Abstract
Only d-allose, among various rare monosaccharides tested, induced resistance to Xanthomonas oryzae pv. oryzae in susceptible rice leaves with defence responses: reactive oxygen species, lesion mimic formation, and PR-protein gene expression. These responses were suppressed by ascorbic acid or diphenylene iodonium. Transgenic rice plants overexpressing OsrbohC, encoding NADPH oxidase, were enhanced in sensitivity to d-allose. d-Allose-mediated defence responses were suppressed by the presence of a hexokinase inhibitor. 6-Deoxy-d-allose, a structural derivative of d-allose unable to be phosphorylated, did not confer resistance. Transgenic rice plants expressing Escherichia coli AlsK encoding d-allose kinase to increase d-allose 6-phosphate synthesis were more sensitive to d-allose, but E. coli AlsI encoding d-allose 6-phosphate isomerase expression to decrease d-allose 6-phosphate reduced sensitivity. A d-glucose 6-phosphate dehydrogenase-defective mutant was also less sensitive, and OsG6PDH1 complementation restored full sensitivity. These results reveal that a monosaccharide, d-allose, induces rice resistance to X. oryzae pv. oryzae by activating NADPH oxidase through the activity of d-glucose 6-phosphate dehydrogenase, initiated by hexokinase-mediated conversion of d-allose to d-allose 6-phosphate, and treatment with d-allose might prove to be useful for reducing disease development in rice.
Key words: d-Allose, d-glucose 6-phosphate dehydrogenase, hexokinase, NADPH oxidase, Oryza sativa L, rare sugar.
Introduction
Rare sugars are a group of ~50 monosaccharides that are present in very low amounts in the natural world (Izumori, 2002, 2006). Studies of rare sugars were limited by a lack of methods to produce these sugars on a bulk scale until the development of methodology for rare sugar production by Izumori’s group (Izumori, 2002, 2006). Recently, biological functions and metabolic pathways of one of these rare sugars, d-allose, for several organisms have been reported. In animals, d-allose can have an immunosuppressive effect (Hossain et al., 2000) and a protective effect against liver damage (Hossain et al., 2003). It can also inhibit cancer cell proliferation and production of reactive oxygen species (ROS) in neutrophils (Murata et al., 2003; Sui et al., 2005). Hamster fibroblasts form d-allose 6-phosphate (A6P) from d-allose, indicating that d-allose is transported and internally converted (Ullrey and Kalckar, 1991). In microbes, hexokinases of yeast and Thermus caldophilus can phosphorylate several monosaccharides including d-allose (Chenault et al., 1997; Bae et al., 2005). In Aerobacter aerogenes and Escherichia coli, d-allose is incorporated into the glycolytic pathway through conversion to d-fructose 6-phosphate (F6P) (Gibbins and Simpson, 1964; Kim et al., 1997). Although d-allose has been detected in tissues of some plants (Perold et al., 1973; Chari et al., 1981; Jensen et al., 1981; Weckwerth et al., 2004), its function and metabolism in plants have not been well understood.
Recently, it was demonstrated in rice that application of d-allose inhibits the gibberellin signal transduction pathway downstream of the SLR1 protein step; thus, gibberellin-dependent responses, such as growth of seedlings and elongation of the second leaf sheath, were inhibited (Fukumoto et al., 2011, 2013). Because defence-related genes were up-regulated after d-allose treatment in an expression analysis using a rice microarray (Kano et al., 2010) and in a quantitative reverse transcription–PCR (qRT–PCR) analysis of Arabidopsis (Narusaka et al., 2009), treatment with d-allose might prove to be useful for reducing disease development (Kano et al., 2010), but its mode(s) of action in defence induction has not been elucidated. In the present study, the discovery of the mechanism and function of d-allose in plant defence induction is described; phosphorylation of d-allose to A6P in d-allose-treated rice is essential to induce defence with lesion mimic formation initiated by the generation of ROS by NADPH oxidase, which is activated by NADPH supplied from d-glucose 6-phosphate dehydrogenase (G6PDH).
Materials and methods
Chemicals
Rare sugars and their derivatives (Supplementary Fig. S1 available at JXB online) with respective purities of 100% were prepared by the Rare Sugar Research Center at Kagawa University using methods described previously (Izumori, 2002, 2006). Common sugars, enzymes, and other reagents used in buffers, solvents, and reaction mixtures described in the respective sections were purchased from Wako (Tokyo, Japan) unless noted otherwise.
Plant materials, sugar treatments, and bacterial inoculation
Rice plants (Oryza sativa L.) cv. Nipponbare were used as the wild type (WT) in the respective experiments. G6PDH1 mutants, selected from a search of the Tos17 rice mutant database (Miyao et al., 2003) (http://tos.nias.affrc.go.jp/), were obtained from the National Institute of Agrobiological Sciences (NIAS), Japan. WT, mutants overexpressing target genes (OsrbohC, Alsk, or AlsI), and Tos17 mutants were grown to the six-leaf stage at 25 °C (14h light/10h dark) in plastic pots (9cm diameter×9cm height) with a small hole (1cm diameter) at the bottom to absorb water from a tray (20×14×7cm) containing 1 litre of water (Kano et al., 2010, 2011). Plants were then placed for 2 d on a tray containing either 1 litre of water or a sugar solution to observe lesion mimic formation and to measure lesion lengths. When ascorbic acid (AsA) or N-acetyl-d-glucosamine (GlcNAc) was used, 5mM AsA or 5mM GlcNAc was added to water with/or without 5mM d-allose. Plants for 3,3′-diaminobenzidine (DAB) staining and phosphorylated sugar detection were incubated with sugars and/or chemicals for 24h. For observation of lesion mimics, plants were placed in another tray with only water for 3 d after the 2 d sugar treatment. For the measurement of lesion length, plants were inoculated with a virulent race of Xanthomonas oryzae pv. oryzae (Xoo) (strain T7174) (~1×106 CFU ml–1) after the 2 d sugar treatment, then incubated with water for 10 d as described previously (Kano et al., 2010, 2011).
Chemical treatment for rice cut leaves
To visualize H2O2 accumulation, leaf tissues were stained with DAB (Sigma, St Louis, USA) as described by Torres et al. (2005). After treatment with 5mM d-allose alone or with 5mM AsA, 5mM GlcNAc, 25 µM diphenylene iodonium (DPI), or 25mM Na3PO4 for 24h, fully opened fifth leaf blades were immediately vacuum-infiltrated with 0.1% (w/v) DAB solution containing 0.1% (v/v) Triton X-100 and kept in the dark overnight. Coloured leaves were photographed after overnight destaining of excess DAB in ethanol/chloroform (4:1, v/v).
DPI was dissolved in 0.1% (v/v) dimethylsulphoxide (DMSO), and Na3PO4 was dissolved in distilled water and the solution was neutralized. Fifth leaf blades were removed from rice plants, and cut ends were placed in a solution of either 0.1% DMSO, 25 µM DPI (containing 0.1% DMSO), a mixture of 0.1% DMSO and 5mM d-allose, a mixture of 25 µM DPI, 25mM Na3PO4, and 5mM d-allose, or a mixture of 25mM Na3PO4 and 5mM d-allose, at 25 °C for 24h, before DAB staining.
Detection of sugars and phosphorylated sugars by HPLC using ABEE labelling
The p-aminobenzoic acid ethyl ester (ABEE) labelling was performed as described by Yasuno et al. (1999) with modifications. Sugar-treated rice leaves (100mg) were ground in liquid nitrogen with a mortar and pestle. The powder-like tissues were mixed with 500 µl of extraction buffer (30mM potassium phosphate buffer, pH 7.6, containing 1mM EDTA), and centrifuged at 13 000rpm for 10min at 4 °C. The supernatant was passed through an Ultrafree-MC centrifugal filter unit (Millipore, Billerica, MA, USA) (0.22 µm). In the case of phosphatase treatment, the extracts (44 µl) were passed through the filter unit, then mixed with 1 µl (20U) of alkaline phosphatase (Takara, Shiga, Japan) and 5 µl of 10× buffer (in the enzyme kit), and incubated at 37 °C for 1h. For the recombinant enzyme assay, the reaction mixtures with the respective sugar substrate were passed through the filter units.
A 10 µl sample as prepared above was added to 40 µl of ABEE reagent solution (J-Oil Mills, Tokyo, Japan) with borane–pyridine complex (in the kit) and heated at 80 °C for 1h as per the manufacturer’s instructions. After the mixture cooled to room temperature, 200 µl each of distilled water and chloroform were added. After centrifugation of the mixture at 3000rpm for 5min, the upper aqueous layer was used for high-performance liquid chromatography (HPLC).
The layer containing ABEE-labelled sugars (10 µl) was analysed with an HPLC system (Prominence; Shimadzu, Kyoto, Japan) using an Xbridge C18 column (4.6mm ID×250mm) (Waters, Milford, MA, USA). A 50min separation at a flow rate of 1.0ml min–1 at 30 °C with a running solvent system of 0.2mM of potassium borate buffer (pH 8.9)/acetonitrile (93/7) was followed by a 20min wash with 0.02% trifluoroacetic acid/acetonitrile (50/50) and equilibration for 15min with the running solvent. The peaks were monitored with the fluorescence detector (RF-10A XL, Shimadzu) with emission of 360nm and excitation of 305nm.
Cloning strategies
The coding region of AlsK and AlsI was amplified from Escherichia coli JM109 DNA by PCR using specific primers (Supplementary Table S1 at JXB online). The coding region of OsHXK5, OsHXK6, OsrbohC, OsG6PDH1, and OsG6PDH2 was amplified from the respective cDNA clones that were provided by the Rice Genome Resource Center, Japan, using PCR and specific primers (Supplementary Table S1). The signal peptide regions of OsHXK5 (135bp after the initiation codon) and OsHXK6 (129bp after the initiation codon) were excluded. The DNA fragments were inserted into the pBI333-EN4 vector (Nishizawa et al., 1999), pET32 vector (Novagen, Frankfurter, Germany), or pUC18-sGFP vector (Niwa et al., 1999).
Recombinant enzyme production and purification
The DNA fragment containing the coding region of OsHXK5, OsHXK6, AlsK, OsG6PDH1, or OsG6PDH2 was subcloned in-frame into the pET32 vector (Novagen), and overexpressed in E. coli SoluBL21 (Genlantis, San Diego, CA, USA) according to the manufacturer’s instructions. The recombinant proteins were purified using a HisTrap HP column (GE Healthcare, Wauwatosa, WI, USA) as per the manufacturer’s instructions and dialysed against 0.2M TRIS-HCl buffer (pH 7.6) containing MgCl2 (5mM).
Kinase assays
d-Glucose kinase activity of OsHXKs was measured spectrophotometrically at 340nm by coupling production of d-glucose 6-phosphate (G6P) to reduction of NADP via G6PDH reaction as described by Miller and Raines (2005). Reaction mixtures with 0.05–10mM d-glucose contained 0.2M TRIS (pH 7.6), NADP+ (0.5mM), dithiothreitol (DTT; 1mM), ATP (25mM), MgCl2 (5mM), and G6PDH (7.5U). d-Allose kinase activity of OsHXKs was determined spectrophotometrically (340nm) at 25 °C by coupling production of ADP to oxidation of NADH via pyruvate kinase (15U) (Oriental Yeast, Tokyo, Japan) and lactate dehydrogenase (25U) (Oriental Yeast) reactions as described by Miller and Raines (2005). Reaction mixtures with 0.1mM to 1M d-allose contained 0.2M TRIS (pH 7.6), NADH (0.5mM), DTT (1mM), phosphoenolpyruvate (5mM), ATP (25mM), MgCl2 (5mM), and KCl (5mM).
Recombinant G6PDH assays
The activity of recombinant rice G6PDHs was measured spectrophotometrically (340nm) at 25 °C by detecting NADP reduction via G6PDH reaction, which is coupled with G6P production, as described by Wakao and Benning (2005). Reaction mixtures with 0.01–10mM G6P contained 0.2M TRIS (pH 7.6), NADP+ (0.01–10mM), and MgCl2 (5mM). DTT (10mM) was incubated with the reaction mixture for 1h to test its activity.
G6PDH activity determination in protein extracts from rice leaves
Protein, extracted from rice leaves as described by Gibon et al. (2004), was added to dehydrogenase assay buffer (50mM TRIS-HCl, 5mM MgCl2, 0.5mM G6P, 1mM 6-phosphogluconate, 1mM NADP+, pH 7.6), and 6-phosphogluconate dehydrogenase (6PGD) assay buffer (50mM TRIS-HCl, 5mM MgCl2, 1mM 6-phosphogluconate, and 1mM NADP+, pH 7.6). The reduction of NADP+ to NADPH was assessed by absorbance change at 340nm. G6PDH activity was calculated as dehydrogenase activity minus 6PGD activity (Liu et al., 2007).
Rice transformation
The binary vector pBI333-EN4 (Nishizawa et al., 1999) containing the target overexpression or complementation genes were introduced into Agrobacterium tumefaciens EHA101 by electroporation (Shen and Forde, 1989). Rice was transformed as described by Hiei et al. (1994). Second-generation plants were used for Xoo inoculation or various tests to determine the effect on d-allose-induced responses described in other sections.
RT–PCR and qRT–PCR analysis
Total RNA was isolated from rice leaves with Trizol Reagent Kit (Invitrogen, San Diego, CA, USA). RT–PCR was performed with OneStep RT-PCR Kit (Qiagen, Hilden, Germany) for transgenic and Tos17-inserted rice with gene-specific primers (Supplementary Table S1 at JXB online) as described previously (Gomi et al., 2010). For qRT–PCR, reverse transcription was performed using the Prime Script RT Reagent Kit (Takara) with specific primers (Supplementary Table S1) by a Thermal Cycler Dice TP800 (Takara) and SYBR Premix Ex Taq Mixture (Takara). The transcript level was normalized by comparison with actin (AK060893), and the obtained data were analysed as described previously (Kano et al., 2010, 2011).
Results
Effect of rare sugars on induction of rice disease resistance to X. oryzae pv. oryzae
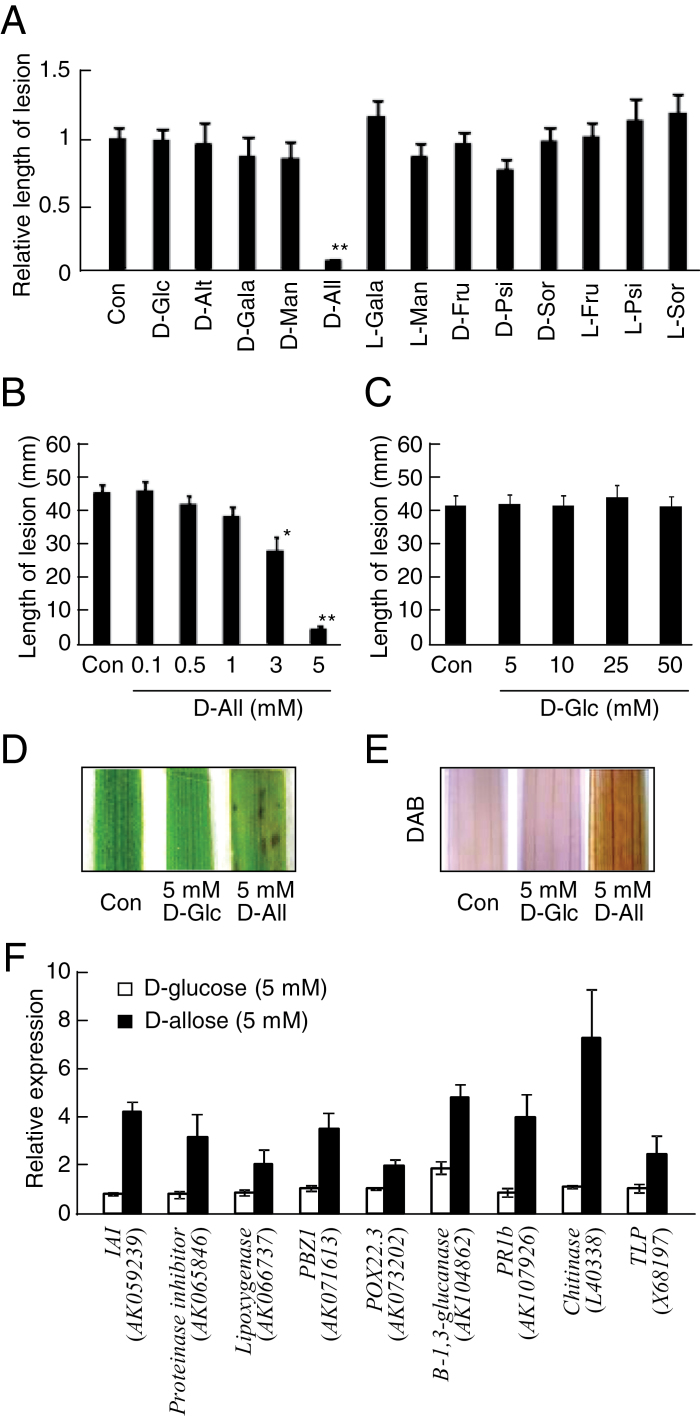
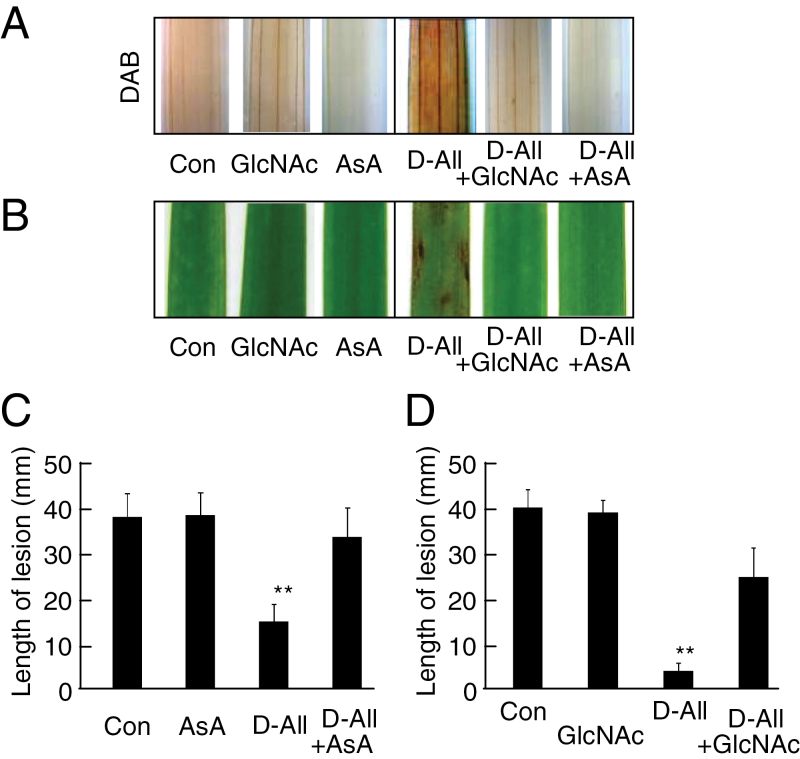
Nine rare sugars and four common sugars (Supplementary Fig. S1 at JXB online) were tested for their ability to induce disease resistance after sugar-treated rice leaves were inoculated with Xoo (Fig. 1). The mean length of lesions was only inhibited after treatment with 5mM d-allose (Fig. 1A), but did not differ significantly after mock or other sugar treatments (Fig. 1A). Disease resistance caused by the d-allose treatment was induced in a dose-dependent manner, with the reduction of lesion development starting at 3mM, while d-glucose produced no inhibition even at 50mM (Fig. 1B, C). The d-allose-specific induction of resistance to Xoo was associated with formation of lesion mimics on the rice leaves (Fig. 1D). Since the lesion mimic after a hypersensitive response is often induced by production of ROS and is associated with induction of disease resistance to Xoo in rice (Yin et al., 2000; Ono et al., 2001; Torres et al., 2005), hydrogen peroxide (H2O2) production was monitored as an indicator of ROS generation by staining leaf tissues with DAB after the d-allose treatment (Fig. 1E). The level of H2O2 was significantly higher in d-allose-treated rice leaves than in d-glucose-treated or mock-treated leaves (Fig. 1E), and expression of defence-related PR-protein genes was also induced in the d-allose-treated rice leaves (Fig. 1F). d-Allose had no visible effect on growth of Xoo (Supplementary Fig. S2). The d-allose-mediated induction of ROS accumulation, lesion mimic formation, and resistance to Xoo was suppressed by simultaneous treatment with AsA, a scavenger of ROS (Fig. 2A–C).
Fig. 1.
Rare sugar effects on induction of rice resistance to Xoo. Mean lesion length (±SE, n=12) on leaves treated for 2 d before Xoo inoculation with (A) 5mM sugars, (B) 0.1–5mM d-allose, or (C) 5–50mM d-glucose. Lesion development 10 d after Xoo inoculation is indicated as values relative to control (A) or lesion lengths (B, C) (*P < 0.05, **P < 0.01). (D) Lesion mimic development on leaves 3 d after a 2 d treatment with 5mM d-glucose or d-allose. (E) DAB detection of H2O2 accumulation in leaves after 24h treatment with 5mM d-glucose or d-allose. (F) Expression of defence-related genes at 2 d after treatment with 5mM d-allose or d-glucose. Fold (±SE, n=4) expression relative to control (no sugar) is shown. The following abbreviations are used in all figures and tables: d-Glc, d-glucose; d-Alt, d-altrose; d-Gala, d-galactose; d-Man, d-mannose; d-All, d-allose; l-Gala,l-galactose; l-Man, l-mannose; d-Fru, d-fructose; d-Psi, d-psicose; d-Sor, d-sorbose; l-Fru, l-fructose; l-Psi, l-psicose; l-Sor, l-sorbose; Con, control; and DAB, 3,3′-diaminobenzidine. (This figure is available in colour at JXB online.)
Fig. 2.
Ascorbic acid (AsA) or N-acetyl-d-glucosamine (GlcNAc) effect on d-allose-induced resistance. (A) DAB detection of H2O2 accumulation in leaves after 24h treatment with 5mM AsA or GlcNAc with or without 5mM d-allose. (B) Lesion mimic development on leaves 3 d after a 2 d treatment with 5mM AsA or GlcNAc with or without 5mM d-allose. (C and D) Mean lesion length (±SE, n=12) on leaves pre-treated with 5mM AsA (C) or GlcNAc (D) with or without 5mM d-allose (**P < 0.01). (This figure is available in colour at JXB online.)
OsrbohC is involved in d-allose-induced resistance to Xoo
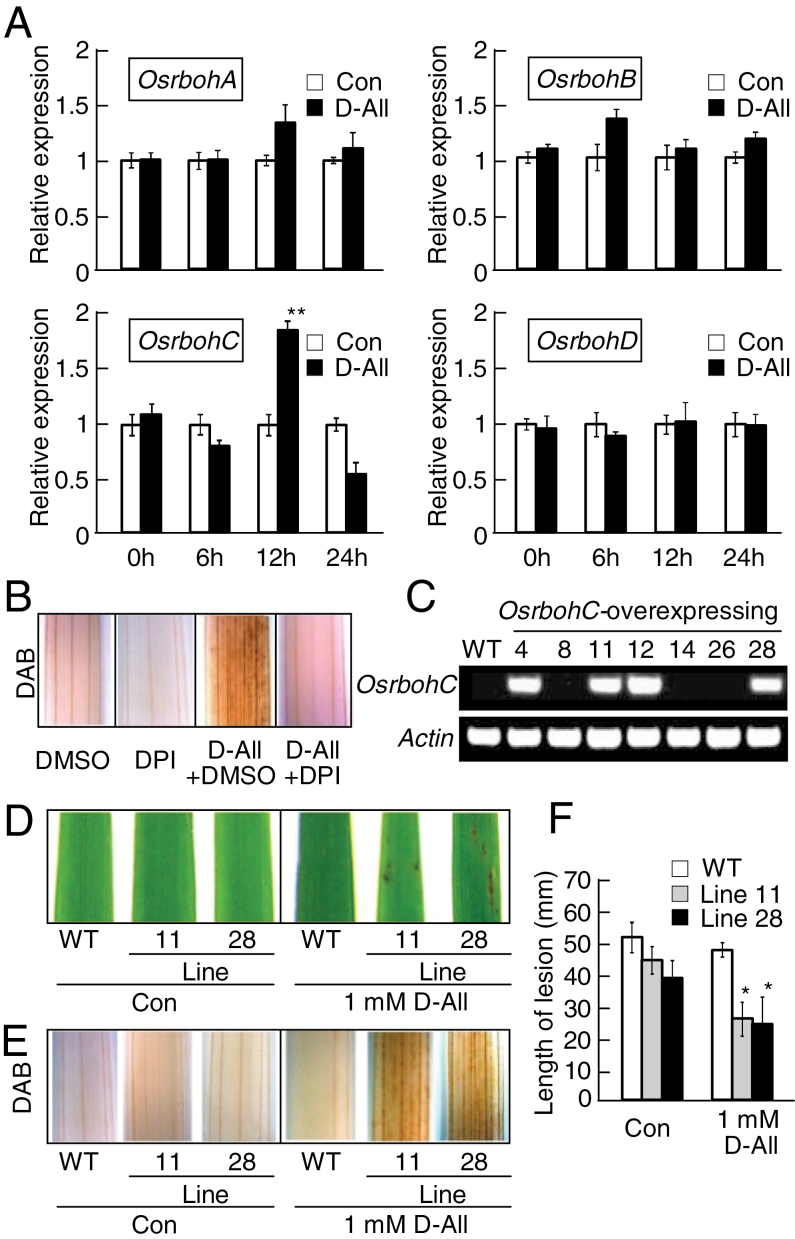
d-Allose induced ROS accumulation (Figs 1E, 2A). NADPH oxidase, encoded by members of the Respiratory burst oxidase homolog (Rboh) gene family, is a known generator of ROS during the defence response of many plants including rice (Doke, 1985; Torres et al., 2005; Sagi and Fluhr, 2006). Thus, induction patterns in d-allose-treated leaves of rice Rboh genes (OsrbohA–OsrbohD) were examined by qRT–PCR analysis (Fig. 3A). Quantitative analysis over time indicated that only OsrbohC was induced at 12h after d-allose treatment (Fig. 3A). Treatment with DPI, an NADPH oxidase inhibitor (Kawasaki et al., 1999), inhibited the accumulation of H2O2 in d-allose-treated leaves (Fig. 3B).
Fig. 3.
OsrbohC is involved in d-allose-induced resistance to Xoo. (A) Osrboh gene expression in leaves at 0–24h after treatment with 5mM d-allose or no sugar (control) was calculated as values (±SE, n=3) relative to control. Accessions: OsrbohA (AK103747), OsrbohB (AK065117), OsrbohC (AK120905), and OsrbohD (AK072353) (**P < 0.01). (B) Effect of diphenylene iodonium (DPI) treatment on d-allose-induced H2O2 accumulation in leaves. (C) RT–PCR detection of OsrbohC and actin gene expressions in leaves from WT and OsrbohC-overexpressing rice. (D) Lesion mimic development in leaves from WT and OsrbohC-overexpressing rice 3 d after a 2 d treatment with 1mM d-allose. (E) DAB detection of H2O2 accumulation after 24h treatment with 1mM d-allose in leaves from WT and OsrbohC-overexpressing rice. (F) Mean lesion lengths (±SE, n=8) 10 d after Xoo inoculation in leaves pre-treated for 2 d with 1mM d-allose from WT and OsrbohC-overexpressing rice (*P < 0.05). (This figure is available in colour at JXB online.)
To examine further the contribution of OsrbohC to ROS generation in d-allose-treated leaves, transgenic rice plants overexpressing OsrbohC were generated (Supplementary Fig. S3A at JXB online). Two-independent lines (lines 11 and 28) were selected from multiple transgenic rice plants expressing OsrbohC (Fig. 3C), and the second generation of these lines was tested further. The overexpression of OsrbohC did not influence growth or any visible phenotype of rice (Supplementary Fig. S3B), and the excess OsrbohC did not change the sensitivity to Xoo with/or without d-glucose treatment (Supplementary Fig. S3C).
When the OsrbohC-overexpressing plants were treated with even 1mM d-allose, lesion mimics formed on the leaves, but not on the treated WT (Fig. 3D). H2O2 generation was much stronger in leaves of transgenic plants treated with 1mM d-allose than in those of the WT (Fig. 3E). When the transgenic plants were inoculated with Xoo, blight lesions were significantly shorter on leaves of 1mM d-allose-treated transgenic plants than on those of the WT (Fig. 3F).
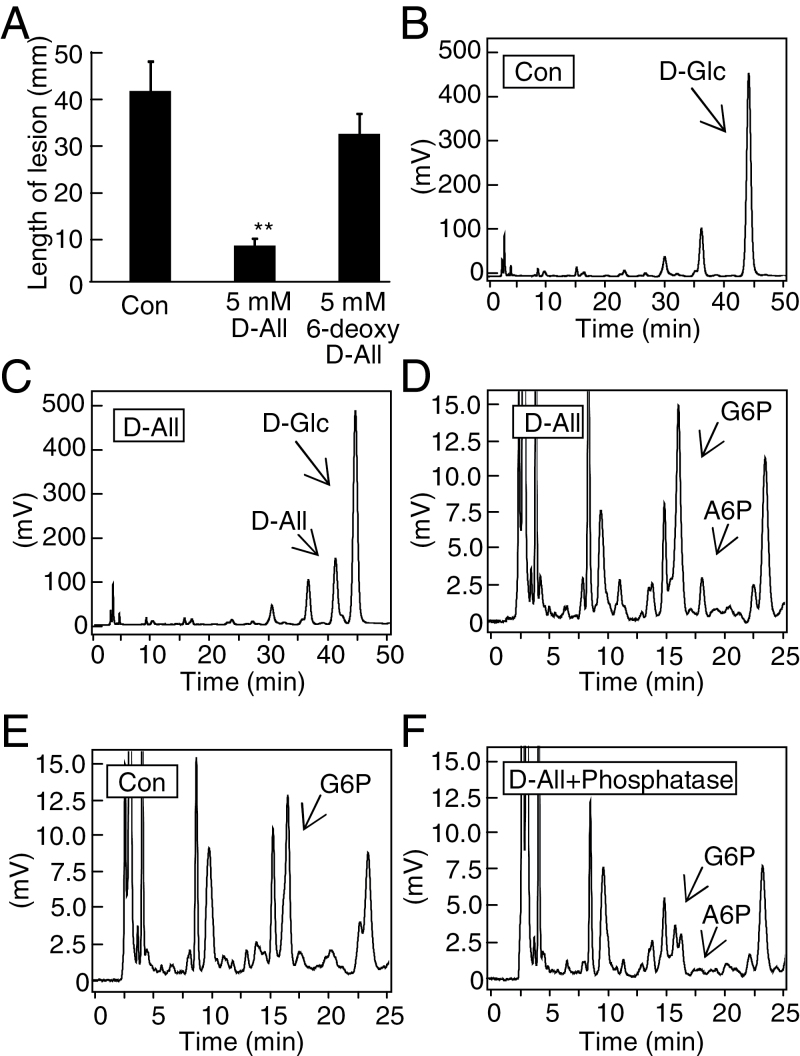
Phosphorylation of d-allose at carbon 6 is important for rice resistance induction
6-Deoxy-d-allose, a derivative of d-allose with a methyl group provided by conversion of a hydroxyl group to hydrogen on carbon 6 (Supplementary Fig. S1 at JXB online), did not confer resistance to Xoo (Fig. 4A). Since the hydroxyl group is often a phosphorylation site on sugars, HPLC was used to check for phosphorylated d-allose in d-allose-treated leaves. The major peak in extracts of mock-treated rice tissue was d-glucose (Fig. 4B), while a d-allose peak was detected in d-allose-treated leaves (Fig. 4C). In addition, a peak of A6P (retention time 17.5min) was detected in extracts from d-allose-treated rice leaves (Fig. 4D), but not from mock-treated leaves (Fig. 4E). Alkaline phosphatase addition to the extracts significantly reduced the peak size of A6P and G6P (Fig. 4F).
Fig. 4.
Detection of phosphorylated d-allose in d-allose-treated leaves. (A) Mean lesion length (±SE, n=12) 10 d after Xoo inoculation of leaves pre-treated for 2 d with 5mM d-allose or 6-deoxy-d-allose (**P < 0.01). (B–E) HPLC detection of ABEE-labelled monosaccharides (Yasuno et al., 1999) in extracts from leaves treated with (C and D) or without d-allose (B and E) for 24h. (D) Close-up of the chart to show phosphorylated sugars of (C). (E) Close-up of (B). (F) Reduction of phosphorylated sugars by phosphatase addition in extracts from leaves treated with d-allose. A comparative scale view to (D) is shown. Calculated values of sugars in leaves were 852ng g FW–1 for d-allose and 2.7 µg g FW–1 for d-glucose in (C). The following abbreviations are used in all figures and tables: A6P, d-allose 6-phosphate; G6P, d-glucose 6-phosphate.
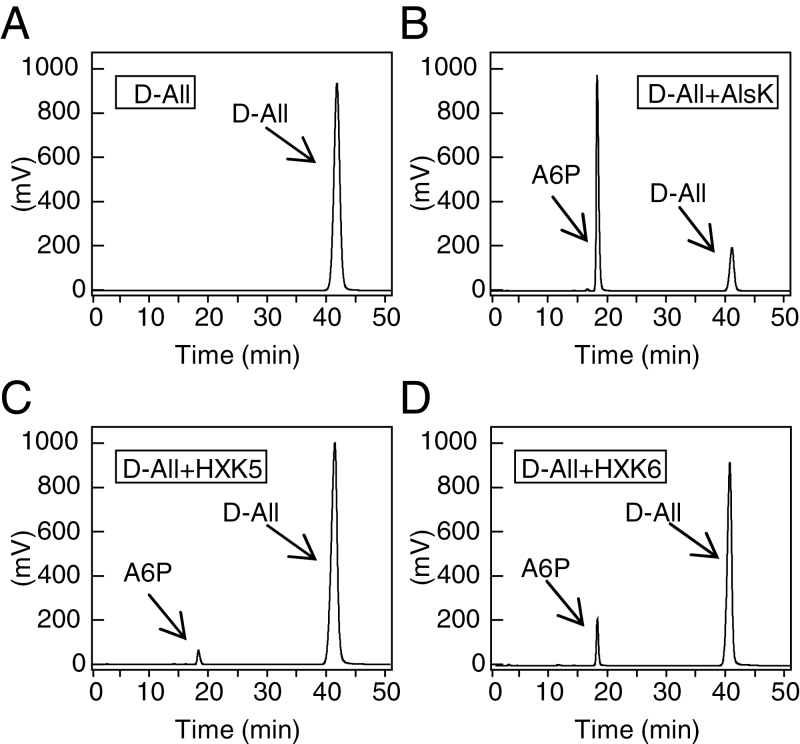
Hexokinases of yeast and T. caldophilus use several sugars including d-allose as substrates (Chenault et al., 1997; Bae et al., 2005). A6P levels were thus measured after supplying d-allose as a substrate for two main rice hexokinases, OsHXK5 and OsHXK6 (Cho et al., 2009) using their respective recombinants OsHXK5 and OsHXK6 (Fig. 5) or a recombinant d-allose kinase (AlsK) of E. coli (Miller and Raines, 2005) (Supplementary Fig. S4 at JXB online) as the positive control for A6P production (Fig. 5). K m values of OsHXK5 and OsHXK6 for d-allose differed by two orders of magnitude from those for d-glucose, but the difference in affinity was lower than that of AlsK for d-glucose (Table 1) (Miller and Raines, 2005). Based on comparisons of the k cat/K m values, enzymatic activity of AlsK for d-allose conversion to A6P was >100 times more efficient than that of OsHXK5 or OsHXK6, but both OsHXK5 and OsHXK6 were also highly active in converting d-allose to A6P (Fig. 5, Table 1). Moreover, d-allose-mediated induction of Xoo resistance was suppressed by treatment with GlcNAc, an inhibitor of HXK, as were ROS generation and subsequent lesion mimic development (Fig. 2A, B, D).
Fig. 5.
d-Allose phosphorylation by recombinant kinases. (A–D) Products of recombinant d-allose kinase (AlsK) and rice hexokinases (HXK5 and HXK6) reacted with 5mM d-allose were labelled by ABEE (Yasuno et al., 1999) and determined by HPLC. (A) d-Allose alone without any recombinant enzyme. (B) AlsK reacted with d-allose. (C) HXK5 reacted with d-allose. (D) HXK6 reacted with d-allose.
Table 1.
Rice hexokinase OsHXK5 and OsHXK6 can use d-allose as substrate
| Substrate | k cat (s–1) | K m (M) | k cat/K m (M–1 s–1) | Reference |
|---|---|---|---|---|
| OsHXK5 | ||||
| d-Glucose | 205 | 1.9×10–4 | 1.1×106 | This study |
| d-Allose | 13 | 3.8×10–2 | 3.4×102 | This study |
| OsHXK6 | ||||
| d-Glucose | 106.5 | 2.0×10–4 | 5.3×105 | This study |
| d-Allose | 18 | 3.7×10–2 | 4.9×102 | This study |
| Allose kinase | ||||
| d-Glucose | 1.5 | 1.0×10–1 | 1.5×101 | Miller and Raines (2005) |
| d-Allose | 17 | 2.6×10–4 | 6.5×104 | Miller and Raines (2005) |
Sugar kinase activity was determined by the methods of Miller and Raines (2005).
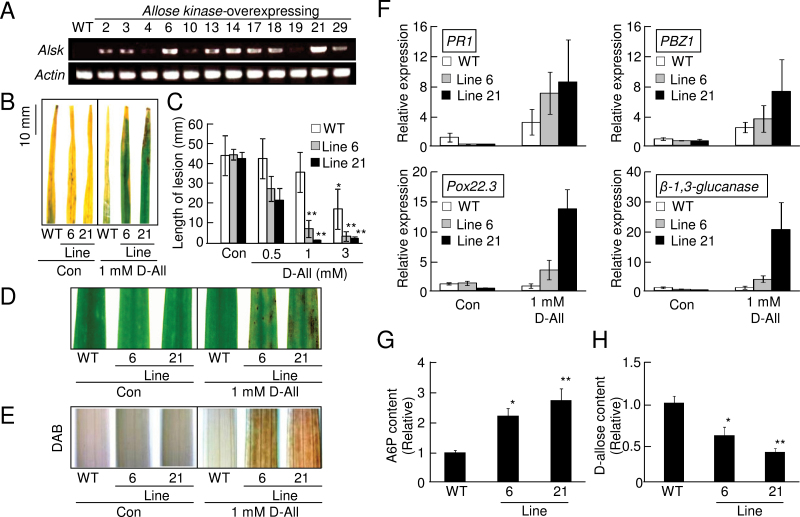
Overexpression of AlsK increased sensitivity to d-allose in rice plants
Since the phosphorylation of d-allose by E. coli AlsK was much more efficient than that by OsHXK5 and OsHXK6 (Table 1), transgenic rice plants were generated that constitutively expressed E. coli AlsK (Miller and Raines, 2005) (Supplementary Fig. S5A at JXB online) to enhance the efficiency of d-allose conversion to A6P. Two independent lines (lines 6 and 21) were selected among transgenic rice plants that were confirmed by RT–PCR to express AlsK (Fig. 6A), and the second generation of these lines was used for further experiments. Overexpression of AlsK did not influence growth or any visible trait of rice (Supplementary Fig. S5B), and excess AlsK did not change the sensitivity to Xoo with/or without d-glucose treatment (Supplementary Fig. S5C). There was also no significant difference in the ratios of inherent d-glucose and G6P contents between the AlsK-overexpressing plants and the WT (Supplementary Fig. S5D, E). However, when these rice plants were treated with even 1 mM d-allose, the blight lesions on the AlsK-expressing plants were significantly shorter than those on the WT (Fig. 6B, C). The enhanced resistance was associated with lesion mimic formation in the AlsK-expressing plants treated with 1mM d-allose (Fig. 6D), and H2O2 accumulation was also enhanced (Fig. 6E). Expression of PR-protein genes including probenazole-inducible protein (PBZ1), pathogenesis-related protein 1b (PR1b), peroxidase (Pox22.3), and β-1,3-glucanase, which are known to be induced strongly by >5mM d-allose in the WT (Fig. 1F), were significantly induced even by 1mM d-allose (Fig. 6F).
Fig. 6.
d-Allose sensitivity increased by AlsK overexpression in rice. (A) RT–PCR detection of AlsK and actin expression in leaves from WT and different lines of AlsK-overexpressing rice. (B) Typical lesion development 10 d after Xoo inoculation of WT and AlsK-overexpressing lines pre-treated with or without 1mM d-allose. (C) Mean lesion length (±SE, n=8) 10 d after Xoo inoculation in leaves pre-treated for 2 d with 0.5–3mM d-allose (**P < 0.01 compared with the WT without d-allose treatment). (D) Lesion mimic development in leaves from WT and AlsK-overexpressing rice at 3 d after a 2 d treatment with 1mM d-allose. (E) DAB detection of H2O2 accumulation at 24h after treatment with 1mM d-allose in leaves from WT and AlsK-overexpressing rice plants. (F) Expression of defence-related genes at 2 d after treatment with 1mM d-allose. Fold (±SE, n=4) expression relative to control (no sugar) is shown. Accession numbers are given in Fig. 1. (G, H) d-Allose 6-phosphate (A6P) (G) or d-allose (H) content detected by HPLC in leaves from WT and AlsK-overexpressing lines at 24h after treatment with 5mM d-allose. Values are relative (±SE, n=3) to the WT (*P < 0.05, **P < 0.01). The calculated value of d-allose content was 756ng g FW–1 in (H). (This figure is available in colour at JXB online.)
The HPLC peak area corresponding to A6P in both lines (6 and 21) of the d-allose-treated AlsK-overexpressing plants was significantly higher than in the WT (Fig. 6G; Supplementary Fig. S5G, I, K at JXB online), and those for d-allose were lower in the transgenic plants than in the WT (Fig. 6H; Supplementary Fig. S5F, H, J).
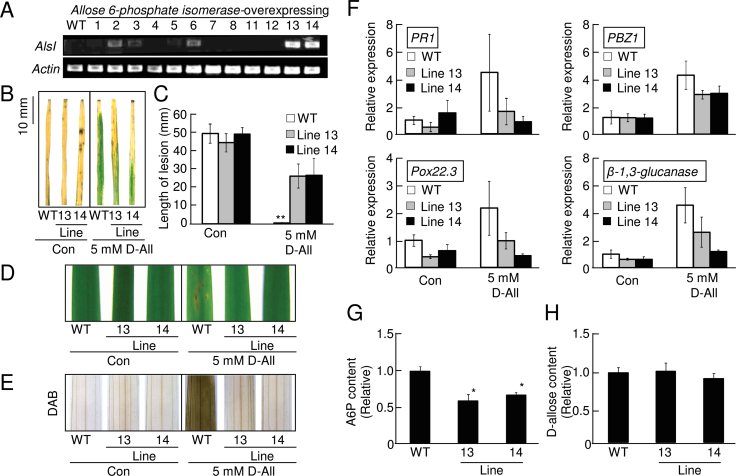
Overexpression of AlsI decreased sensitivity to d-allose in rice plants
Since E. coli d-allose 6-phosphate isomerases (AlsI) is known to convert A6P to d-psicose 6-phosphate (P6P) (Kim et al., 1997) (Supplementary Fig. S4 at JXB online), transgenic rice plants constitutively expressing E. coli AlsI were generated (Supplementary Fig. S6A) to decrease A6P by conversion to P6P. Two independent lines (lines 13 and 14) expressing AlsI were selected (Fig. 7A), and the second generation of these lines was used for further experiments. Overexpression of AlsI did not affect growth or any visible trait of rice (Supplementary Fig. S6B). When these AlsI-expressing rice plants were treated with 5 mM d-allose and inoculated with Xoo, d-allose-induced resistance was reduced, and blight lesion formation was significantly increased (Fig. 7B, C). The reduced d-allose-induced resistance to Xoo in the AlsI-expressing plants was associated with reduced lesion mimic formation (Fig. 7D), accumulation of H2O2 (Fig. 7E), and expression of the PR-protein gene (Fig. 7F).
Fig. 7.
d-Allose sensitivity decreased by AlsI overexpression in rice. (A) RT–PCR detection of AlsI and actin expression in leaves from the WT and different lines of AlsI-overexpressing rice. (B) Typical lesion development at 10 d after Xoo inoculation of the WT and AlsI-overexpressing lines pre-treated with or without 5mM d-allose. (C) Mean lesion length (±SE, n=8) at 10 d after Xoo inoculation of leaves pre-treated for 2 d with 5mM d-allose (**P < 0.01 compared with the WT without d-allose treatment). (D) Lesion mimic development in leaves from WT and AlsI-overexpressing rice 3 d after a 2 d treatment with 5mM d-allose. (E) DAB detection of H2O2 accumulation 24h after treatment with 5mM d-allose in leaves from WT and AlsI-overexpressing plants. (F) Expression of defence-related genes at 2 d after treatment with 5mM d-allose. Fold (±SE, n=4) expression relative to the control (no sugar) is shown. (G, H) d-Allose 6-phosphate (A6P) (G) or d-allose (H) content detected by HPLC in leaves from the WT and AlsI-overexpressing lines at 24h after treatment with 5mM d-allose. Values are relative (±SE, n=3) to the WT (*P < 0.05). The calculated value of d-allose content was 823ng g FW–1 in (H). (This figure is available in colour at JXB online.)
The HPLC peak area corresponding to A6P in both lines (13 and 14) of the d-allose-treated AlsI-overexpressing plants was significantly lower than in the WT (Fig. 7G; Supplementary Fig. S6D, F, H at JXB online), and those for d-allose did not change (Fig. 7H; Supplementary Fig. S6C, E, G).
Reduced sensitivity to d-allose in the G6PDH-defective rice mutant
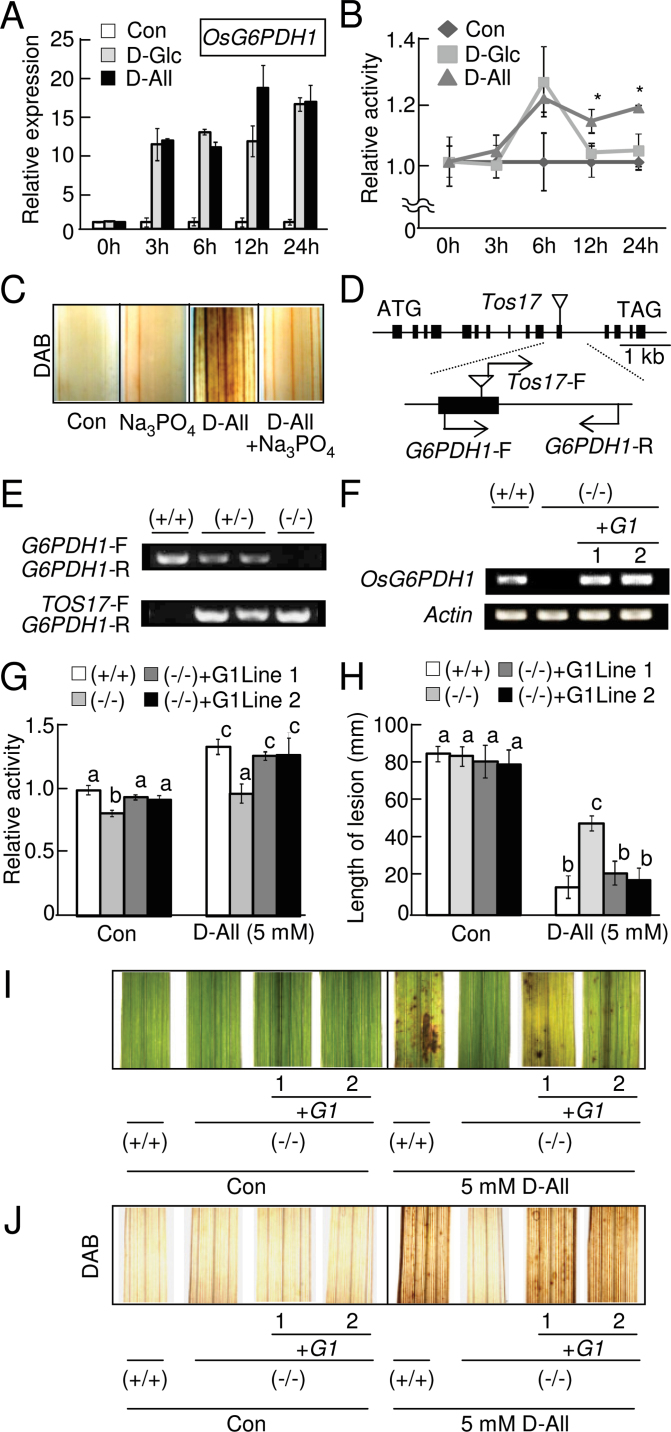
In this study, it was found that rice hexokinases can catalyse the conversion of d-allose to A6P (Fig. 5, Table 1), which accumulates and leads to the induction of defence responses (Figs 2A, B, D, 4, 6, 7). Since the hexokinase product (G6P) from d-glucose can serve as the substrate of G6PDH, which can supply NADPH to NADPH oxidase for ROS generation (e.g. Scharte et al., 2009; Gutpe et al., 2009; Spencer et al., 2011), the involvement of G6PDH in d-allose signal transduction was examined. Among five genes encoding G6PDH in the rice genome (OsG6PDH1–OsG6PDH5), expression of OsG6PDH1 (Fig. 8A), OsG6PDH3, and OsG6PDH5 (Supplementary Fig. S7A at JXB online) was induced as soon as 3h after treatment with d-glucose or d-allose, and expression of OsG6PDH1 at 12h (Fig. 8A) and of OsG6PDH3 at 12h and 24h after d-allose treatment (Supplementary Fig. S7A) was higher than with d-glucose.
Fig. 8.
Role of OsG6PDH1 in the d-allose signalling pathway. (A) OsG6PDH1 gene (accession no. AK073697) expression in leaves at 0–24h after treatment with 5mM d-allose or d-glucose, calculated relative (±SE, n=4) to the control (no sugar). (B) Total G6PDH activity in extracts from leaves at 0–24h after treatment with 5mM d-allose or d-glucose, calculated as values relative (±SE, n=4) to the control (no sugar) (*P < 0.05). The calculated value of the enzymatic activity for the control at 0h was 99 µmol NADPH min–1 g FW–1. (C) DAB detection of H2O2 accumulation at 24h after treatment with 5mM d-allose with or without a G6PDH inhibitor (Na3PO4). (D) Exon and intron organization of OsG6PDH1 and the Tos17 insertion site with locations of specific primers. Solid boxes and lines indicate exons and introns; triangles indicate the position of Tos17 insertion. (E) Genotypic determination for wild homozygote (+/+), heterozygote (+/–), or homozygote for Tos17 insertion (–/–) in the Tos17 mutant line NC8489 by genomic PCR with primer pairs in (D). (F) OsG6PDH1 transcript accumulations in the leaves by RT–PCR. Abbreviations in (F–J): (+/+) wild homozygote, (–/–) homozygote for Tos17 insertion, or (+G1) two lines of the OsG6PDH1 complementation mutant in Tos17 mutant line NC8489, respectively. (G) Total G6PDH activity in extracts from leaves of (+/+), (–/–), or (+G1) at 0 or 24h after treatment with 5mM d-allose, calculated as values relative (±SE, n=4) to (+/+) (no sugar). Means with different letters are significantly different at P < 0.05. (H) Mean lesion length (±SE, n=8) 10 d after Xoo inoculation in leaves pre-treated for 2 d with or without 5mM d-allose. Means with different letters are significantly different at P < 0.05. (I) Lesion mimic development in leaves from (+/+), (–/–), or (+G1) at 3 d after a 2 d treatment with 5mM d-allose. (J) DAB detection of H2O2 accumulation at 24h after treatment with 5mM d-allose in leaves from (+/+), (–/–), or (+G1) rice plants. (This figure is available in colour at JXB online.)
Phylogenic analyses of OsG6PDH genes against Arabidopsis G6PD genes encoding G6PDH predicted that OsG6PDH1 and OsG6PDH2 are in the cytoplasm (Supplementary Fig. S7B at JXB online) because Arabidopsis G6PD5 and G6PD6 in the same clade are cytoplasmic (Wakao et al., 2008). There is no typical sorting signal present in OsG6PDH1 and OsG6PDH2, and green fluorescent protein (GFP)-tagged OsG6PDH1 and G6PDH2 in the bombarded tobacco leaf cells localized in the cytoplasm (Supplementary Fig. S7C).
Recombinant proteins of OsG6PDH1 and OsG6PDH2 had enzymatic activity with G6P and NADP+, and OsG6PDH1 had higher activity based on k cat/K m values (Table 2). OsG6PDH1 and OsG6PDH2 suffered tight feedback inhibition by NADPH (Supplementary Table S2 at JXB online), and DTT did not affect activity with G6P (Supplementary Table S3) as described for Arabidopsis G6PDs (Wakao and Benning, 2005). Neither enzyme used A6P as a substrate (Supplementary Table S3). Interestingly, when total OsG6PDH activity was measured using protein extracts of rice leaf tissues at various times after either d-glucose or d-allose treatment, G6PDH activity had increased by 6h after d-glucose and d-allose treatments (Fig. 8B). However, activity dropped to the control level after 12h with d-glucose, but activity did not drop with d-allose even after 24h (Fig. 8B). Na3PO4, an inhibitor of G6PDH (Liu et al., 2007), significantly reduced H2O2 accumulation in d-allose-treated leaves (Fig. 8C), also implicating G6PDH in d-allose signal transduction.
Table 2.
Enzymatic profiles for OsG6PDH1 and OsG6PDH2 recombinant proteins using G6P as a kinetic parameter
| Enzyme | k cat (s–1) | K m G6P (M) | k cat/K m G6P (M–1 s–1) |
|---|---|---|---|
| G6PDH1 | 3.04 | 6.06×10–4 | 5.02×103 |
| G6PDH2 | 1.06 | 1.05×10–3 | 1.01×103 |
Kinetic parameters were determined using a G6PDH-coupled assay for G6P (Wakao and Benning, 2005).
To examine further the role of cytosolic OsG6PDH1 and OsG6PDH2 in d-allose signalling, several retrotransposon Tos17 insertion lines were obtained and line NC8489 was examined for an OsG6PDH1 mutation. Several Tos17-insertion mutants for OsG6PDH1 were found (Supplementary Fig. S8A at JXB online), but none for OsG6PDH2. Among mutant lines, Tos17 was inserted at the target site in exon 11 in NC8489, which was then examined further (Fig. 8D; Supplementary Fig. S8A).
In the homozygous NC8489 line (–/–), OsG6PDH1 was not amplified from genomic DNA (Fig. 8E) or mRNA (Fig. 8F). Total enzyme activity of G6PDHs in leaf extracts was reduced in the line with/or without 5mM d-allose (Fig. 8G), and d-allose-induced resistance to Xoo was lower than in the WT (+/+) (Fig. 8H). The reduced d-allose-induced resistance to Xoo in the line was associated with reductions of lesion mimic formation (Fig. 8I) and H2O2 accumulation (Fig. 8J). To confirm that this reduction in d-allose sensitivity was caused by the loss of OsG6PDH1, the intact OsG6PDH1 gene was introduced into line NC8489 (–/–) for a complementation analysis. When the OsG6PDH1 promoter region (2496bp) connected to OsG6PDH1 (Supplementary Fig. S8B at JXB online) was introduced (+G1 line 1 and line 2) (Fig. 8F–J), transcription and protein function of OsG6PDH1 was recovered in two independent complementation lines (+G1 line 1 and line 2) (Fig. 8F, G), and their sensitivities for d-allose were nearly equal to that of the WT (+/+) (Fig. 8H–J).
Discussion
Natural oligosaccharides and salicylic acid are known as plant defence activators that induce PR-protein gene expression and defence responses in many different plants (e.g. Dixon and Lamb, 1990; Ebel and Cosio, 1994; Klessig and Malamy, 1994). Some monosaccharides, mainly d-glucose and d-fructose at high concentrations, can also regulate growth of higher plants (Rolland et al., 2006); however, monosaccharides have never been reported to be deeply involved in the induction of a plant defence system. As far as is known, the present finding that d-allose induced a rice defence reaction against Xoo that included lesion mimic formation and PR-protein gene expression initiated by ROS generation is a novel effect of this particular monosaccharide in plants, and thus d-allose might be a candidate agent to test for reduction of disease development in rice (Kano et al., 2010).
ROS are generated by NADPH oxidase in defence responses in many plants (Doke, 1985; Yin et al., 2000; Ono et al., 2001; Torres et al., 2005; Sagi and Fluhr, 2006), and rice Osrboh genes encoding an NADPH oxidase have been identified (Wong et al., 2007). Shimamoto’s group (Kawasaki et al., 1999; Wong et al., 2007) showed that ROS functions in a regulatory mechanism by forming a multiprotein complex with OsrbohB. In this study, the involvement of rbohs in the d-allose-induced rice defence induction was also identified because treatment with DPI, an NADPH oxidase inhibitor (Kawasaki et al., 1999), inhibited ROS accumulation in d-allose-treated leaves, and the expression of the OsrbohC gene was typically induced after d-allose treatment. It was then found that the OsrbohC-overexpressing plants were more sensitive to d-allose for induction of ROS accumulation. Overexpression of OsrbohC did not result in constitutive ROS production, perhaps because rboh is known to require post-transcriptional regulation for ROS generation that is induced only after a trigger by various stresses (Doke, 1985; Kawasaki et al., 1999; Sagi and Fluhr, 2006; Wong et al., 2007). For example, Ca2+ influx into the cytoplasm and changes in protein phosphorylation are implicated in activating rboh (Sagi and Fluhr, 2006), and many other proteins including small GTPase Rac/Rop are involved in regulating the OsrbohB complex (Wong et al., 2007; Nakashima et al., 2008). Although candidate proteins for the putative complex that includes OsrbohC are not clear yet, Shimamoto’s group (Wong et al., 2007) reported that Rac2, Rac6, and Rac7 could directly interact with OsrbohC in their yeast two-hybrid system, perhaps indicating the involvement of Rac in OsrbohC activation after d-allose treatment.
A6P was detected in rice leaves after d-allose treatment. Although HXK has been known as the first enzyme in the hexose assimilation pathway (Jang et al., 1997; Rolland et al., 2006), the presence of plant enzymes responsible for the phosphorylation of d-allose has never been reported. However, some HXKs from yeast and T. caldophilus have the potential to phosphorylate various aldohexoses including d-allose (Chenault et al., 1997; Bae et al., 2005), and it was established that rice HXKs, known to possess a glucose kinase function (Cho et al., 2009), can also catalyse d-allose phosphorylation. Rice OsHXK5 and OsHXK6 were selected to test as the target HXKs because these rice HXKs are considered to be comparable in function with Arabidopsis AtHXK1 (Cho et al., 2009), and a loss-of-function mutant of AtHXK1 [glucose-insensitive2 (gin2) mutant] had a d-allose-insensitive phenotype for inhibition of vegetative growth of Arabidopsis seedlings (Fukumoto et al., 2011, 2013).
Reduced conversion of d-allose to A6P by HXK inhibition and a modification of d-allose at carbon 6 (6-deoxy-d-allose) to block phosphorylation prevented any defence responses, indicating the importance of d-allose conversion to A6P in d-allose signal transduction. Thus, overexpression of E. coli AlsK was tested, which is more efficient for A6P production than OsHXK5 or OsHXK6 but less efficient for G6P production from d-glucose, and the conversion of A6P from d-allose increased in AlsK-overexpressng rice, as did sensitivity to d-allose for inducing defence responses including ROS induction, lesion mimic formation, PR-protein gene expression, and disease resistance against Xoo. Escherichia coli AlsI was also overexpressed to convert the accumulated A6P to P6P; defence induction was reduced, further elucidating the importance of A6P in d-allose signal transduction. Together these results indicate that HXK is the initial contact site for d-allose in rice cells, and the conversion of d-allose to A6P is essential for the defence responses in rice (Supplementary Fig. S9 at JXB online).
Many monosaccharides play a role in signal transduction for cellular functions through their phosphorylation during sugar metabolism (e.g. Rolland et al., 2006; Chu et al., 2010). During glycolysis, phosphorylated d-glucose G6P can be converted to F6P by G6P isomerase and converted to 6-phosphogluconolactone by G6PDH in the pentose-phosphate cycle (Rolland et al., 2006). The pentose-phosphate cycle, which generates NADPH and is related to redox regulations (Kruger and von Schaewen, 2003; Wakao and Benning, 2005; Ratcliffe and Shachar-Hill, 2006), is also considered to be involved in plant defence systems because inhibiting G6PDH reduces ROS generation induced by elicitor treatment (Pugin et al., 1997) and because cytosolic overexpression of the P2 type of G6PDH leads to the induction of disease resistance with ROS generation via NADPH oxidase (Scharte et al., 2009). G6PDH also seems to be involved in various cellular regulations via post-transcriptional modifications (Bulteau et al., 2001; Gupte et al., 2009), but the exact roles of this enzyme other than as the initial enzyme of the pentose-phosphate pathway are not clear. Plant G6PDH can be regulated by redox balance (Wakao and Benning, 2005), and a complex formation of the P0 type of G6PDH leads to a change in localization of other P1-type G6PDHs (Meyer et al., 2011).
In this study, it was found that a G6PDH inhibitor reduced d-allose-derived ROS generation, the defence responses of a Tos17-inserted mutant of OsG6PDH1 were less sensitive to d-allose, and lines complemented with OsG6PDH1 recovered full sensitivity to d-allose. These results revealed that cytosolic OsG6PDH1 is involved in d-allose signal transduction to induce defence responses (Supplementary Fig. S9 at JXB online). Gene expression of plastidic isoforms of the P2 type of OsG6PDH3 and the P0 type of OsG6PDH5 were also induced by d-allose treatment. Similar to the case of OsG6PDH1 at 12h after d-allose treatment, OsG6PDH3 expression was induced more by d-allose than by d-glucose. Although involvement of these plastidic rice G6PDHs in ROS generation caused by plasma membrane-localized NADPH oxidase is not known yet, further study will be fascinating because formation of a protein complex with G6PDH isoforms was reported to lead to a change in the localization of other G6PDH isoforms (Meyer et al., 2011), and a plastidic type of G6PDH was reported to be involved in NADPH oxidase-dependent ROS generation induced by an elicitor in tobacco (Asai et al., 2011). Multiple reports describe NADPH derived from G6PDH reaction for ROS generation (e.g. Scharte et al., 2009; Gutpe et al., 2009; Spencer et al., 2011), but recombinant OsG6PDH1 did not use A6P as a substrate, indicating that d-allose-triggered ROS generation by NADPH oxidase is probably not caused by simple activation of OsG6PDH1 reaction by HXK-derived A6P. Interestingly, G6PDH activity in d-allose-treated leaves increased at 6h after treatment and the level was maintained even after 24h; however, G6PDH activity in d-glucose-treated leaves had increased by 6h after the treatment and returned to the control level by 12h. Since plant G6PDH is an unstable protein (Wakao and Benning, 2005), it was hypothesized that the G6PDH activity after 12h in the d-allose-treated leaves may be due to A6P stabilizing G6PDH. Various phosphorylated monosaccharides can interact with G6PDH (Scott and Tatum, 1971), and these substrates and cofactors also help stabilize the G6PDH (Puchkaev et al., 2002; Wang and Engel, 2009). Phosphorylated monosaccharides are known to increase protein stability or activation. For example, glycogen synthase can bind G6P, which is not a substrate or cofactor of this enzyme, thus rearranging the subunit interface and facilitating catalysis by freeing the active site (Baskaran et al., 2010). G6PDH can also be regulated by phosphorylation (Bulteau et al., 2001; Gupte et al., 2009), and protein kinases are post-transcriptionally involved as regulatory proteins of G6PDH (Gupte et al., 2009; Santo et al., 2012). A6P might interact with regulatory proteins other than G6PDH to activate or maintain the stability of G6PDH to generate NADPH by G6P usage as well. This body of information indicates that the exact targets and functions of A6P on the d-allose effects described here will provide more insight into novel roles for phosphorylated sugars in the future.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Fisher projections of various monosaccharide structures used in this study.
Figure S2. Effect of d-allose concentration on Xoo growth in liquid culture.
Figure S3. OsrbohC overexpression in rice.
Figure S4. Metabolic pathway of d-allose in Escherichia coli.
Figure S5. E. coli d-allose kinase (AlsK) overexpression in rice.
Figure S6. E. coli d-allose 6-phosphate isomerase (AlsI) overexpression in rice.
Figure S7. Characterization of rice G6PDH genes.
Figure S8. Characterization of Tos17 mutants for OsG6PDH1 and its complementation.
Figure S9. Schematic model of d-allose signal transduction for induction of rice resistance to Xoo.
Table S1. Primers used in this study.
Table S2. Enzymatic profiles for OsG6PDH1- and OsG6PDH2-recombinant proteins using NADP+ as a kinetic parameter.
Table S3. Property summary for OsG6PDH1- and OsG6PDH2-recombinant proteins.
Acknowledgements
We thank Dr H. Kaku, NIAS, Tsukuba, Japan, for rice seeds and Xoo, and the Rice Genome Resource Center at NIAS for providing Tos17 lines. This work was supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry.
References
- Asai S, Yoshioka M, Nomura H, et al. 2011. A plastidic glucose-6-phosphate dehydrogenase is responsible for hypersensitive response cell death and reactive oxygen species production. Journal of General Plant Pathology 77, 152–162 [Google Scholar]
- Bae J, Kim D, Choi Y, et al. 2005. A hexokinase with broad sugar specificity from a thermophilic bacterium. Biochemical and Biophysical Research Communications 334, 754–763 [DOI] [PubMed] [Google Scholar]
- Baskaran S, Roach PJ, DePaoli-Roach AA, Hurley TD. 2010. Structural basis for glucose-6-phosphate activation of glycogen synthase. Proceedings of the National Academy of Sciences, USA 107, 17563–17568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulteau AL, Verbeke P, Petropoulos I, Chaffotte AF, Friguet B. 2001. Proteasome inhibition in glyoxal-treated fibroblasts and resistance of glycated glucose-6-phosphate dehydrogenase to 20 S proteasome degradation in vitro . Journal of Biological Chemistry 276, 45662–45668 [DOI] [PubMed] [Google Scholar]
- Chari VM, Grayer-Barkmeijer RJ, Harborne JB, Öesterdahl BG. 1981. An acylated allose-containing 8-hydroxyflavone glycoside from Veronica filiformis . Phytochemistry 20, 1977–1979 [Google Scholar]
- Chenault HK, Mandes RF, Hornberger KR. 1997. Synthetic utility of yeast hexokinase. Substrate specificity, cofactor regeneration, and product isolation. Journal of Organic Chemistry 62, 331–336 [DOI] [PubMed] [Google Scholar]
- Cho J-I, Ryoo N, Eom J-S, et al. 2009. Role of the rice hexokinases OsHXK5 and OsHXK6 as glucose sensors. Plant Physiology 149, 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Noh H-N, Kim S, Kim KH, Hong S-W, Lee H. 2010. Enhanced drought tolerance in Arabidopsis via genetic manipulation aimed at the reduction of glucosamine-induced ROS generation. Plant Molecular Biology 74, 493–502 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Lamb CJ. 1990. Molecular communication in interactions between plants and microbial pathogens. Annual Review of Plant Physiology and Plant Molecular Biology 41, 339–367 [Google Scholar]
- Doke N. 1985. NADPH-dependent O2 – generation in membrane fractions isolated from wounded potato tubers inoculated with Phytophtora infestans . Physiological Plant Pathology 27, 311–322 [Google Scholar]
- Ebel J, Cosio EG. 1994. Elicitors of plant defense responses. International Review of Cytology 148, 1–36 [Google Scholar]
- Fukumoto T, Kano A, Ohtani K, et al. 2011. Rare sugar d-allose suppresses gibberellin signaling through hexokinase-dependent pathway in Oryza sativa L. Planta 234, 1083–1095 [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kano A, Ohtani K, et al. 2013. Phosphorylation of d-allose by hexokinase involved in regulation of OsABF1 expression for growth inhibition in Oryza sativa L. Planta 237, 1379–1391 [DOI] [PubMed] [Google Scholar]
- Gibbins LN, Simpson FJ. 1964. The incorporation of d-allose into the glycolytic pathway by Aerobacter aerogenes . Canadian Journal of Microbiology 10, 829–836 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, et al. 2004. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. The Plant Cell 39, 847–862 [DOI] [PubMed] [Google Scholar]
- Gomi K, Satoh M, Ozawa R, et al. 2010. Role of hydroperoxide lyase in white-backed planthopper (Sogatella furcifera Horváth)-induced resistance to bacterial blight in rice, Oryza sativa L. The Plant Journal 61, 46–57 [DOI] [PubMed] [Google Scholar]
- Gupte RS, Floyd BC, Kozicky M, et al. 2009. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radical Biology and Medicine 47, 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal 6, 271–282 [DOI] [PubMed] [Google Scholar]
- Hossain MA, Izuishi K, Maeta H. 2003. Protective effects of d-allose against ischemia reperfusion injury of the liver. Journal of Hepato-Biliary-Pancreatic Sciences 10, 218–225 [DOI] [PubMed] [Google Scholar]
- Hossain MA, Wakabayashi H, Goda F, Kobayashi S, Maeba T, Maeta H. 2000. Effect of immunosuppressants FK506 and d-allose on allogenic orthotopic liver transplantation in rats. Transplantation Proceedings 32, 2021–2023 [DOI] [PubMed] [Google Scholar]
- Izumori K. 2002. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 89, 120–124 [DOI] [PubMed] [Google Scholar]
- Izumori K. 2006. Izumoring: a strategy for bioproduction of all hexoses. Journal of Biotechnology 124, 717–722 [DOI] [PubMed] [Google Scholar]
- Jang LC, Leon P, Zhou L, Sheen J. 1997. Hexokinase as a sugar sensor in higher plants. The Plant Cell 9, 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SR, Mikkelsen CB, Nielsen BJ. 1981. Iridoid mono- and di-glycosides in Mentzelia . Phytochemistry 20, 71–83 [Google Scholar]
- Kano A, Gomi K, Yamasaki-Kokudo, et al. 2010. A rare sugar, d-allose, confers resistance to rice bacterial blight with upregulation of defense-related genes in Oryza sativa . Phytopathology 100, 85–90 [DOI] [PubMed] [Google Scholar]
- Kano A, Hosotani K, Gomi K, et al. 2011. d-Psicose induces upregulation of defense-related genes and resistance in rice against bacterial blight. Journal of Plant Physiology 168, 1852–1857 [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Henmi K, Ono E, et al. 1999. The small GTP-binding protein Rac is a regulator of cell death in plants. Proceedings of the National Academy of Sciences, USA 96, 10922–10926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Song S, Park C. 1997. The d-allose operon of Escherichia coli K-12. Journal of Bacteriology 179, 7631–7637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Malamy J. 1994. The salicylic acid signal in plants. Plant Molecular Biology 26, 1439–1458 [DOI] [PubMed] [Google Scholar]
- Kruger NJ, von Schaewen A. 2003. The oxidative pentose phosphate pathway: structure and organization. Current Opinion in Plant Biology 6, 236–246 [DOI] [PubMed] [Google Scholar]
- Liu YG, Wu RR, Wan Q, Xie GQ, Bi YR. 2007. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant and Cell Physiology 48, 511–522 [DOI] [PubMed] [Google Scholar]
- Meyer T, Holscher C, Schwoppe C, von Schaewen A. 2011. Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. The Plant Journal 66, 745–758 [DOI] [PubMed] [Google Scholar]
- Miller BG, Raines RT. 2005. Reconstitution of a defunct glycolytic pathway via recruitment of ambiguous sugar kinases. Biochemistry 44, 10776–10783 [DOI] [PubMed] [Google Scholar]
- Miyao A, Tanaka K, Murata K, et al. 2003. Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. The Plant Cell 15, 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Sekiya K, Watanabe Y, et al. 2003. A novel inhibitory effect of d-allose on production of reactive oxygen species from neutrophils. Journal of Bioscience and Bioengineering 96, 89–91 [DOI] [PubMed] [Google Scholar]
- Nakashima A, Chen L, Thao NP, et al. 2008. RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. The Plant Cell 20, 2265–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka Y, Narusaka M, Abe H, et al. 2009. High-throughput screening for plant defense activators using a β-glucuronidase-reporter gene assay in Arabidopsis thaliana . Plant Biotechnology 26, 345–349 [Google Scholar]
- Nishizawa Y, Nishio Z, Nakazono K, et al. 1999. Enhanced resistance to blast (Magnaporthe grisea) in transgenic japonica rice by constitutive expression of rice chitinase. Theoretical and Applied Genetics 99, 383–390 [DOI] [PubMed] [Google Scholar]
- Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H. 1999. Non-invasive quantitative detection and applications of nontoxic-, S65T-type green fluorescent protein in living plants. The Plant Journal 18, 455–463 [DOI] [PubMed] [Google Scholar]
- Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. 2001. Essential role of the small GTPase Rac in disease resistance of rice. Proceedings of the National Academy of Sciences, USA 98, 759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perold GW, Beylis P, Howard AS. 1973. Metabolites of Proteaceae: 8. The occurrence of (+)- d-allose in nature: rubropilosin and pilorubrosin from Protea rubropilosa Beard. Journal of the Chemical Society 6, 643–649 [DOI] [PubMed] [Google Scholar]
- Puchkaev AV, Vlasov AP, Metelitza DI. 2002. Stability of glucose 6-phosphate dehydrogenase complexed with its substrate or cofactor in aqueous and micellar environment. Applied Biochemistry and Microbiology 38, 36–44 [PubMed] [Google Scholar]
- Pugin A, Frachisse J-M, Tavernier E, et al. 1997. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. The Plant Cell 9, 2077–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe RG, Shachar-Hill Y. 2006. Measuring multiple fluxes through plant metabolic networks. The Plant Journal 45, 490–511 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology 57, 675–709 [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. 2006. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology 141, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo SD, Stampfl H, Krasensky J, et al. 2012. Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis . The Plant Cell 24, 3380–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharte J, Schön H, Tjaden Z, Weis E, von Schaewen A. 2009. Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proceedings of the National Academy of Sciences, USA 106, 8061–8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WA, Tatum EL. 1971. Purification and partial characterization of glucose 6-phosphate dehydrogenase from Neurospora crassa . Journal of Biological Chemistry 246, 6347–6352 [PubMed] [Google Scholar]
- Shen W-J, Forde BG. 1989. Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Research 17, 8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NY, Yan Z, Boudreau RL, et al. 2011. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. Journal of Biological Chemistry 286, 8977–8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Dong Y, Watanabe Y, et al. 2005. The inhibitory effect and possible mechanisms of d-allose on cancer cell proliferation. International Journal of Oncology 27, 907–912 [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. 2005. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana . Nature Genetics 37, 1130–1134 [DOI] [PubMed] [Google Scholar]
- Ullrey DB, Kalckar HM. 1991. Search for cellular phosphorylation products of d-allose. Proceedings of the National Academy of Sciences, USA 88, 1504–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao S, Benning C. 2005. Genome-wide analyses of glucose-6-phosphate dehydrogenases in Arabidopsis. The Plant Journal 41, 243–256 [DOI] [PubMed] [Google Scholar]
- Wakao S, Andre C, Benning C. 2008. Functional analyses of cytosolic glucose-6-phosphate dehydrogenases and their contribution to seed oil accumulation in Arabidopsis. Plant Physiology 146, 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-T, Engel PC. 2009. Clinical mutants of human glucose 6-phosphate dehydrogenase: impairment of NADP+ binding affects both folding and stability. Biochimica et Biophysica Acta 1792, 804–809 [DOI] [PubMed] [Google Scholar]
- Weckwerth W, Loureiro ME, Wenzel K, Fiehn O. 2004. Differential metabolic networks unravel the effects of silent plant phenotypes. Proceedings of the National Academy of Sciences, USA 101, 7809–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, et al. 2007. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. The Plant Cell 19, 4022–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno S, Kokubo K, Kamei M. 1999. New method for determining the sugar composition of glycoproteins, glycolipids, and oligosaccharides by high-performance liquid chromatography. Bioscience, Biotechnology, and Biochemistry 63, 1353–1359 [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen J, Zeng L, et al. 2000. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Molecular Plant-Microbe Interactions 13, 869–876 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.