriosis | Endometriosis↑, proestrus>estrus | Endometriosis increased vaginal hyperalgesia when E2 was high in cycling rats (proestrus). No estrous cycle effect in normal rats. |
| Berkley (Berkley et al., 2007) | Rat/ OVx, estropause | VD→ escapes (Vaginal Hyperalgersia); Endometriosis | Intact: Endo ↑VH. OVx: ↑VH, E2↓VH; OVx + Endo: VH, ↓ by E2 | Endometriosis hyperalgesia is centrally mediated, OVx hyperalgesia is peripherally mediated; OVx + Endo had opposing effects. |
| Bradshaw (Bradshaw and Berkley, 2002) | Rat/ F,OVx,+E2 | VD→escapes (Vaginal Hyperalgesia) | OVx>E2 | E2 is antinociceptive. Ovariectomy induced vaginal hyperalgesia, relieved by E2 replacement. |
| Giamberardino (Giamberardino et al., 1997) | Rats/ F, cycle | Ureteral stone→ crises | met/di>p/e | E2 is antinociceptive |
| Cao (Cao et al., 2012) | Rat/ OVx,ERβ agonist | CRD→VMR | ERβ agonist ↓VMR | ERβ activation is antinociceptive, opposes ERα. |
Behavior: only those aspects of paper relevant to sex differences or hormonal modulation of behavioral responses are listed.
Abbreviations: VMR: visceromotor response; CRD: colorectal distention; UBD: urinary bladder distention; VD: vaginal distention; PnUR: pelvic nerve to urethra reflex; M,F,p,e,met,di: intact male, intact female, proestrous, estrous, metestrous, diestrous; OVx: ovariectomized; +E2: OVx with E2 replacement; GDx: gonadectomy in males; SD: sex difference; ↑/↓: increase/decrease. PPT: ERα agonist; Pn: pelvic nerve
Hollow organ (e.g., colon, bladder, uterus) distention is the prototypical model of visceral pain. A balloon is placed inside the organ (or the organ forms the balloon, e.g., bladder) and distended to different pressures allowing different types of measurements. Hollow organ distention causes pseudoaffective reflex contraction of the abdominal muscles that can be quantified by recording the electromyogram in awake or lightly anesthetized animals (the visceromotor response). Less often distention-evoked elevation of the hindquarters, pressor responses or intraabdominal/intracolonic pressure is measured, or escape responses/learned behaviors quantified. Neuronal responses can be recorded from primary afferents, dorsal horn neurons and supraspinal neurons. The extent of activation can be quantified by immunocytochemical labeling for cellular markers (e.g., c-Fos, pERK, pCREB). Animal imaging techniques have advanced such that PET and fMRI can be used to study brain networks that process nociceptive information. This has facilitated the study of sex differences and the role of gonadal hormones in deep tissue pain.
3.1.1. Sex differences in visceral pain
Our lab studies sex differences in visceral pain using the colorectal distention model. We and others have reported sex differences in the visceromotor response to colorectal distention (Figure 1) with the majority of studies indicating females have greater sensitivity (greater magnitude visceromotor response) than males in both rats and mice (Holdcroft et al., 2000; Ji et al., 2006; Ji et al., 2012b; Kamp et al., 2003). Similar results were reported using alternate responses (hindquarter elevation) to colorectal distention (Bourdu et al., 2005). Different conclusions were reported in studies using different measures. Male mice had greater visceral sensitivity compared to females, but the EMG was only half the duration of the distending stimulus making interpretation difficult (Larsson et al., 2003). Two studies reported no sex difference (Larauche et al., 2012a; Wang et al., 2009), but the Larauche study reported intracolonic pressure, not the visceromotor response. Sex differences in bladder pain were similar in direction to the majority of the colorectal pain studies. The magnitude of the visceromotor response to urinary bladder distention was greater in female rats compared to males (Ball et al., 2010; Ness et al., 2001).
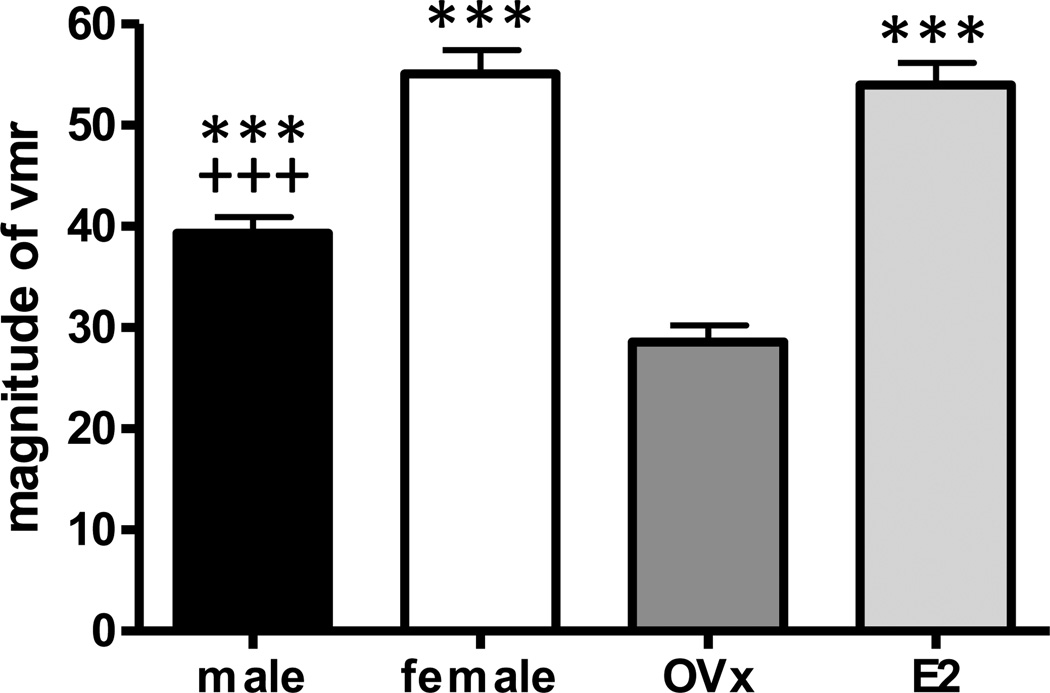
Figure 1.
Sex difference and effect of hormone modulation on the magnitude of the visceromotor response (vmr) to colorectal distention. The vmr was significantly greater in female rats compared to males. Ovariectomy significantly decreased the vmr compared to intact females and replacement E2 restored the vmr to a magnitude similar to intact rats. One way ANOVA p<0.0001, n=55–61/group. *** p<0.001 vs. OVx; +++ p<0.001 vs. female.
3.1.2. Pronociceptive effects of E2 on visceral pain
Studies in female rats suggest estrogen likely contributes to the sex difference in deep tissue pain. The magnitude of the visceromotor response to colorectal distention fluctuates with the estrous cycle, greatest in proestrus and least in met/diestrus (Gustafsson and Greenwood-Van Meerveld, 2011; Holdcroft et al., 2000; Ji et al., 2008; Sapsed-Byrne et al., 1996). This pattern reflects fluctuations in estradiol (E2) and progesterone plasma concentrations during the estrous cycle, suggesting the level of gonadal hormones or changing hormone concentrations are important in modulating the visceromotor response. Hormone depletion by ovariectomy decreased the magnitude of the visceromotor response to colorectal distention and systemic replacement by bolus injection of E2 four to 48 hours earlier restored the response (Figure 1). The plasma E2 concentration had peaked and was declining 48 hrs following administration supporting the idea that changing levels of E2 are important (Ji et al., 2003). Similarly, the visceromotor response to bladder distention was decreased by ovariectomy and restored by E2 replacement (Robbins et al., 2010). Importantly, Robbins and colleagues demonstrated that E2 replacement in ovariectomized rats by bolus injection with testing 24 hours later increased the visceromotor response to bladder distention, but chronic E2 replacement produced by pellets implanted a week earlier did not increase the visceromotor response. When the pellets were removed, the visceromotor response increased 24 hours later, suggesting E2 withdrawal likely increased visceral sensitivity (Robbins et al., 2010). Further studies showed that E2, acting at the level of the spinal cord could account for the effects of systemic E2. Spinal administration of the estrogen receptor antagonist ICI-182,780 attenuated the visceromotor response to colorectal distention in intact female rats and spinal administration of E2 to ovariectomized rats facilitated the visceromotor response similar to systemic E2 (Ji et al., 2011; Ji et al., 2003).
In a model of mechanical stimulation of the uterine-cervix in rats to emulate labor, ovariectomy decreased the visceromotor response which was reversed by E2 replacement and the response of the innervating hypogastric nerve afferents increased in OVx+E2 rats via activation of TRPV1 (Liu et al., 2005; Yan et al., 2007). Correspondingly, uterine-cervix afferent activity increased as estrogen levels increased during pregnancy (Liu et al., 2008).
NMDA receptor activity could partially account for the sex difference induced by estrogen in deep tissue pain. Spinal administration of the NDMA receptor antagonist APV dose-dependently attenuated the visceromotor response to colorectal distention with greater effect in males. This corresponded with greater cell membrane expression of the GluN1 subunit of the NMDA receptor in the dorsal horn in females, suggesting females had more functional NMDA receptors (Ji et al., 2012b). This was likely due to E2 modulation of NMDA receptor activity. Ovariectomy increased the potency of APV in modulating the visceromotor response to colorectal distention and E2 increased GluN1 and GluN2B expression and PKA-mediated GluN1 phosphorylation in the spinal cord (Ji et al., 2012a; Tang et al., 2008). These data suggest a role for E2 modulation of NMDA receptors in sex differences in visceral pain. This is supported by studies of cross organ sensitization using the pelvic nerve to urethra reflex. Stimulation of the pelvic nerve (either electrically or via colonic inflammation) increased urethra activity. The reflex was more robust in rats in proestrus compared to metestrus and was decreased by ovariectomy (Peng et al., 2010; Peng et al., 2008). The reflex was blocked by APV with greater effect in ovariectomized rats (Lin et al., 2006).
In contrast to the pronociceptive effect of estrogen in these distention models progesterone replacement had no effect on increasing the visceromotor response following ovariectomy, but dampened the effect of E2 when coadministered (Ji et al., 2005). However, the progesterone metabolite 3 alpha-hydroxy- 5 alpha- pregnan-20-one was antinociceptive to duodenal distention (Winfree et al., 1992).
3.1.2.1. Visceral inflammation
Hollow organ distention (e.g., colorectal distention) mimics natural stimuli, such as obstruction without inflammation or a tumor that stretches the organ wall evoking pain. A sensitizing agent increases visceral pain, generally by inducing inflammation, but how does this affect sex differences and hormonal modulation? There are several models that induce colonic or bladder hypersensitivity lasting from hours to weeks in the absence or presence of inflammation. Female hypersensitivity to colorectal distention was reported following mustard oil irritation of the colon and the magnitude of the increase was greater in females compared to males (Ji et al., 2012b; Lu et al., 2007). Similarly, hypersensitivity that persisted several weeks in the absence of colonic inflammation was greater in female rats (Bourdu et al., 2005). Similar to noninflamed animals, estrogen modulated the magnitude of response in females. Ovariectomy reduced the visceromotor response to intracolonic mustard oil which was restored by E2 replacement (Ji et al., 2005; Lu et al., 2007). Intracolonic 5HTP, a serotonin precursor, induced colonic hypersensitivity without noticeable inflammation that was attenuated by ovariectomy and facilitated by E2 (Lu et al., 2009). OVx+E2 had a similar effect on colorectal pain in an inflammatory colitis model (Fan et al., 2009). In a model of bladder inflammation females developed behavioral responses faster than males, but the peak response was the same (Bon et al., 1997). In another model of bladder inflammation the visceromotor response to bladder distention increased during proestrus, when E2 was high, compared to diestrus, when E2 was low (Ball et al., 2010). These data suggest that visceral inflammation increases sensitivity, but does not alter the modulation by gonadal hormones.
3.1.3. Antinociceptive effects of E2 on visceral pain
In contrast to the pronociceptive effects of estrogen providing a possible explanation for sex differences to colon or bladder distention, estrogen appears antinociceptive in a model of ureteral stones. Although female rats spent a greater percentage of time in crises following ureteral stone implant compared to males (Affaitati et al., 2011), this was negatively correlated with estrogen levels. When E2 was high or decreasing during proestrus/estrus, there were significantly fewer ureteral crises than when E2 was low during met/di-estrus (Giamberardino et al., 1997). However, estrogen receptor or androgen receptor antagonists decreased the number of crises in females, with no effect in males (Affaitati et al., 2011). These paradoxical results might be due to the antagonists acting at subtypes of receptors for estrogens and androgens. This could unmask activity of other receptors. For example, ICI-182,780 antagonizes ERα and ERβ, but is an agonist at the G-protein coupled receptor, GPER (Prossnitz et al., 2008).
Increasing E2 to supraphysiological levels in intact female rats in the ureteral stone model decreased crises in females, but not males, while testosterone had no effect (Aloisi et al., 2010). Since these experiments were conducted in intact rats, the possibility of interactions between multiple hormones and receptors may have contributed to the fluctuations in the level of nociception/antinociception. Indeed, elevated levels of E2 and progesterone observed during pregnancy or pseudopregnancy are analgesic (Dawson-Basoa and Gintzler, 1996; Gintzler and Bohan, 1990). In addition, experimental conditions may have contributed to the observed outcome; ureteral crises were observed following stone implantation which required surgery and there was likely uncontrolled postoperative pain.
The role of estrogen on nociceptive sensitivity during hollow organ distention of female reproductive organs differs from bladder and colon. The percentage of escape responses to uterine distention with a balloon was greater during periods of lower circulating estradiol: metand diestrus compared to proestrus (Bradshaw et al., 1999). Correspondingly, ovariectomy increased escape responses to vaginal distention which was reversed by estrogen replacement (Bradshaw and Berkley, 2002). This hyperalgesia was likely peripherally mediated, resulting from an ovariectomy-induced decrease in vaginal wall thickness and/or increase in innervation density contributing to increased vaginal hypersensitivity and referred hyperalgesia (Berkley et al., 2007; Pessina et al., 2006; Ting et al., 2004). However, the distention threshold of primary afferents in the hypogastric nerve innervating the uterus and pelvic nerve innervating the vagina was lowest in proestrus (Robbins et al., 1992). Part of these contradictory results might be related to reproductive function vs. nociceptive sensitivity as afferent activity is not necessarily related to escape behaviors evoked by noxious stimuli.
Endometriosis is a condition in which uterine endometrium grows outside the uterine cavity. Clinically, this often results in pain. Endometriosis is modeled in rats by autotransplanting pieces of uterine horn to the abdominal mesentery. Vaginal hyperalgesia in endometriosis is estrogen modulated; greatest when E2 levels were highest during proestrus and least during estrus (Cason et al., 2003). Since the endometrial implants become innervated by sympathetic and sensory fibers from the thoracolumbar spinal cord and dorsal root ganglion (DRG) it is likely the vaginal hyperalgesia was centrally mediated (Berkley et al., 2004; McAllister et al., 2012). In contrast, in the absence of endometriosis, ovariectomy induced vaginal hyperalgesia which was reversed by estrogen replacement. However, ovariectomy had no effect on rats with endometrial hyperalgesia, but the hyperalgesia was attenuated by E2, suggesting peripheral antinociceptive effects and central pronociceptive effects partially offset (Berkley et al., 2007).
In mice, long term ovariectomy increased mechanosensitivity in the area of referred pain from pelvic organs and visceral pain behavior was increased following administration of capsaicin into the colon. There was a corresponding increase in pERK in the spinal cord. Treatment that decreased pERK decreased hypersensitivity and vice versa. Furthermore, the referred pain only occurred at the level of the lumbosacral spinal cord (Klinger et al., 2011; Sanoja and Cervero, 2005; Sanoja and Cervero, 2008), suggesting the ovariectomy-induced hypersensitivity was peripherally mediated, perhaps a consequence of vaginal atrophy. Alternatively, the long duration loss of E2 may have led to osteoporosis-like symptoms. Similarly, rats ovariectomized for 4 weeks had greater mechanical and thermal sensitivity of the hindpaw and tail compared to ovariectomized rats with chronic E2 replacement (Sarajari and Oblinger, 2010). However, there was no change in the magnitude of the visceromotor response to colorectal distention in rats measured weekly for 8 weeks following ovariectomy (Traub et al., 2013).
3.1.4. Summary
The literature focusing on sex differences and hormonal modulation of acute and inflammatory visceral pain is complex. Overall, the majority of studies suggest E2 is pronociceptive and we contend it could partially account for the sex difference in visceral sensitivity. The majority of sex difference studies listed in table 1 (not all studies from the same lab drawing the same conclusion are listed) support greater nociceptive sensitivity in females. The remaining studies reported no sex difference or greater sensitivity in males. However, these latter studies measured different endpoints. The studies focusing on hormones indicate elevated levels of circulating E2 or withdrawal from the elevated levels likely contributed to the greater female visceral sensitivity; approximately 80% of the studies concluded that nociceptive responses were greater in cycling rats in proestrus compared to other cycle phases or that ovariectomy decreased responses and E2 replacement restored the response. In these cases, nociceptive sensitivity was determined hours to days following E2 replacement, when E2 levels were declining from peak values. Although several studies concluded E2 is antinociceptive these studies might have had a confounding peripheral component such as recent surgery or vaginal atrophy. The remaining studies used models with multiple conditions where the test stimulus and pathological condition were separate or focused on female-specific organs.
3.2. Sex differences and gonadal hormone modulation of deep somatic pain (Table 2)
Table 2.
Sex differences and hormonal modulation of deep somatic stimuli a
| Ref | Species/prep | Test (stim→response) | Result | Additional results and Notes |
|---|---|---|---|---|
| Sex difference | ||||
| (Burnes et al., 2008) | Mouse/ M,F,OVx, +testosterone | Muscle fatigue; ASIC3 wildtype and KO | Wt: F>M=OVx+T. KO: Fwt=MKO>Mwt. | Greater muscle fatigue (pain) in females. Testosterone was protective against muscle pain (low pH) but dependent on ASIC3. |
| Niu (Niu et al., 2012) | Rat/ M,F | masseter CFA→ mechanosensitivity | M=F. CB1R agonist: antihyperalgsia M>F | No SD in muscle hyperalgesia, but SD in peripheral modulation of muscle pain by CB1R agonist. Speculate testosterone is antinociceptive/protective. |
| Cairns (Cairns et al., 2002) | Rat/ M,F,OVx,GDx, +E2 | Glu, m.oil in TMJ → jaw muscle activity | F>M (Glu); OVx↓,+E2↑; GDx,+E2 no change. m.oil: ↑activity but no SD. | Glutamate sensitized TMJ to further inflammation w/o SD. E2 in OVx was pronociceptive to glutamate. |
| Gaumond (Gaumond et al., 2002) | Rat/ M,F,GDx,OVx | Hindpaw formalin response | F>M: I, interphase, II; F>OVx: interphase; GDx>M: I, late II; OVx=GDx. | Formalin evoked greater responses in females, suggests E2 was antinociceptive by decreasing interphase inhibition; Testosterone was protective; GDx eliminated sex difference. |
| Coulombe (Coulombe et al., 2011) | Mice/ M,F, OVx+α/β agonist, ERαKO, ERβKO | Hindpaw formalin response | Interphase: ERβKO↓, β ag↑; Phase I: ERαKO↑, α ag↓. ↑ | In females, ERα was antinociceptive, ERβ was pronociceptive (↓ inhibitory mechanisms). Minimal effect in males. |
| Zhang (Zhang et al., 2012) | Rat/ M,F,OVx,+E2, antags | Hindpaw Mech Threshold, Thermal Threshold; Formalin response | E2 ↓MTh, ↓TTh: M=F=OVx. Formalin males: ↓ by ICI, G-15, ATD | Spinal E2 was pronociceptive in M and F (no SD); mERs mediated effects of E2. |
| Joseph (Joseph and Levine, 2003) | Rat/ M,F,OVx,+E2 | Chemotherapy-Induced Peripheral Neuropathy | F>M; OVx↓SD; E2 ↑SD; GDx: no effect. Inhibit PKCε: ↓hyp in M=OVx>>E2,F | CIPN F>M; SD was E2 dependent, E2 was pronociceptive. PKCε modulated pain but did not explain SD or E2 effect. |
| Spooner (Spooner et al., 2007) | Mice/ M,F, ERβKO | Hindpaw formalin response; Hot plate | Wt>KO; female effect only; Fos: wt>KO; HP:F=FβKO>M=MβKO | E2 was pronociceptive via ERβ dampening endogenous pain inhibitory mechanism→ ↑ spinal nociceptive activity. Females more sensitive to HP, ERβ independent. |
| Gaumond (Gaumond et al., 2005) | Rat/ M,F,GDx,OVx +E2, +T for 3wks | Hindpaw formalin response | GDx ↑phase I, II, T↓ I,II; OVx ↓ interphase; E+Prog restored interphase; E2, Prog had no effect. | GDx increased formalin response, reversed by testosterone (antinociceptive). OVx increased interphase inhibition, reversed by E2+prog (anti-inhibitory). |
| Pajot (Pajot et al., 2003) | Rat/ M,F,GDx,OVx | Formalin response to lip, foot | Lip: OVx>F; GDx=M. Foot: OVx=F, GDx=M. | E2 was antinociceptive to lip formalin, not hindpaw. Orofacial region more sensitive than hindpaw. |
| Ma (Ma et al., 2011) | Rat/ M,F,OVx,+E2, GDx | OVx induced Mech hyperalgesia, ATP antag in hindpaw | Mhyp: OVx>E2; GDx:no effect. ATPantag: reversed OVx Mhyp; GDx:no effect. | E2 was antinociceptive. E2 in females modulated P2X3 signal transduction, pain. Genomic mechanism. OVx↑, E2↓ P2X mRNA, protein in DRG. |
| Fischer (Fischer et al., 2008) | Rat/M,F,OVx,+E2, GDx | Glutamate/formalin in TMJ | di>p,M; OVx=di>F | E2 was antinociceptive to intra TMJ glutamate or formalin. No effect of estrous cycle phases during formalin test. |
| Hormone modulation | ||||
| Aloisi (Aloisi et al., 2003) | Rat/ M, GDx | Hindpaw formalin response, repeated injections | GDx increased formalin response over time | Testosterone was protective |
| Ceccarelli (Ceccarelli et al., 2006) | Rat/ F, OVx | Hindpaw formalin response, repeated injections | F>OVx | Cycling hormones were pronociceptive vs. OVx. Females had greater Fos expression in the arcuate nucleus. |
| Ceccarelli (Ceccarelli et al., 2003) | Rat/ F, longterm OVx (6 months) | Hindpaw formalin; thermal pain | Formalin licking: OVx>F; flinching, flexing OVx=F; | Replacement E2 antinociceptive to supraspinal (licking), but not spinal (flick, flex) responses to formalin. |
| Kuba (Kuba et al., 2005) | Rat/ OVx, +E2 | Hindpaw formalin response | OVx > E2 (phase II) E2 capsules ↓ late phase but only high concentration of formalin | E2 was antinociceptive. Chronic E2 from capsules potentially anti-inflammatory in periphery. |
| Mannino (Mannino et al., 2007) | Rat/ F cycling, OVx, +E2 capsules | Hindpaw formalin response | OVx> p=E2 | E2 was antinociceptive |
| Sanoja (Sanoja and Cervero, 2005; Sanoja and Cervero, 2008) | Mice/F,OVx,+E2 | Mech hyp; Therm hyp; colonic capsaicin | Mhyp: OVx>E2. Thyp: no effect. Cap behavior: OVx>E2. No effect of estrous cycle. | E2 was antinociceptive. OVx increased hyperalgesia which was reversed by E2. |
| Fischer (Fischer et al., 2007) | Rat/M,F,GDx,OVx,+T | TMJ formalin response | GDx>M; OVx=OVx+T; GDx=GDx+E2>GDx+T | Testosterone was protective decreasing the formalin response in males. E2 had no effect in GDx. Testosterone had no effect in GDx. |
Behavior: only those aspects of paper relevant to sex differences or hormonal modulation of behavioral responses are listed.
Abbreviations: M,F,p,e,met,di: intact male, intact female, proestrous, estrous, metestrous, diestrous; OVx: ovariectomized; +E2: OVx with E2 replacement; GDx: gonadectomy in males; +T: GDx + testosterone replacement; SD: sex difference; ↑/↓: increase/decrease. ag: agonist; antag: antagonist; Glu: glutamate; KO: knockout; mER: membrane bound estrogen receptor; Pn: pelvic nerve; PPT: ERα agonist; m.oil: mustard oil; wt: wildtype; ICI: ICI-182,780; G-15: GPER antagonist; ATD: aromatase inhibitor;
Sex differences and hormonal modulation of deep somatic pain has been most extensively studied in the orofacial region examining muscles of mastication and the temporomandibular joint. While models of hindpaw inflammation can be considered superficial or subcutaneous and not necessarily deep tissue, we have included the formalin model due to the extensive literature on sex differences.
3.2.1. Orofacial pain
Temporomandibular disorders are more prevalent in women and inflammation of the masseter muscles or temporomandibular joint (TMJ) is used to model aspects of human temporomandibular disorder. In rats, masseter muscle or TMJ inflammation increased mechanosensitivity of the orofacial region and Fos expression in the medullary dorsal horn, but there was no sex difference (Bereiter et al., 2005b; Niu et al., 2012). However, following TMJ inflammation there was greater excitatory amino acid release in the trigeminal nucleus caudalis/upper cervical cord (Vc/C2) in males compared to females (Bereiter and Benetti, 2006). In the periphery, glutamate injected into the TMJ evoked greater jaw muscle activity in female rats compared to males. Subsequent mustard oil injected into the TMJ further increased jaw muscle activity but without a sex difference (Cairns et al., 2002), suggesting a sex difference in glutamate receptor activity but not TRPA1. This is supported by greater NMDA receptor expression in the spinal cord of females (section 3.1.2) and greater NMDA receptor activity in female DRG neurons (section 3.4.1).
Underlying these sex differences could be sexually dimorphic effects of gonadal hormones. Masseter muscle inflammation-induced mechanical hyperalgesia was more robust following E2 replacement in ovariectomized rats (Liverman et al., 2009b; Traub et al., 2013). Jaw muscle activity evoked by glutamate injected into the TMJ was attenuated by ovariectomy and increased by E2 replacement. However, orchiectomy and E2 replacement had no effect (Cairns et al., 2002). Following TMJ inflammation by CFA, E2 dose-dependently upregulated NF-κB and downstream inflammatory cytokines in the synovial fluid and increased nociceptive behaviors as measured by a decrease in food intake (Kou et al., 2011).
In contrast, E2-evoked antinociception in TMJ inflammatory models has also been reported. TMJ inflammation induced by formalin produced a similar number of nociceptive behaviors in males and proestrous females, albeit lower than diestrous females and E2 reduced nociceptive behaviors in ovariectomized rats (Fischer et al., 2008). Similarly, TMJ inflammation induced by CFA evoked greater nociception in OVx+saline rats as measured by a different feeding behavior compared to OVx+E2 rats (Kramer and Bellinger, 2009).
It has been suggested that E2 antinociception in somatic inflammatory pain models is peripherally mediated via nongenomic signaling mechanisms (Favaro-Moreira et al., 2009) and several models of TMJ inflammation support an anti-inflammatory effect of E2. E2 attenuated plasma extravasation and neutrophil migration in the TMJ following TMJ inflammation in proestrus rats and OVx+E2 rats (Flake et al., 2006; Torres-Chavez et al., 2012). E2 and testosterone also attenuated these inflammatory measures in orchiectomized rats (Torres-Chavez et al., 2012). However, while E2 decreased CFA-induced TMJ inflammation (plasma extravasation) it increased TMJ afferent activity (Flake et al., 2005).
3.2.2. Hindpaw inflammatory pain
A number of studies have examined sex differences and the effects of gonadal hormones in models of hindpaw inflammation, most notably the formalin model. Formalin injected into the hindpaw produces an immediate 5–10 minute period of licking or shaking the injected limb (phase I; nociceptor activation) followed by a 5–10 minute lower activity interphase (thought due to an increase in descending inhibition) followed by a 30–40 minute phase II increase in excitatory activity (inflammation and central sensitization). Female rats displayed more licking/shaking in all 3 phases of the formalin response compared to males suggesting greater sensitivity (Gaumond et al., 2002). Formalin also evoked a more robust response in dorsal horn neurons during phase II in female rats and conditioning electrical stimulation more effectively attenuated the formalin response in males (You et al., 2006) similar to greater CPM in men. How this is modulated by gonadal hormones is unclear. In male rats, formalin-induced nociceptive responses were decreased by spinal administration of an ERα/β antagonist, GPER antagonist or aromatase inhibitor, suggesting pronociceptive activity at multiple membrane estrogen receptors at the spinal level (Zhang et al., 2012a). In female rats, ovariectomy decreased the nociceptive behavior during the interphase. This was blocked by replacing E2 and progesterone (E2/P) (Ceccarelli et al., 2006; Gaumond et al., 2002; Gaumond et al., 2005). Since the interphase is thought due to an increase in inhibitory processing, the positive correlation between E2/P and nociceptive responses suggest E2/P cause a deficit in inhibitory processing. Studies in female, but not male mice, further suggest this anti-inhibitory effect was due to activation of ERβ (Coulombe et al., 2011; Spooner et al., 2007).
This pronociceptive or anti-inhibitory effect of estrogen in the formalin test is contradicted by another series of reports that ovariectomy increased the response during phase II (excitatory response) of the formalin test compared to intact rats in proestrus or ovariectomized rats with steady state replacement of E2 (Hunter et al., 2011; Kuba et al., 2005; Mannino et al., 2007). Since the increase in nociceptive behavior in ovariectomized rats was only apparent following a high concentration of formalin (5%), it was possible that E2 had a peripheral anti-inflammatory effect reducing nociceptive behaviors in phase II. In agreement, ERα and ERβ selective agonists attenuated late phase 2 responses in mice (Coulombe et al., 2011).
Following longterm ovariectomy (6 months) licking behavior to 5% formalin was greater than in intact females. However, spinal reflexes, flinching and flexing, were not different. In addition, the flexion reflex (paw withdrawal from noxious heat) was unaffected by longterm ovariectomy suggesting that gonadal hormones modulated supraspinal rather than spinal processing of noxious stimuli (Ceccarelli et al., 2003a).
Conclusions drawn from the formalin model are further complicated by another study that reported that the biphasic response of formalin, this time injected into the lip, was increased by ovariectomy, but not orchiectomy, and accompanied by an increase in ERα in the trigeminal subnucleus caudalis and C1-C2 dorsal horn. This same study reported no effect of ovariectomy on responses to intraplantar injection of formalin compared to intact rats (1.5% formalin was used in the lip and hindpaw suggesting greater modulation in the orofacial region). Furthermore, there was no sex difference to formalin at either test site (Pajot et al., 2003).
3.2.3. Cancer Pain
There is no compelling evidence for a sex difference in cancer pain in humans (Edrington et al., 2004; Fillingim et al., 2009) and limited data regarding sex differences in pain resulting from cancer treatment. In rats mechanosensitivity in chemotherapy induced peripheral neuropathy was greater in females compared to males. Ovariectomy decreased and E2 replacement restored the hypersensitivity. A PKCε inhibitor decreased the neuropathy in males and ovariectomized females, but not intact or E2 treated females (Joseph and Levine, 2003).
3.2.4. Summary
Of the studies that looked at somatic deep tissue pain in males and females most found females more sensitive than males and two found no sex difference. This is consistent with the overall impression that females are more sensitive to pain than males. However, in contrast to visceral pain models, most studies focusing on the effects of hormones indicated E2 was antinociceptive, comparing intact females with the effects of ovariectomy and OVx+E2. It is possible the antinociceptive effects of E2 may have resulted from peripheral anti-inflammatory effects as antinociception mostly observed in inflammatory pain models.
3.3. The role of gonadal hormone receptor subtypes
3.3.1. Estrogen receptors
Behavioral and cellular studies support both genomic and non-genomic or rapid estrogen signaling in pain and nociception. ERα and ERβ are the classical estrogen receptors that function as ligand bound transcription factors. In addition, these classical receptors and GPER are also localized to plasma and intracellular membranes. E2 binding these receptors activates second messenger signaling pathways to modulate ion channels and receptors affecting neuronal activity and activating transcription factors within seconds to minutes (see (Gintzler and Liu, 2012; McEwen, 2001; Prossnitz and Barton, 2011; Srivastava et al., 2011; Vasudevan and Pfaff, 2008) for review).
Little is known about the role of different estrogen receptors in deep tissue pain. Our lab has reported opposing effects of selective activation of the classical estrogen receptors ERα and ERβ in ovariectomized rats (Cao et al., 2012; Ji et al., 2011)(FIGURE 2). Selective activation of ERα either systemically or at the level of the spinal cord increased the magnitude of the visceromotor response to colorectal distention four hours later, mimicking the effect of E2. A shorter duration response was noted for ERα, but not ERβ. Activation of ERα with a selective agonist facilitated the visceromotor response to colorectal distention within 15 minutes, suggesting the facilitation was via a nongenomic mechanism (Ji et al., 2011). In contrast, selective activation of ERβ decreased the magnitude of the visceromotor response to colorectal distention. The inhibition was not observed for 3–4 hrs after agonist administration leaving open the possibility this was a genomic process (Cao et al., 2012). When the ERβ agonist was administered to intact female rats overstimulating the ERβ system, the visceromotor response to colorectal distention was attenuated, suggesting the facilitatory effect of ERα activation masked the inhibitory effect of ERβ activation in intact rats.
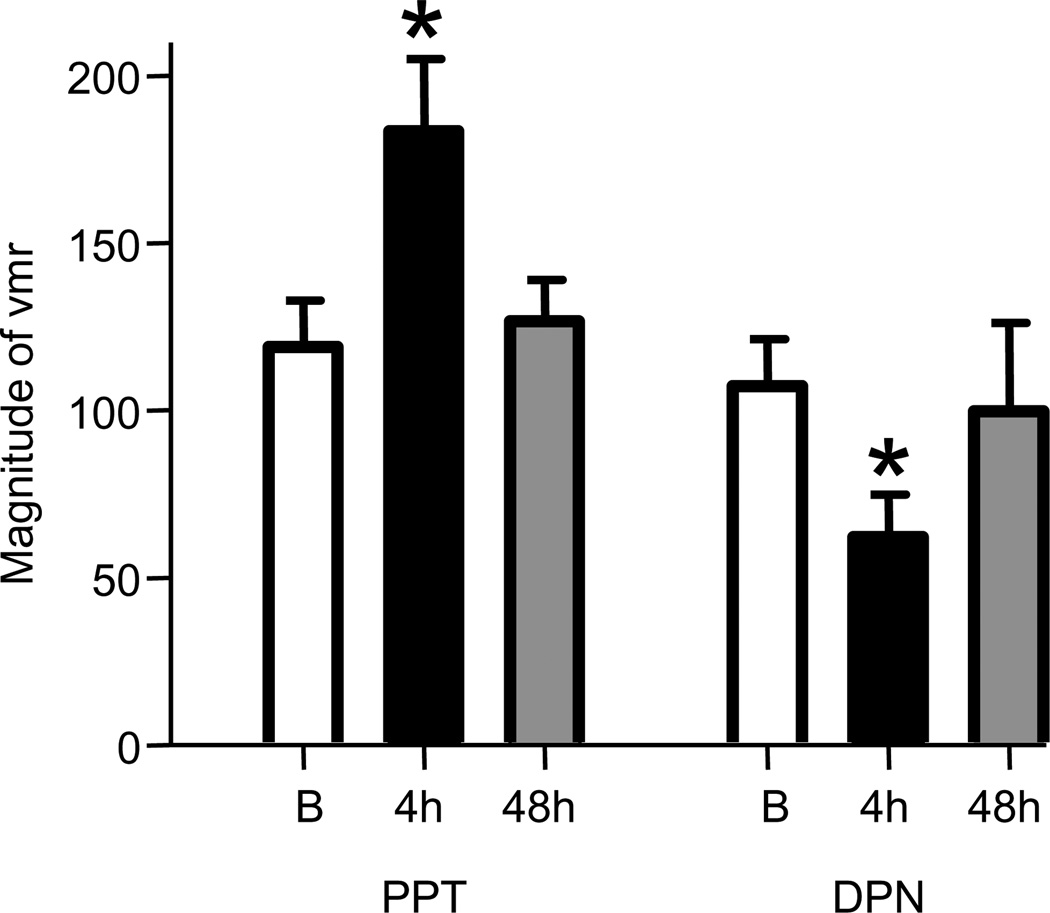
Figure 2.
The effect of s.c. administration of the ERα agonist PPT and the ERβ agonist DPN on the visceromotor response. The effect of each agonist was significantly greater than baseline four hours after injection and returned to baseline by 48 hours (1 way RM ANOVA). * p<0.05 vs. baseline (B) prior to agonist administration. Figures are derived from data originally published in (Ji et al., 2011) and (Cao et al., 2012) with permission.
The antinociceptive effect of ERβ activation supports a series of reports, in which an ERβ agonist inhibited hyperalgesia in several models of chemical, acute or persistent inflammatory pain and neuropathic pain, leading the authors to speculate ERβ mediated anti-inflammatory effects of estrogen (Gardell et al., 2008; Leventhal et al., 2006; Piu et al., 2008). In a different model of endometriosis than described above, in which human endometrial tissue is transplanted into athymic nude mice, an ERβ agonist decreased the size of the cysts in the abdominal cavity further supporting anti-inflammatory activity, but no effects on nociceptive processing were reported (Harris et al., 2005). In rat models of spontaneously developing inflammatory bowel disease or adjuvant-induced arthritis, an ERβ agonist attenuated diarrhea and reduced inflammatory scores in both models leading the authors to suggest ERβ modulates the immune system (Harris et al., 2003).
GPER is a G-protein coupled receptor that binds E2 activating several different intracellular signaling pathways (Prossnitz et al., 2007). Selective activation of GPER mediated visceral hypersensitivity induced by the 5-HT precursor 5HTP (Lu et al., 2009) and selective activation of GPER increased superficial pain related behaviors in mice (Deliu et al., 2012). GPER is expressed in primary afferents. While no sex difference in expression was reported, expression in females was decreased by ovariectomy (Takanami et al., 2010). In another study E2 binding GPER rather than ERα/β evoked mechanical hyperalgesia by increasing PKCε translocation in DRG cells. This obscured subsequent activation of β2-adrenergic receptor-induced hyperalgesia (Hucho et al., 2006; Khasar et al., 2005; Kuhn et al., 2008). Activation of ERα and GPER, but not ERβ, activated ERK in trigeminal ganglion neurons and contributed to masseter muscle pain (Liverman et al., 2009a; Liverman et al., 2009b) and G-1, a GPER agonist, rapidly increased Ca2+ in trigeminal ganglion neurons from female rats (Fehrenbacher et al., 2009).
In the central nervous system testosterone is converted into neurosteroidal E2 by aromatase, providing a mechanism for local and rapid modulation of nociceptive processing by E2 binding ERα, ERβ or GPER. Intrathecal administration of aromatase inhibitors attenuated nociceptive processing to noxious cutaneous stimuli in Japanese quail within minutes supporting rapid signaling in addition to slower evolving genomic modulation (Evrard, 2006; Evrard and Balthazart, 2004). In rats, spinal aromatase inhibitors shifted morphine antinociception from kappa opioid receptor dependent to independent. This was likely via rapid signaling as it occurred within 15 minutes (Liu et al., 2011). Clinically, aromatase inhibitors are used to treat pain from endometriosis (see (Ferrero et al., 2011; Nothnick, 2011; Pavone and Bulun, 2012) for review).
3.3.2. Androgen receptors
A general conclusion from many studies is that testosterone, presumably via androgen receptors, is protective or antinociceptive and the greater testosterone plasma concentration in males supports this hypothesis. Testosterone replacement in gonadectomized male rats was analgesic in the tailflick test (Frye and Seliga, 2001). Likewise, orchiectomy in males increased phase I and II responses to 2% formalin in rats which was reversed by testosterone replacement (Gaumond et al., 2005). There was no difference in nociceptive behaviors to a single injection of 5% formalin comparing intact and gonadectomized male rats. However, following repeated formalin injections in males, nociceptive behaviors decreased in intact, but not gonadectomized rats (Aloisi et al., 2003; Ceccarelli et al., 2003b). In intact rats, daily testosterone injections attenuated formalin-induced licking behavior in females, but not males (Aloisi et al., 2004), possibly a ceiling effect of testosterone in males. In males, orchiectomy increased nociceptive responses to formalin injected in the TMJ which was reversed by testosterone, but not E2 (Fischer et al., 2007). In a mouse model of muscle fatigue males showed less fatigue than females. In ovariectomized mice testosterone reduced fatigue, similar to males (Burnes et al., 2008). In rats, there was no sex difference in mechanical hyperalgesia following masseter muscle inflammation, but peripheral antinociceptive mechanisms were sexually dimorphic. A cannabinoid 1 receptor (CB1R) agonist produced greater antihyperalgesia in males than females which correlated with CB1R expression in the trigeminal ganglion. Orchiectomy reduced the CB1R mRNA in males with no effect of ovariectomy in females. Testosterone reversed the decrease in CB1R expression in males, but had no effect in females (Niu et al., 2012).
3.3.3. Summary
While not as extensively studied as estradiol, the majority of studies support an antinociceptive or protective role for testosterone. Although testosterone is aromatized into E2 in the brain and spinal cord, only a few investigators have addressed this aspect of testosterone function in deep tissue pain and nociception. More is known about estrogen receptors, both the classical genomic receptors (ERα and ERβ) and the more recently recognized GPER. Studies support both genomic and nongenomic roles of ERα and ERβ but specific contributions to nociceptive processing remain unclear. E2 acting at GPER activates second messenger pathways that either modulate neuronal excitability or modulate transcription.
3.4. Where do sex differences originate?
Sex differences in the response to noxious stimuli in conscious animals can occur at the level of primary afferents, the dorsal horn or at supraspinal levels. Sex differences in the response of primary afferents were reported in several models. Similarly, differences were reported at spinal and supraspinal levels contributing to differences in perception and descending modulation. While there are possible alternate explanations, data support sex differences in primary afferent activity. In contrast, sex differences in dorsal horn neuron activity could be intrinsic to the spinal cord or could result from sex differences in supraspinal processing and descending modulation.
3.4.1. Primary afferents
Estrogen receptors are expressed in primary afferents and estrogen modulates primary afferent activity. Estimates of estrogen receptor expression in DRG and trigeminal ganglion (TG) neurons range from 20% to “most cells” (Bereiter et al., 2005a; Papka and Storey-Workley, 2002; Taleghany et al., 1999). Generally, expression is greatest in the L6-S2 DRG and ERβ is expressed in more cells than ERα. Estrogen receptors are expressed in 20–70% of bladder afferents (Bennett et al., 2003; Ji et al., 2011) and in uterine-cervix afferents (Papka et al., 2001; Papka and Storey-Workley, 2002), but there is a lack of estrogen receptor alpha expression in rat lumbosacral colonic afferents (Ji et al., 2011).
There are only a handful of studies examining sex differences in the response to noxious stimuli of primary afferents innervating deep tissue. There was no sex difference in the evoked response of colonic afferents to colorectal distention (Ji et al., 2012b). One reason for this might be the observation that ovariectomy did not change the response of colonic afferents to colorectal distention compared to intact female rats, suggesting a lack of hormonal modulation of colonic afferents (Ji et al., 2011). However, males had greater spontaneous activity in lumbosacral (pelvic nerve) and thoracolumbar (splanchnic nerves) colonic afferent fibers in the dorsal roots (Ji et al., 2012b). Since females were more sensitive to colorectal distention than males (Figure 1), these observations suggest central processing rather than differences in primary afferent input accounts for the observed differences in behavior. This could reflect sex differences in transmitter release or receptor expression/function or hormonal influences within the CNS, either at the level of the spinal cord or at supraspinal sites. A similar conclusion was reported for muscle afferents; female rats had greater behavioral mechanosensitivity/lower thresholds than males, but muscle afferent fibers from males had lower mechanical thresholds than those from females (Hendrich et al., 2012).
Modulation of primary afferents by gonadal hormones has been studied in primary afferents that innervate sex-specific organs and common organs. Threshold for uterine and vaginal afferents innervated by the hypogastric and pelvic nerves, respectively, was lowest during proestrus and highest during diestrus. As previously mentioned this contradicts the behavioral studies from this group, but is thought to be related to reproductive function rather than nociception (Robbins et al., 1992).
Sex differences in excitability of primary afferents innervating tissues common to males and females may be due to genomic mechanisms or estrogen modulation by several different nongenomic signaling mechanisms. NMDA or glutamate injected into muscles of mastication evoked greater primary afferent discharges in female rats compared to males. Ovariectomy decreased the NMDA-evoked response which was reversed by E2 replacement (Cairns et al., 2001; Cairns et al., 2002; Dong et al., 2007). There was a greater percentage of GluN2B expressing masseter afferents in DRG from female rats with elevated E2 levels (OVx+E2, intact) compared to males, supporting other studies that E2 increases NMDA receptor activity (section 3.1.2.). Likewise, glutamate evoked greater activity in female rat facial cutaneous mechanoreceptors compared to males that was attenuated by an NMDA receptor antagonist (Gazerani et al., 2010). In unidentified DRG cells, NMDA evoked current was greater in cells from female rats compared to males and was E2 dependent (McRoberts et al., 2007). In cultured TG neurons bradykinin increased E2-dependent PLC signaling in female, but not male rats. Correspondingly, in ovariectomized rats, locally injected E2 increased bradykinin evoked hypersensitivity (Rowan et al., 2010). In support, E2 increased excitability of afferents innervating the TMJ which was further increased by inflammation (Flake et al., 2005). E2 increased pCREB in cultured DRG neurons, but not in autonomic pelvic ganglion neurons, via activation of pERK (Purves-Tyson and Keast, 2004). However, the specific estrogen receptors involved are unknown.
In contrast to the facilitatory effect of E2 in primary afferents described above, ovariectomy but not orchiectomy, increased locally injected ATP evoked mechanosensitivity of the hindpaw that was reversed by E2 (Ma et al., 2011). Consistent with this, E2 decreased ATP-induced calcium currents and P2X3 receptor mRNA in intact rat DRG cells (Ma et al., 2005). Similarly in mice, E2 attenuated ATP-induced Ca2+ currents by blocking L-type voltage gated calcium channels which was dependent on ERα activation (Chaban et al., 2011; Chaban et al., 2003; Cho and Chaban, 2012). This apparently involved estrogen receptor interaction with mGlu2/3 receptors (Chaban et al., 2011).
Of note, the inhibitory effect of E2 on ATP induced calcium currents was reported in physiologically unidentified DRG cells. Since visceral afferents constitute approximately 10% of DRG neurons, it is likely the aforementioned neurons innervated somatic tissue. However, in an animal model of colitis, E2 had an excitatory interaction with P2X receptors. Ovariectomy decreased visceral sensitivity and P2X3 receptor expression in DRG neurons which was reversed by E2 replacement, and E2 increased the magnitude of ATP-induced current in DRG cells (Fan et al., 2009). One possibility is the direction of modulation by E2 on P2X function changes during chronic inflammation. Indeed, colonic afferents from noninflamed rats do not express ERα and estrogen does not modulate colonic afferent activity (Ji et al., 2011). Following inflammation there may be an upregulation of ERα or a facilitatory effect mediated by ERβ or GPER may be unmasked. E2 might also have different effects on DRG cells that innervate viscera compared to somatic tissue.
3.4.2. Dorsal horn neurons
Following intrathecal administration, ER agonists/antagonists can modulate sensory and/or motor systems to affect responses. Recording from dorsal horn neurons provides evidence for second order sensory processing. Windup is a gain in the response of dorsal horn neurons to repetitive primary afferent stimulation. It is centrally mediated, NMDA receptor dependent and constitutes one of the most basic forms of dorsal horn neuronal plasticity. Electrical stimulation in the periphery evoked more robust windup in female rat dorsal horn neurons compared to males (Figure 3). Furthermore, only 37% of dorsal horn neurons in male rats showed windup vs. 82% in females (Chi-Square, p<0.001, unpublished observation). Windup is thought to be the mechanism underlying temporal summation in humans and the greater windup in female rats is consistent with greater temporal summation in women (see section 2.1.).
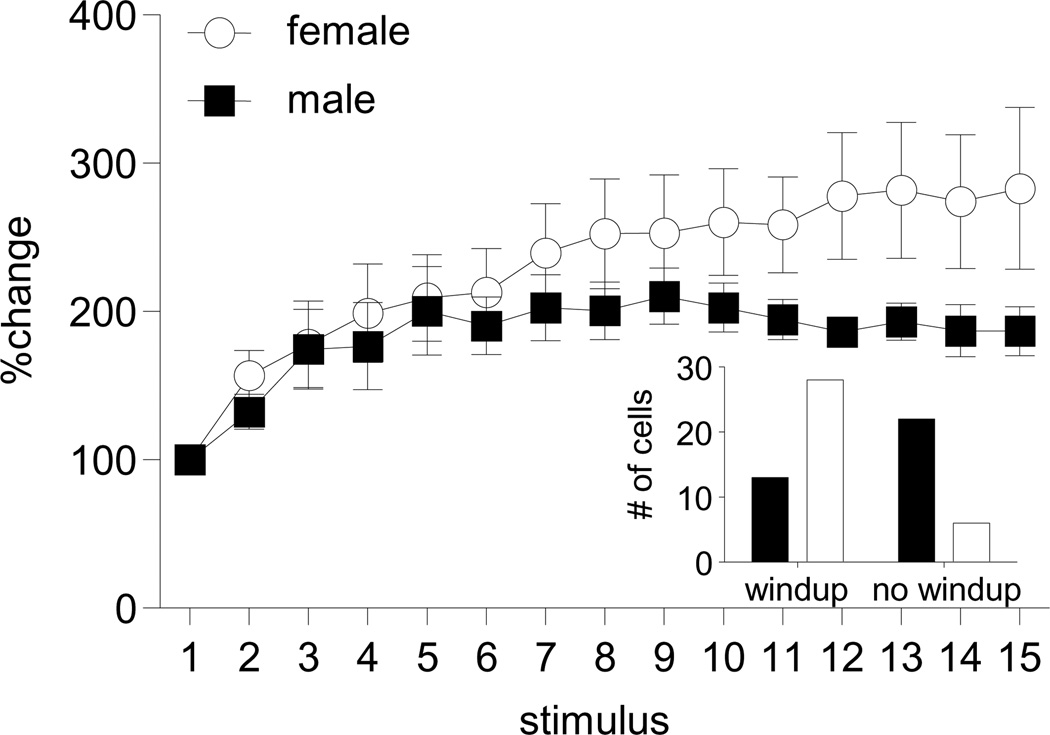
Figure 3.
Sex difference in magnitude and incidence of windup. Response to electrical stimulation at 1 Hz normalized to response to the first stimulus. Data are mean ± sem. There is a time × sex interaction, p<0.005, n=13 male, 28 female. Inset: Significantly more cells showed windup in female rats compared to males (Chi-square: p<0.001). This parallels greater temporal summation in women.
Several studies examining medullary or spinal dorsal horn neuronal responses to noxious deep tissue stimuli are similar to the behavioral outcomes providing a site for sex differences and hormonal modulation. The response of dorsal horn neurons to colorectal distention was greater in female rats compared to males (Ji et al., 2012b). Ovariectomy decreased the response of dorsal horn neurons to colorectal distention and E2 replacement recovered the response (Ji et al., 2003). Similarly, the response of medullary dorsal horn neurons responsive to temporomandibular joint stimulation/inflammation was more robust in proestrous rats compared to diestrous rats and ovariectomy decreased responses which were increased by E2 replacement (Okamoto et al., 2003; Tashiro et al., 2007). Surprisingly, there was no sex difference in the response of spinal dorsal horn neurons to cardiac stimulation with algogenic substances (Little et al., 2011). However, colonic inflammation induced a sexually dimorphic response of dorsal horn neurons. Compared to neurons that responded to colorectal distention in noninflamed rats, one phenotype of dorsal horn neuron was facilitated in females with no change in males. A second phenotype was facilitated in males and inhibited in females (Ji et al., 2012b). These data suggest sex differences in spinal processing of deep tissue stimuli contribute to behavioral differences in pain perception.
Most of these previous studies used estrogen replacement over days or bolus injections and examined responses hours to days later, suggesting the possibility of transcriptional effects underlying differences in neuronal responses. E2 also acts at membrane bound receptors initiating a rapid signaling cascade to alter cellular excitability. In ovariectomized rats E2 applied to the surface of the brainstem attenuated the response of medullary dorsal horn neurons to TMJ stimulation within minutes. An ERα agonist mimicked the inhibitory effect of E2, but an ERβ agonist facilitated neuronal responses, indicating receptor subtype selectivity in nociceptive processing (Tashiro et al., 2012). In a slice preparation, E2 potentiated synaptic signaling in the spinal cord via ERβ with no effect of ERα stimulation (Zhang et al., 2012b). Two points should be made here. First, the Tashiro paper supports membrane receptor initiated rapid signaling following selective activation of ERα and ERβ, but a nonselective agonist such as E2 evoked an overall inhibitory response. In contrast, the same group reported E2 replacement over several days in ovariectomized rats facilitated the response of dorsal horn neurons in the same TMJ model (Tashiro et al., 2007). This suggests that genomic and nongenomic signaling might result in different outcomes in neuronal activity and overall behavioral response. Second, it is unclear what the phenotype of the medullary dorsal horn neuron was and whether it expressed ER. ERα is co-expressed with enkephalin providing a mechanism for antinociceptive activity of E2 in the spinal cord and presumably medullary dorsal horn (Amandusson et al., 1996).
3.4.3. Supraspinal processing
Supraspinal processing of nociceptive stimuli occurs at many sites throughout the brain. While there is significant literature addressing supraspinal processing of deep tissue pain, most experiments were done in males or in females without consideration of estrous status. Only recently have studies begun to examine sex differences in supraspinal processing or modulation by gonadal hormones.
Imaging studies in humans suggest chronic pain activates limbic pathways to a greater extent in women and cortical structures to a greater extent in men. Imaging of deep tissue pain in animals reported similar findings. Colorectal distention in female rats evoked greater changes in the ventromedial prefrontal cortex and broader limbic/paralimbic areas that are involved in the affective aspect of visceral pain. In contrast, male rats had greater cortical activation that lead to greater cortical inhibitory effects on limbic structures (Wang et al., 2009).
In ovariectomized rats TMJ inflammation induced E2-dependent hyperalgesia and upregulation of NGF and TRPV1 in the hippocampus, but not in the amygdala, prefrontal cortex or thalamus. Inhibition of hippocampal NGF or TRPV1 attenuated this hyperalgesia (Wu et al., 2012; Wu et al., 2010). However, E2 does modulate amygdala activity and nociceptive sensitivity. Site specific administration of E2 pellets to the amygdala increased the visceromotor response to colorectal distention, but had no effect on somatic sensation (Myers et al., 2010). However, activation of the amygdala by corticosterone reduced the estrous cycle fluctuation in the visceromotor response to colorectal distention, but cutaneous mechanosensitivity was greater during proestrus/estrus (Gustafsson and Greenwood-Van Meerveld, 2011). In contrast, E2 or E2 + P injected into the amygdala decreased nociceptive behavior on the hotplate and increased the tailflick latency (Frye and Walf, 2004; Walf and Frye, 2003).
Injection of nitroglycerin to model migraine induced sexually dimorphic Fos expression in multiple brain nuclei. Male rats had greater Fos expression in the paraventricular nucleus (PVH), supraoptic nucleus (SON) and nucleus trigeminalis caudalis (SPVC). In females, ovariectomy decreased nitroglycerin-induced Fos expression in the PVH, SON, central nucleus of the amygdala, nucleus tractus solitarius, area postrema and SPVC. Orchiectomy reduced Fos expression only the SPVC in males (Greco et al., 2012).
In contrast, there was no sex difference in Fos expression in second order neurons in the spinal or medullary dorsal horn following colorectal distention or TMJ inflammation (Bereiter et al., 2005b; Ji et al., 2012b; Murphy et al., 2009). However, there was significantly greater colorectal distention-induced Fos expression in retrogradely labeled spinal neurons that projected to the parabrachial nucleus (PBn) in male rats although there was no sex difference in the total number of spino-PBn neurons (Murphy et al., 2009). The PBn is a vital link in visceral nociceptive circuitry between the spinal cord and supraspinal sites including the amygdala and PAG. Sexually dimorphic activation of the PBn could contribute to sex differences in the affective component of pain and descending modulation. Indeed, a sex difference was observed in the functional projection of PAG neurons to the RVM following hindpaw inflammation. While no sex difference was noted in Fos expression in the PAG by persistent inflammatory pain, significantly more PAG-RVM cells were activated in males in comparison with females even though females had a greater anatomical projection from the PAG to the RVM (Loyd and Murphy, 2006). Since the PAG-RVM pathway is well known for its role in descending pain modulation, the sex difference in spino-PBn and PAG-RVM circuit activation may contribute to the sexually dimorphic prevalence of some deep tissue pain conditions.
3.4.3.1. Stress and deep tissue pain
Women are usually more susceptible to stress-related disorders than men. Chronic stress is one of the many triggers of IBS, TMD and other functional pain syndromes. In many cases stress triggers the onset of or worsens the symptoms. Stress induces visceral hypersensitivity, but there are few papers examining sex differences or the role of gonadal hormones. Acute or mild chronic stress activates the emotional arousal circuit. Hypothalamic release of corticotropin releasing factor (CRF) and related peptides stimulate the hypothalamic-pituitary-adrenal (HPA) axis increasing circulating corticosterone (cortisol in humans) as well as activating the sympathetic nervous system. This increases visceral nociceptive processing at several levels of the nervous system (see (Dalla et al., 2011; Larauche et al., 2012b) for review).
In female rats, stress induced visceral hypersensitivity is estrogen dependent. Partial restraint stress in adult females induced visceral hypersensitivity that was decreased by ovariectomy and reversed by E2 replacement (Bradesi et al., 2002; Bradesi et al., 2003). In a new preparation modeling comorbidity of TMD, stress and IBS, visceral hypersensitivity persisted much longer in ovariectomized rats regularly injected with E2 compared to vehicle (Traub et al., 2013). In a mouse model of gastritis the inflammatory response was greater in females while the circulating levels of corticosterone decreased by more than half in females and not at all in males. Similarly, measures of anxiety increased in females, but not in males. However, there was no modulation of any responses in females across the estrous cycle (Painsipp et al., 2007).
Early life adversity has detrimental effects in adults that are sexually dimorphic. Maternal separation of all neonatal pups in a litter for 2 hours per day for 2 weeks induced visceral hypersensitivity to colorectal distention in male and female adults. When only half the litter was separated there was visceral hypersensitivity in adult females, but not males. Partial restraint stress alone in adults or following maternal separation increased the visceral hypersensitivity in females, with no effect in males (Rosztoczy et al., 2003). In contrast, a different strain of male rats was hypersensitive to colorectal distention following maternal separation and water avoidance stress as adults (Coutinho et al., 2002). In a different model, exposing neonatal rats to an unpredictable pairing of an odor and shock resulted in visceral hypersensitivity to colorectal distention in adult females, but not males. Furthermore, estrogen modulated the sensitivity in the adult females; ovariectomy decreased the visceral hypersensitivity which was reversed by E2 replacement (Chaloner and Greenwood-Van Meerveld, 2013).
Neonatal bladder inflammation also induced colorectal hypersensitivity in adult females. Following neonatal insult colorectal hypersensitivity in estrous/diestrous rats was normalized to the level observed in proestrous rats (Miranda et al., 2011). This may reflect the greater visceral hypersensitivity in proestrous rats without neontatal insult. This model also suggests neonatal insult in one viscus can modulate sensitivity in other viscera in adults contributing to increased comorbid pain conditions.
3.4.4. Summary
Sex differences in deep tissue pain can occur at multiple levels of the neuraxis. The majority of animal studies show that female gonadal hormones exert an excitatory effect on primary afferents, spinal and supraspinal neurons. In primary afferents the interaction of E2/ERs and additional specific receptors appears to increase or decrease afferent excitability. E2 attenuates P2X receptor responses to ATP. This could be interpreted as another example of the peripheral anti-nociceptive/anti-inflammatory effect of E2. On the other hand, E2 increases NMDA receptor activity which could occur in the absence of inflammation or perhaps the activity in DRG cells in vitro reflects what happens in the central terminal of primary afferents; increased NMDA receptor activity increasing transmitter release.
Sex differences at the level of the spinal cord are present, and apparently are independent of primary afferent differences. However, whether this represents the source of sex differences in deep tissue pain is unknown at present. In addition, the supraspinal studies suggest sex differences could arise in several sites in the brain. Stress results in sexually dimorphic visceral hypersensitivity that is E2 dependent.
4. Conclusion
That there exists a sex difference in deep tissue pain is well supported. The majority of studies concluded the prevalence and severity of chronic pain conditions is disproportionately greater in women, and the majority of animal studies, especially in rodents, support greater nociceptive processing in females. Much evidence suggests gonadal hormones contribute to sex differences, but underlying mechanisms are unclear. Hormones can modulate nociceptive processing at many levels of the neuraxis or in the periphery. Often the same stimulus and hormonal complement has different effects depending on the site of action or underlying conditions. Possible peripheral anti-inflammatory/anti-nociceptive effects of E2 could oppose pro-nociceptive activity within CNS circuitry. In addition, it is still not clear if the absolute or changing concentrations of hormones are more important in determining pro- or anti-nociceptive direction. Many studies in women suggest deep tissue pain symptoms are exaggerated in the perimenstrual period or during menses. This can be interpreted as evidence that gonadal hormones are antinociceptive since pain is greater when hormone levels are low. However, it can also be interpreted to suggest that withdrawal of hormone facilitates transcriptional regulation affecting nociceptive processing that takes several days to fully manifest. Unfortunately, the rapidly changing hormonal levels during the rodent estrous cycle make clarification of this question difficult. On the other hand, animal experimentation allows us to address questions about underlying mechanisms that cannot be addressed in humans. The role of membrane receptor initiated rapid signaling, perhaps by locally synthesized hormones, neurosteroids, is just beginning to be carefully examined in pain modulation. While evidence supports a role in nociceptive processing, the relative importance of rapid signaling vs. classical genomic mechanisms is unknown. Future studies will hopefully address some of these issues in determining the mechanisms underlying sex differences in pain.
Highlights.
Humans and animal studies support greater deep tissue pain in females
The sex difference is partially dependent on gonadal hormones
Gonadal hormones are pro- or anti-nociceptive depending on organ/condition
Acknowledgements
We thank Drs. Joel Greenspan and Jin Ro for critical comments on the manuscript. Supported by NIH grants NS37424 and DE022235.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch. Intern. Med. 2000;160:221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment. Pharmacol. Ther. 2010;32:738–755. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affaitati G, Ceccarelli I, Fiorenzani P, Rossi C, Pace MC, Passavanti MB, Aurilio c, Sorda G, Danielli B, Giamberardino MA, Aloisi AM. Sex differences in the analgesic effects of ICI 182,780 and Flutamide on ureteral calculosis in rats. Horm. Behav. 2011;59:9–13. doi: 10.1016/j.yhbeh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin. J Pain. 2003;19:168–174. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Affaitati G, Ceccarelli I, Fiorenzani P, Lerza R, Rossi C, Pace MC, Chiefari M, Aurilio C, Giamberardino MA. Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. Eur. J. Pain. 2010;14:602–607. doi: 10.1016/j.ejpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I, Fiorenzani P. Gonadectomy Affects Hormonal and Behavioral Responses to Repetitive Nociceptive Stimulation in Male Rats. Ann. N. Y. Acad. Sci. 2003;1007:232–237. doi: 10.1196/annals.1286.022. 232–237. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci Lett. 2004;361:262–264. doi: 10.1016/j.neulet.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Alonso C, Loevinger BL, Muller D, Coe CL. Menstrual cycle influences on pain and emotion in women with fibromyalgia. J Psychosom. Res. 2004;57:451–458. doi: 10.1016/j.jpsychores.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Amandusson A, Hermanson O, Blomqvist A. Colocalization of oestrogen receptor immunoreactivity and preproenkephalin mRNA expression to neurons in the superficial laminae of the spinal and medullary dorsal horn of rats. Eur. J. Neurosci. 1996;8:2440–2445. doi: 10.1111/j.1460-9568.1996.tb01207.x. [DOI] [PubMed] [Google Scholar]
- Ball CL, Ness TJ, Randich A. Opioid blockade and inflammation reveal estrous cycle effects on visceromotor reflexes evoked by bladder distention. J Urol. 2010;184:1529–1535. doi: 10.1016/j.juro.2010.05.090. [DOI] [PubMed] [Google Scholar]
- Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton. Neurosci. 2003;105:90–100. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- Benson S, Kotsis V, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S. Behavioural and neural correlates of visceral pain sensitivity in healthy men and women: does sex matter? Eur. J Pain. 2012;16:349–358. doi: 10.1002/j.1532-2149.2011.00027.x. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Benetti AP. Amino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female rats. Pain. 2006;126:175–183. doi: 10.1016/j.pain.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch. Oral Biol. 2005a;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005b;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav. Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11094–11098. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ, McAllister SL, Accius BE, Winnard KP. Endometriosis-induced vaginal hyperalgesia in the rat: effect of estropause, ovariectomy, and estradiol replacement. Pain. 2007;(132 Suppl. 1):S150–S159. doi: 10.1016/j.pain.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur. J. Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am. J Physiol Regul. Integr. Comp Physiol. 2006;291:R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- Bitzer J. Hormone withdrawal-associated symptoms: overlooked and underexplored. Gynecol. Endocrinol. 2013 doi: 10.3109/09513590.2012.760194. [DOI] [PubMed] [Google Scholar]
- Bon K, Lanteri-Minet M, Menetrey D, Berkley KJ. Sex, time-of-day and estrous variations in behavioral and bladder histological consequences of cyclophosphamide-induced cystitis in rats. Pain. 1997;73:423–429. doi: 10.1016/S0304-3959(97)00134-6. [DOI] [PubMed] [Google Scholar]
- Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, Dechelotte P, Bommelaer G, Eschalier A, Ardid D. Rectal Instillation of Butyrate Provides a Novel Clinically Relevant Model of Noninflammatory Colonic Hypersensitivity in Rats. Gastroenterology. 2005;128:1996–2008. doi: 10.1053/j.gastro.2005.03.082. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Bueno L. Acute and chronic stress differently affect visceral sensitivity to rectal distension in female rats. Neurogastroenterol. Motil. 2002;14:75–82. doi: 10.1046/j.1365-2982.2002.00305.x. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain. 2003;102:227–234. doi: 10.1016/S0304-3959(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Berkley KJ. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41:157–165. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am. J Physiol Regul. Integr. Comp Physiol. 2008;294:R1347–R1355. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J. Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sim Y, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002;957:338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- Cao DY, Ji Y, Tang B, Traub RJ. Estrogen Receptor beta Activation Is Antinociceptive in a Model of Visceral Pain in the Rat. J. Pain. 2012;13:685–694. doi: 10.1016/j.jpain.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm. Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Fiorenzani P, Massafra C, Aloisi AM. Long-term ovariectomy changes formalin-induced licking in female rats: the role of estrogens. Reprod. Biol. Endocrinol. 2003a;1:24. doi: 10.1186/1477-7827-1-24. 24- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli I, Fiorenzani P, Massafra C, Aloisi AM. Repeated nociceptive stimulation induces different behavioral and neuronal responses in intact and gonadectomized female rats. Brain Res. 2006;1106:142–149. doi: 10.1016/j.brainres.2006.05.089. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Massafra C, Aloisi AM. The behavioral and neuronal effects induced by repetitive nociceptive stimulation are affected by gonadal hormones in male rats. Pain. 2003b;104:35–47. doi: 10.1016/s0304-3959(02)00460-8. [DOI] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J. Neurosci Res. 2011;89:1707–1710. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chaloner A, Greenwood-Van Meerveld B. Sexually Dimorphic Effects of Unpredictable Early Life Adversity on Visceral Pain Behavior in a Rodent Model. J Pain. 2013 doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Chang L, Berman S, Mayer EA, Suyenobu B, Derbyshire S, Naliboff B, Vogt B, FitzGerald L, Mandelkern MA. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am. J. Gastroenterol. 2003a;98:1354–1361. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Berman S, Mayer EA, Suyenobu B, Derbyshire S, Naliboff B, Vogt B, FitzGerald L, Mandelkern MA. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol. 2003b;98:1354–1361. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Naliboff BD. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R277–R284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- Cho T, Chaban VV. Interaction between P2X3 and oestrogen receptor (ER)alpha/ERbeta in ATP-mediated calcium signalling in mice sensory neurones. J Neuroendocrinol. 2012;24:789–797. doi: 10.1111/j.1365-2826.2011.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe MA, Spooner MF, Gaumond I, Carrier JC, Marchand S. Estrogen receptors beta and alpha have specific pro- and anti-nociceptive actions. Neuroscience. 2011;184:172–182. doi: 10.1016/j.neuroscience.2011.02.057. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am. J. Physiol Gastrointest. Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Craft RM. Modulation of pain by estrogens. Pain. 2007;(132 Suppl. 1):S3–s12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dalla c, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr. Top. Behav. Neurosci. 2011;8:97–118. doi: 10.1007/7854_2010_94. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa ME, Gintzler AR. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain. 1996;64:608–615. doi: 10.1016/0304-3959(96)87175-2. [DOI] [PubMed] [Google Scholar]
- Deliu E, Brailoiu GC, Arterburn JB, Oprea TI, Benamar K, Dun NJ, Brailoiu E. Mechanisms of G Protein-Coupled Estrogen Receptor-Mediated Spinal Nociception. The Journal of Pain. 2012;13:742–754. doi: 10.1016/j.jpain.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J. Pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Lovick TA. Differential activation of the periaqueductal gray by mild anxiogenic stress at different stages of the estrous cycle in female rats. Neuropsychopharmacology. 2010;35:1174–1185. doi: 10.1038/npp.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrington JM, Paul S, Dodd M, West c, Facione N, Tripathy D, Koo P, Schumacher K, Miaskowski C. No evidence for sex differences in the severity and treatment of cancer pain. J Pain Symptom. Manage. 2004;28:225–232. doi: 10.1016/j.jpainsymman.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R291–R299. doi: 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- Evrard Hc, Balthazart J. Aromatization of androgens into estrogens reduces response latency to a noxious thermal stimulus in male quail. Horm. Behav. 2004;45:181–189. doi: 10.1016/j.yhbeh.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Fan J, Yu Lh, Zhang Y, Ni X, Ma B, Burnstock G. Estrogen altered visceromotor reflex and P2X3 mRNA expression in a rat model of colitis. Steroids. 2009;74:956–962. doi: 10.1016/j.steroids.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Favaro-Moreira NC, Torres-Chavez KE, Fischer L, Tambeli CH. Peripheral estradiol induces temporomandibular joint antinociception in rats by activating the nitric oxide/cyclic guanosine monophosphate signaling pathway. Neuroscience. 2009;164:724–732. doi: 10.1016/j.neuroscience.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher Jc, Loverme J, Clarke W, Hargreaves KM, Piomelli D, Taylor BK. Rapid pain modulation with nuclear receptor ligands. Brain Res. Rev. 2009 doi: 10.1016/j.brainresrev.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero S, Gillott DJ, Venturini PL, Remorgida V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod. Biol. Endocrinol. 2011;9:89-. doi: 10.1186/1477-7827-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Clemente J, Tambeli C. The Protective Role of Testosterone in the Development of Temporomandibular Joint Pain. The Journal of Pain. 2007;8:437–442. doi: 10.1016/j.jpain.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, Arruda Veiga Mc, Tambeli CH. The Influence of Sex and Ovarian Hormones on Temporomandibular Joint Nociception in Rats. J Pain. 2008;9:630–638. doi: 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake NM, Hermanstyne TO, Gold MS. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R343–R348. doi: 10.1152/ajpregu.00835.2005. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect. Behav. Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav. Neurosci. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Hyldtoft L, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Brann MR, Olsson R, Piu F. Differential modulation of inflammatory pain by a selective estrogen receptor beta agonist. Eur. J. Pharmacol. 2008;592:158–159. doi: 10.1016/j.ejphar.2008.06.107. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Res. 2005 doi: 10.1016/j.brainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Dong X, Wang M, Kumar U, Cairns BE. Sensitization of rat facial cutaneous mechanoreceptors by activation of peripheral N-methyl-d-aspartate receptors. Brain Res. 2010;1319:70–82. doi: 10.1016/j.brainres.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Ge HY, Madeleine P, Arendt-Nielsen L. Sex differences in temporal characteristics of descending inhibitory control: an evaluation using repeated bilateral experimental induction of muscle pain. Pain. 2004;110:72–78. doi: 10.1016/j.pain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Ge HY, Madeleine P, Cairns BE, Arendt-Nielsen L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: a potential experimental model of gender-specific differences. Clin. J Pain. 2006;22:37–44. doi: 10.1097/01.ajp.0000149799.01123.38. [DOI] [PubMed] [Google Scholar]
- George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J. Pain. 2007;8:2–10. doi: 10.1016/j.jpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Gerstner G, Ichesco E, Quintero A, Schmidt-Wlcke T. Changes in regional gray and white matter volume in patients with myofascial-type temporomandibular disorders: a voxel-based morphometry study. J. Orofac. Pain. 2011;25:99–106. [PubMed] [Google Scholar]
- Giamberardino MA, Affaitati G, Valente R, Iezzi S, Vecchiet L. Changes in visceral pain reactivity as a function of estrous cycle in female rats with artificial ureteral calculosis. Brain Res. 1997;774:234–238. doi: 10.1016/s0006-8993(97)81711-8. [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Bohan MC. Pain thresholds are elevated during pseudopregnancy. Brain Res. 1990;507:312–316. doi: 10.1016/0006-8993(90)90288-m. [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Liu NJ. Importance of sex to pain and its amelioration; relevance of spinal estrogens and its membrane receptors. Front Neuroendocrinol. 2012 doi: 10.1016/j.yfrne.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco R, Tassorelli c, Mangione AS, Smeraldi A, Allena M, Sandrini G, Nappi G, Nappi RE. Effect of Sex and Estrogens on Neuronal Activation in an Animal Model of Migraine. Headache. 2012 doi: 10.1111/j.1526-4610.2012.02249.x. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;(132 Suppl. 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Traub RJ. Gender differences in pain and its relief. In: McMahon S, Koltenburg M, Tracey I, Turk D, editors. Wall and Melzack’s Textbook of Pain: Expert Consult-Online and Print. 6th edition. Amsterdam: Elsevier; 2013. [Google Scholar]
- Gustafsson JK, Greenwood-Van Meerveld B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am. J Physiol Gastrointest. Liver Physiol. 2011;300:G1080–G1085. doi: 10.1152/ajpgi.00349.2010. [DOI] [PubMed] [Google Scholar]
- Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented Central Pain Processing in Vulvodynia. The Journal of Pain. 2013;14:579–589. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum. Reprod. 2005;20:936–941. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC. Neurosci. 2012;13:60. doi: 10.1186/1471-2202-13-60. doi: 10.1186/1471-2202-13-60., 60–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend. Med. 2009;(6 Suppl 2):152–167. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Jarrett ME. Update on irritable bowel syndrome and gender differences. Nutr. Clin. Pract. 2008;23:275–283. doi: 10.1177/0884533608318672. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Joseph EK, Ferrari LF, Chen X, Levine JD. In Vivo and in Vitro Comparison of Female and Male Nociceptors. J Pain. 2012;13:1224–1231. doi: 10.1016/j.jpain.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin. J. Pain. 2010;26:104–109. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcroft A, Berkley KJ. Sex and gender differences in pain and its relief. Textbook of Pain. 2006:1167–1198. [Google Scholar]
- Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. Br. J. Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci. Lett. 1999;262:61–64. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur. J Neurosci. 2006;24:527–534. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- Hunter DA, Barr GA, Amador N, Shivers KY, Kemen L, Kreiter CM, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol-induced antinociceptive responses on formalin-induced nociception are independent of COX and HPA activation. Synapse. 2011;65:643–651. doi: 10.1002/syn.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth. Analg. 2008;107:309–317. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T. Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache. 2012;52:441–454. doi: 10.1111/j.1526-4610.2011.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. doi: 10.1186/1744-8069-8-32., 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152:1182–1191. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Cao DY, Bai G, Traub RJ. Estrogen modulation of spinal GluN2B subunit in a visceral pain model in the rat. Society for Neuroscience Abstract Viewer 2012. 2012a [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Estrogen Modulates the Visceromotor Reflex and Responses of Spinal Dorsal Horn Neurons to Colorectal Stimulation in the Rat. J. Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tang B, Cao DY, Wang G, Traub RJ. Sex differences in spinal processing of transient and inflammatory colorectal stimuli in the rat. Pain. 2012b;153:1965–1973. doi: 10.1016/j.pain.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. Estrogen increases and progesterone decreases behavioral and neuronal responses to colorectal distention following colonic inflammation in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism for protein kinase c epsilon signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;119:831–838. doi: 10.1016/s0306-4522(03)00203-3. [DOI] [PubMed] [Google Scholar]
- Kamp EH, Jones RC, Tillman SR, III, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G434–G444. doi: 10.1152/ajpgi.00324.2002. [DOI] [PubMed] [Google Scholar]
- Kano M, Farmer AD, Aziz Q, Giampietro VP, Brammer MJ, Williams SC, Fukudo S, Coen SJ. Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am. J Physiol Gastrointest. Liver Physiol. 2013;304:G687–G699. doi: 10.1152/ajpgi.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Wlson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J. Pharmacol. Exp. Ther. 1999;289:1370–1375. [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Estrogen regulates adrenal medullary function producing sexual dimorphism in nociceptive threshold and beta-adrenergic receptor-mediated hyperalgesia in the rat. Eur. J Neurosci. 2005;21:3379–3386. doi: 10.1111/j.1460-9568.2005.04158.x. [DOI] [PubMed] [Google Scholar]
- King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., III Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143:172–178. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MB, Sacks S, Cervero F. A role for extracellular signal-regulated kinases 1 and 2 (ERK 1/2) in the maintenance of persistent mechanical hyperalgesia in ovariectomized mice. Neuroscience. 2011;172:483–493. doi: 10.1016/j.neuroscience.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney c, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav. Brain Res. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- Kou XX, Wu YW, Ding Y, Hao T, Bi RY, Gan YH, Ma X. 17beta-estradiol aggravates temporomandibular joint inflammation through the NF-kappaB pathway in ovariectomized rats. Arthritis Rheum. 2011;63:1888–1897. doi: 10.1002/art.30334. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba T, Kemen LM, Quinones-Jenab V. Estradiol administration mediates the inflammatory response to formalin in female rats. Brain Res. 2005;1047:119–122. doi: 10.1016/j.brainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Dina OA, Goswami c, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur. J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- Kurland JE, Coyle WJ, Winkler A, Zable E. Prevalence of irritable bowel syndrome and depression in fibromyalgia. Dig. Dis. Sci. 2006;51:454–460. doi: 10.1007/s10620-006-3154-7. [DOI] [PubMed] [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larauche M, Mulak A, Kim YS, Labus J, Million M, Tache Y. Visceral analgesia induced by acute and repeated water avoidance stress in rats: sex difference in opioid involvement. Neurogastroenterol. Motil. 2012a;24:e1031–e547. doi: 10.1111/j.1365-2982.2012.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp. Neurol. 2012b;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Arvidsson S, Ekman c, Bayati A. A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice -- effects of opioid receptor agonists. Neurogastroenterol. Motil. 2003;15:371–381. doi: 10.1046/j.1365-2982.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Larsson MB, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Strom M, Mayer EA, Walter SA. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Brandt MR, Cummons TA, Piesla MJ, Rogers KE, Harris HA. An estrogen receptor-beta agonist is active in models of inflammatory and chemical-induced pain. Eur. J Pharmacol. 2006;553:146–148. doi: 10.1016/j.ejphar.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Lin SY, Chen GD, Liao JM, Pan SF, Chen MJ, Chen JC, Peng HY, Ho YC, Ho YC, Huang PC, Lin JJ, Lin TB. Estrogen modulates the spinal N-methyl-D-aspartic acid-mediated pelvic nerve-to-urethra reflex plasticity in rats. Endocrinology. 2006;147:2956–2963. doi: 10.1210/en.2005-1378. [DOI] [PubMed] [Google Scholar]
- Little JM, Qin c, Farber JP, Foreman RD. Spinal cord processing of cardiac nociception: Are there sex differences between male and proestrous female rats? Brain Res. 2011 doi: 10.1016/j.brainres.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Eisenach JC, Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. J Neurophysiol. 2005;93:2167–2173. doi: 10.1152/jn.01012.2004. [DOI] [PubMed] [Google Scholar]
- Liu B, Tong c, Eisenach JC. Pregnancy increases excitability of mechanosensitive afferents innervating the uterine cervix. Anesthesiology. 2008;108:1087–1092. doi: 10.1097/ALN.0b013e31817302e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal kappa- and mu-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J. Neurosci. 2011;31:11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009a;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERαlpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009b doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J. Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CL, Hsieh Jc, Dun NJ, Oprea TI, Wang PS, Luo Jc, Lin HC, Chang FY, Lee SD. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Lu CL, Hsieh Jc, Tsaur ML, Huang YH, Wang PS, Wu LL, Liu PY, Chang FY, Lee SD. Estrogen rapidly modulates mustard oil-induced visceral hypersensitivity in conscious female rats: A role of CREB phosphorylation in spinal dorsal horn neurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G438–G446. doi: 10.1152/ajpgi.00210.2006. [DOI] [PubMed] [Google Scholar]
- Ma B, Rong W, Dunn PM, Burnstock G. 17beta-estradiol attenuates alpha, beta-meATP-induced currents in rat dorsal root ganglion neurons. Life Sci. 2005;76:2547–2558. doi: 10.1016/j.lfs.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Ma B, Yu LH, Fan J, Cong B, He P, Ni X, Burnstock G. Estrogen modulation of peripheral pain signal transduction: involvement of P2X(3) receptors. Purinergic. Signal. 2011;7:73–83. doi: 10.1007/s11302-010-9212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol Replacement in Ovariectomized Rats Is Antihyperalgesic in the Formalin Test. J. Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Martin VT. New theories in the pathogenesis of menstrual migraine. Curr. Pain Headache Rep. 2008;12:453–462. doi: 10.1007/s11916-008-0077-3. [DOI] [PubMed] [Google Scholar]
- Martin VT. Ovarian hormones and pain response: a review of clinical and basic science studies. Gend. Med. 2009a;(6 Suppl 2):168–192. doi: 10.1016/j.genm.2009.03.006. doi: 10.1016/j.genm.2009.03.006 168–192. [DOI] [PubMed] [Google Scholar]
- Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache. 2007;47:552–563. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- Martin VT. Ovarian hormones and pain response: A review of clinical and basic science studies. Gender Medicine. 2009b;6:168–192. doi: 10.1016/j.genm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Mathew PG, Dun EC, Luo JJ. A cyclic pain: the pathophysiology and treatment of menstrual migraine. Obstet. Gynecol. Surv. 2013;68:130–140. doi: 10.1097/OGX.0b013e31827f2496. [DOI] [PubMed] [Google Scholar]
- McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PLoS. One. 2012;7:e31758-. doi: 10.1371/journal.pone.0031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscerovisceral convergence. Neurogastroenterol. Motil. 2011;23:e683–e281. doi: 10.1111/j.1365-2982.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog. Brain Res. 2010;186:141–157. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci. Biobehav. Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Suckow SK, Johns M, Traub RJ. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiol Behav. 2009;97:205–212. doi: 10.1016/j.physbeh.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Schulkin J, Greenwood-Van Meerveld B. Sex Steroids Localized To The Amygdala Increase Pain Responses To Visceral Stimulation In Rats. J Pain. 2010 doi: 10.1016/j.jpain.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom. Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distention in rats: Sources of variability and effect of analgesics. J. Urol. 2001;165:968–974. [PubMed] [Google Scholar]
- Niu KY, Zhang Y, Ro JY. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain. 2012;153:2283–2291. doi: 10.1016/j.pain.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. J Clin. Psychiatry. 2011;72:219–224. doi: 10.4088/JCP.08m04969blu. [DOI] [PubMed] [Google Scholar]
- Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod. Biol. Endocrinol. 2011;9:87-. doi: 10.1186/1477-7827-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ units in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- Okifuji A, Turk DC. Sex hormones and pain in regularly menstruating women with fibromyalgia syndrome. J. Pain. 2006;7:851–859. doi: 10.1016/j.jpain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Bjornsson E, Thjodleifsson B. Natural history of irritable bowel syndrome in women and dysmenorrhea: a 10-year follow-up study. Gastroenterol. Res. Pract. 2012;2012:534204-. doi: 10.1155/2012/534204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscienc. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajot J, Ressot c, Ngom I, Woda A. Gonadectomy induces site-specific differences in nociception in rats. Pain. 2003;104:367–373. doi: 10.1016/s0304-3959(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Palsson O, Whitehead W, Barghout V, Levy R, Feld A, Von Korff M, Garner M, Drossman D, Turner M. IBS severity and health-related quality of life improve with age in women but not in men. Am. J. Gastroenterol. 2003;98:S272-. [Google Scholar]
- Pamuk ON, Cakir N. The variation in chronic widespread pain and other symptoms in fibromyalgia patients. The effects of menses and menopause. Clin. Exp. Rheumatol. 2005;23:778–782. [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci. Lett. 2002;319:71–74. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil. Steril. 2012;98:1370–1379. doi: 10.1016/j.fertnstert.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Lai CY, Hsieh MC, Hsu HH, Wu HC, Lin TB. PI3K modulates estrogen-dependent facilitation of colon-to-urethra cross-organ reflex sensitization in ovariectomized female rats. J Neurochem. 2010;113:54–66. doi: 10.1111/j.1471-4159.2010.06577.x. [DOI] [PubMed] [Google Scholar]
- Peng HY, Huang PC, Liao JM, Tung KC, Lee SD, Cheng CL, Shyu JC, Lai CY, Chen GD, Lin TB. Estrous cycle variation of TRPV1-mediated cross-organ sensitization between uterus and NMDA-dependent pelvic-urethra reflex activity. American Journal of Physiology - Endocrinology And Metabolism. 2008;295:E559–E568. doi: 10.1152/ajpendo.90289.2008. [DOI] [PubMed] [Google Scholar]
- Pessina MA, Hoyt RF, Goldstein I, Traish AM. Differential Effects of Estradiol, Progesterone, and Testosterone on Vaginal Structural Integrity. Endocrinology. 2006;147:61–69. doi: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Piu F, Cheevers c, Hyldtoft L, Gardell LR, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Schiffer HH, Donello JE, Olsson R, Gil DW, Brann MR. Broad modulation of neuropathic pain states by a selective estrogen receptor beta agonist. Eur. J. Pharmacol. 2008;590:423–429. doi: 10.1016/j.ejphar.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol. Sci. 2008;29:116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Molecular and Cellular Endocrinology. 2007;265–266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri J, Bellinger LL, Kramer PR. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. J. Cell Physiol. 2011;226:3169–3180. doi: 10.1002/jcp.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves-Tyson TD, Keast JR. Rapid actions of estradiol on cyclic amp responseelement binding protein phosphorylation in dorsal root ganglion neurons. Neuroscience. 2004;129:629–637. doi: 10.1016/j.neuroscience.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Quiton RL, Greenspan JD. Sex differences in endogenous pain modulation by distracting and painful conditioning stimulation. Pain. 2007;(132 Suppl. 1):S134–S149. doi: 10.1016/j.pain.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012a;153:602–618. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception -part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012b;153:619–635. doi: 10.1016/j.pain.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rezaii T, Hirschberg AL, Carlstrom K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain. 2012;13:646–655. doi: 10.1016/j.jpain.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Riley JL3, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Riley JL3, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- Robbins MT, Mebane H, Ball CL, Shaffer AD, Ness TJ. Effect of estrogen on bladder nociception in rats. J. Urol. 2010;183:1201–1205. doi: 10.1016/j.juro.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosztoczy A, Fioramonti J, Jarmay K, Barreau F, Wittmann T, Bueno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol. Motil. 2003;15:679–686. doi: 10.1046/j.1350-1925.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Berg KA, Milam SB, Jeske NA, Roberts JL, Hargreaves KM, Clarke WP. 17beta-estradiol rapidly enhances bradykinin signaling in primary sensory neurons in vitro and in vivo. J Pharmacol. Exp. Ther. 2010;335:190–196. doi: 10.1124/jpet.110.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samborski W, Sobieska M, Pieta P, Drews K, Brzosko M. Normal profile of sex hormones in women with primary fibromyalgia. Ann. Acad. Med. Stetin. 2005;51:23–26. [PubMed] [Google Scholar]
- Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Cervero F. Estrogen modulation of ovariectomy-induced hyperalgesia in adult mice. European Journal of Pain. 2008;12:573–581. doi: 10.1016/j.ejpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp. Neurol. 2010;224:163–169. doi: 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J. Orofac. Pain. 2007;21:309–317. [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schwap M, Bristela M, Kundi M, Piehslinger E. Sex-specific differences in patients with temporomandibular disorders. J Orofac. Pain. 2013;27:42–50. doi: 10.11607/jop.970. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Glynn c, Brooks J, McQuay H, Jack T, Chessell I, Bountra c, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006;32:256–265. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotioninduced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc. Natl. Acad. Sci. U. S. A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 2011;12:T12–T26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner MF, Robichaud P, Carrier JC, Marchand S. Endogenous pain modulation during the formalin test in estrogen receptor beta knockout mice. Neuroscience. 2007;150:675–680. doi: 10.1016/j.neuroscience.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kram+ír EA, Shors TJ, Liu F. Rapid Estrogen Signaling in the Brain: Implications for the Fine-Tuning of Neuronal Circuitry. The Journal of Neuroscience. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert. Rev. Neurother. 2012;12:577–585. doi: 10.1586/ern.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Stening KD, Eriksson O, Henriksson KG, Brynhildsen J, Lindh-Astrand L, Berg G, Hammar M, Amandusson A, Blomqvist A. Hormonal replacement therapy does not affect self-estimated pain or experimental pain responses in post-menopausal women suffering from fibromyalgia: a double-blind, randomized, placebo-controlled trial. Rheumatology. 2011;50:544–551. doi: 10.1093/rheumatology/keq348. (Oxford) [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum. Brain Mapp. 2009;30:689–698. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami K, Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor 30 in the spinal somatosensory system. Brain Res. 2010;1310:17–28. doi: 10.1016/j.brainres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J. Neurosci. Res. 1999;57:603–615. [PubMed] [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YR, Yang WW, Wang YL, Lin L. Sex differences in the symptoms and psychological factors that influence quality of life in patients with irritable bowel syndrome. Eur. J Gastroenterol. Hepatol. 2012;24:702–707. doi: 10.1097/MEG.0b013e328351b2c2. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Rapid estrogenic effects on TMJ-responsive brainstem neurons. J. Dent. Res. 2012;91:210–214. doi: 10.1177/0022034511428156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol. 2007;98:3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley c, White L, Khuder SA. Childhood maltreatment and migraine (part III). Association with comorbid pain conditions. Headache. 2010;50:42–51. doi: 10.1111/j.1526-4610.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- Ting AY, Blacklock AD, Smith PG. Estrogen Regulates Vaginal Sensory and Autonomic Nerve Density in the Rat. Biol. Reprod. 2004;71:1397–1404. doi: 10.1095/biolreprod.104.030023. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Zieglgansberger W, Willoch F, Schwaiger M, Conrad B, Bartenstein P. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann. Neurol. 1999;45:40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Torres-Chavez KE, Sanfins JM, Clemente-Napimoga JT, Pelegrini-Da-Silva A, Parada CA, Fischer L, Tambeli CH. Effect of gonadal steroid hormones on formalin-induced temporomandibular joint inflammation. Eur. J Pain. 2012;16:204–216. doi: 10.1016/j.ejpain.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146:47–55. doi: 10.1016/j.pain.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res. 2008;1230:73–79. doi: 10.1016/j.brainres.2008.06.120. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Cao D-Y, Dorsey SG, Dessem D. Stress and estrogen induce persistent visceral hypersensitivity following masseter muscle inflammation: a new model of overlapping pain conditions. Society for Neuroscience Abstract. 2013 [Google Scholar]
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Veale D, Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. Br. J. Rheumatol. 1991;30:220–222. doi: 10.1093/rheumatology/30.3.220. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur. J. Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/or progesterone. Behav. Brain Res. 2003;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145:120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree CJ, Coombs DW, DeLeo JA, Colburn RW. Analgesic effects of intrathecally-administered 3 alpha-hydroxy- 5 alpha- pregnan-20-one in a rat mechanical visceral pain model. Life Sci. 1992;50:1007–1012. doi: 10.1016/0024-3205(92)90095-7. [DOI] [PubMed] [Google Scholar]
- Wu YW, Kou XX, Bi RY, Xu W, Wang KW, Gan YH, Ma XC. Hippocampal Nerve Growth Factor Potentiated by 17beta-Estradiol and Involved in Allodynia of Inflamed TMJ in Rat. J. Pain. 2012;13:555–563. doi: 10.1016/j.jpain.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. 17-{beta}-Estradiol Enhanced Allodynia of Inflammatory Temporomandibular Joint through Upregulation of Hippocampal TRPV1 in Ovariectomized Rats. J. Neurosci. 2010;30:8710–8719. doi: 10.1523/JNEUROSCI.6323-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Liu B, Du D, Eisenach JC, Tong C. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth. Analg. 2007;104:1246–1250. doi: 10.1213/01.ane.0000263270.39480.a2. tables. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory controllike effect): its relevance for acute and chronic pain states. Curr. Opin. Anaesthesiol. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- You HJ, Cao DY, Yuan B, Arendt-Nielsen L. Sex differences in the responses of spinal wide-dynamic range neurons to subcutaneous formalin and in the effects of different frequencies of conditioning electrical stimulation. Neuroscience. 2006;138:1299–1307. doi: 10.1016/j.neuroscience.2005.11.060. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu N, Zhao ZQ, Zhang YQ. Involvement of Estrogen in Rapid Pain Modulation in the Rat Spinal Cord. Neurochem. Res. 2012a doi: 10.1007/s11064-012-0859-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiao X, Zhang XM, Zhao ZQ, Zhang YQ. Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: implications for pain hypersensitivity. J Biol. Chem. 2012b;287:33268–33281. doi: 10.1074/jbc.M112.368142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Callam CS, Woodruff MA, Verne GN. Effects of the N-methyl-D-aspartate receptor on temporal summation of second pain (wind-up) in irritable bowel syndrome. J. Pain. 2011;12:297–303. doi: 10.1016/j.jpain.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]