Abstract
Consistent with their valve-like function in shoot–atmosphere gas exchange, guard cells are smaller than other epidermal cells and usually harbour 2C DNA levels in diploid plants. The paralogous Arabidopsis R2R3 MYB transcription factors, FOUR LIPS and MYB88, ensure that stomata contain just two guard cells by restricting mitosis. The loss of both FLP and MYB88 function in flp myb88 double mutants induces repeated mitotic divisions that lead to the formation of clusters of stomata in direct contact. By contrast, CYCLIN DEPENDENT KINASE B1 function is required for the symmetric division that precedes stomatal maturation. It was found that blocking mitosis by chemically disrupting microtubules or by the combined loss of FLP/MYB88 and CDKB1 function, causes single (undivided) guard cells (sGCs) to enlarge and attain mean DNA levels of up to 10C. The loss of both FLP and CDKB1 function also dramatically increased plastid number, led to the formation of multiple nuclei in GCs, altered GC and stomatal shape, and disrupted the fate of lineage-specific stem cells. Thus, in addition to respectively restricting and promoting symmetric divisions, FLP and CDKB1 together also conditionally restrict the G1/S transition and chloroplast and nuclear number, and normally maintain fate and developmental progression throughout the stomatal cell lineage.
Key words: Endoreplication, FOUR LIPS, microtubules, mitosis, S-phase.
Introduction
Cell differentiation is often associated with defined DNA levels that contribute to the function of specialized cell types (Nagl, 1976; Lee et al., 2009). While many plant cells contain 2C DNA levels throughout their lifespan, others undergo varying degrees of endoreplication meaning DNA replication without mitosis (Lee et al., 2009). Examples of cells and tissues that endoreplicate during development include trichomes, endosperm, epidermal pavement cells in leaves and in the hypocotyl, and suspension cultures (Sugimoto-Shirasu and Roberts, 2003; Lee et al., 2009).
Endoreplicaton occurs either through endocycling or endomitosis. In endocycling, G and S phases occur but not mitotic entry. The latter does take place in endomitosis, and chromosomes start to condense. However, the chromosomes fail to separate and, instead, the cells revert to a phase resembling G1 (Lee et al., 2009). In addition, endomitosis can lead to nuclear division without cytokinesis, resulting in the formation of several nuclei in one cell (Meserve and Duronio, 2012; Pandit et al., 2012).
DNA levels in endoreplicated cells correlate strongly with cell size (Melaragno et al., 1993; Kondorosi et al., 2000; Sugimoto-Shirasu and Roberts, 2003). Moreover, endoreplication can be essential for cell fate acquisition and maintenance as well as for patterning and distributing specialized types of cells (Bramsiepe et al., 2010; Roeder et al., 2010).
Stomata consist of two guard cells (GCs) around a pore, a configuration central to the regulation of shoot–atmosphere gas exchange. Arabidopsis stomata develop from asymmetric divisions of lineage-specific stem cells with the smaller daughter cell, the meristemoid, later developing into a Guard Mother Cell (GMC) precursor (Bergmann and Sack, 2007). The GMC then divides just once which ensures that mature stomata each consist of just two GCs. Diploid Arabidopsis accessions contain GCs that also harbour 2C DNA levels, but adjacent pavement cells in leaves often endoreplicate and reach 16C to 32C DNA levels (Galbraith et al., 1991; Melaragno et al., 1993; see Supplementary Fig. S1E at JXB online).
During Arabidopsis leaf development, a complex containing CDKB1;1 and CYCLINA2;3 (CYCA2;3) promotes division and restricts endoreplication in many cells (Boudolf et al., 2004b, 2009; Imai et al., 2006). However, excessive endoreduplication takes place in whole organs when cyclin-dependent kinase (CDK) activity is inhibited (De Veylder et al., 2011).
During stomatal development, several CDKB1 and CYCA2 genes promote the symmetric division of the GMC precursor, and thus are critical for constructing the mature stomatal valve. The loss-of-function of both CDKB1;1 and CDKB1;2 in double mutants or in a dominant negative form of CDKB1 (pro35S:CDKB1;1 N161) blocks GMC division and induces the formation of abnormal single guard cells (Boudolf et al., 2004a ; Xie et al., 2010). Single guard cells (sGCs) also arise when the function of three CYCA2 genes is compromised (cyca2;2, cyca2:3, and cyca2;4) (Vanneste et al., 2011; see Supplementary Fig. S1A–D at JXB online).
The transcription of the CDKB1;1 and CYCA2;3 genes during stomatal development is regulated in part by the FOUR LIPS (FLP) gene which encodes an R2R3 MYB protein (Xie et al., 2010; Vanneste et al., 2011). Loss-of-function mutations in FLP induce ectopic and extra symmetric divisions that produce clusters of GCs and stomata in direct contact (see Supplementary Fig. S1A–D at JXB online). The MYB88 gene, which is an FLP paralogue, shows no loss-of-function phenotype on its own, but acts synergistically in a flp myb88 double mutant by increasing symmetric divisions and stomatal cluster size (Lai et al., 2005). In a flp myb88 cdkb1;1 cdkb1;2 quadruple mutant, the cdkb1;1 cdkb1;2 phenotype is epistatic to that of flp-1 myb88 resulting in many sGCs that are oval-shaped in face view and that lack a dividing wall (Xie et al., 2010).
It is shown here that guard cells exhibit high levels of endoreplication when the loss of FLP and MYB88 function in restraining Guard Mother Cell division is combined with blocked mitosis. Therefore, these MYB proteins can limit S-phase entry as well as mitosis. Moreover, the loss of these combined functions leads to the fate disruption of several types of epidermal cells and induces the abnormal expression of a stomatal lineage stem cell gene.
Materials and methods
Plant materials
All the Arabidopsis lines used were in the Columbia (Col-0) ecotype including the flp-1 myb88 and cdkb1;1 cdkb1;2 double mutants, and the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant (Lai et al., 2005; Xie et al., 2010). Plants were grown on sterile agar or soil (Promix, Premier Brands) at 22 °ºC with a 16/8h light/dark cycle.
Reporter constructs
The proFAMA:GFP transcriptional fusions were generated by PCR amplification of 3423bp of upstream sequence of the start codon (see Supplementary Table S1 at JXB online), followed by cloning the PCR products into the pENTR/TOPO vector (Invitrogen, Carlsbad, CA), and then by recombination into the pGWB4 destination binary vector (Tanaka et al., 2011). The proFAMA:GFP construct was transformed into wild-type plants (Agrobacterium strain GV3101; Clough and Bent, 1998). Transgenic lines were selected on half-strength MS medium containing 25 µg ml–1 hygromycin.
Measurement of epidermal cell size
To reduce growth variations, different lines were sown at the same time on plates containing half- strength MS medium for each experiment. Cotyledons were harvested 21 d after germination. For clearing, after a water rinse, cotyledons were fixed in acidified methanol (containing 20% methanol and 4% concentrated hydrochloric acid) for 15min in a hot (57 °C) water bath. The acidified methanol was then replaced with a basic solution (7% m/v sodium hydroxide in 60% ethanol) for 30min at room temperature. Samples were then rehydrated in a series of ethanol solutions 40%, 20%, 10%, and incubated for at least 30min at each step. Tissues were then placed in a mixture of 5% ethanol and 25% glycerol for storage at room temperature. The abaxial cotyledon epidermis was visualized using an Olympus AX-70 wide-field light microscope. For sampling, images were captured at two positions along the length of the cotyledon, at one-quarter and at three-quarters of the distance from the tip to the base of the cotyledon. Six images were collected from each cotyledon and six cotyledons were counted for each genotype. Cell areas were measured using ImageJ software.
Phenotypic quantification
The total number of stomatal ‘units’ (SUs) were scored. The ‘units’ can include a normal stoma as well as a group of stomata in direct contact. SU number was scored from differential interference contrast light micrographs of the abaxial epidermis of entire cotyledons. The genotypes analysed included the Col-0 wild type, a flp-1 myb88 double mutant, a cdkb1;1 cdkb1;2 double mutant, and a flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant. Values are means ±SE.
Measurement of nuclear DNA content
Cotyledons were harvested from seedlings grown on plates containing half-strength MS medium and fixed in a solution of 9:1 (v/v) ethanol:acetic acid at –20 °C for at least 1h. For staining, tissues were rinsed once in TN buffer (Tris–NaCl buffer, 50mM sodium chloride, 100mM Tris–hydrochloric acid, pH 8.0) and then stained in the dark for 24h in TN buffer containing 0.1 µg ml–1 4′6-diamidino-2-phenylindole (DAPI).
Nuclear DAPI fluorescence was visualized using mercury lamp excitation. The relative nuclear DNA content was calculated by the total gray values of the nuclear zone using ImageJ software (Boudolf et al., 2004a ). Statistical differences for relative DNA content were determined using a non-parametric test (Mann Whitney test) and Graphpad Prism 5.0 software (San Diego, California, USA).
Cell shape and position were evaluated by bright-field microscopy. To standardize image capture, all fluorescence images from different samples were obtained using comparable microscope settings.
Flow cytometry
First leaves from plants grown for 21 d after germination were harvested from seedlings grown on half-strength MS medium plates. Seeds were sown simultaneously. Nuclei were isolated according to Suda and Travnicek (2006) with only slight modifications. About 20mg of cotyledon tissue was minced with a razor in 600 µl OttoI solution (0.1M citric acid monohydrate, 0.5% v/v TWEEN 20, 0.1% v/v β-mercaptoethanol). The solution was filtered with Miracloth and a 22 µm micropore membrane to separate nuclei from cell and tissue debris. The supernatant was centrifuged at 3000rpm for 3min to spin down nuclei and the pellet was re-suspended in 150 µl OttoI solution. Then 600 µl of OttoII solution (0.4M sodium phosphate dibasic dodecahydrate, 0.05mg ml–1 propidium iodide, 1mg ml–1 RNase) was added to the re-suspended nuclei. Suspensions were analysed using a FACScan flow cytometer (Becton Dickinson). The experiments were repeated independently two or three times with comparable results. The output graph was processed using flowJo software.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from 15–18-d-old Col-0, flp-1 myb88, cdkb1;1 cdkb1;2, and flp-1 myb88 cdkb1;1 cdkb1;2 cotyledons to quantify the expression of cell cycle-related genes using a Plant RNeasy Kit (Qiagen). cDNA was synthesized from 1 µg of DNase-treated total RNA using an oligo (dT)18 primer and superscript III reverse transcriptase enzyme (Invitrogen, Carlsbad, CA, USA). One microlitre of RT product was used for quantitative real-time PCR that used an iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), and then was analysed using an iQ5 real-time PCR machine (Bio-Rad). The Arabidopsis ACTIN2 gene was used to approximate relative levels of constitutive and normalized control expression. Relative changes in gene expression were quantified using the 2–ΔΔCT method (Livak and Schmittgen, 2001) with data obtained from at least two biological replicates. Gene-specific primers are shown in Supplementary Table S1 at JXB online.
Oryzalin treatment
Oryzalin (Sigma) was dissolved in dimethylsulphoxide (DMSO) at 25mM (stock solution), stored at –20 °C, and diluted with sterilized water to 25 µM. Cotyledons from 3-d-old seedlings were submerged in this oryzalin solution for 24h and then removed 1 d later. Plants were then rinsed three times with sterilized water and then cultivated in a growth chamber for 3–5 d. DMSO was used as a control. These experiments were repeated independently three times with similar results.
Microscopy
GFP and propidium iodide fluorescence were viewed and captured using a Nikon Eclipse 80i confocal scanning laser microscope and laser lines 488nm and 543nm with Nikon Plan Apochromat oil immersion objectives (40×1.0 NA, and 60×1.4 NA). An Olympus AX-70 wide-field light microscope was used to capture Differential Interference Contrast images. To visualize nuclear DAPI fluorescence, two-photon microscopy was performed using a Mai-Tai titanium sapphire laser (Spectra-Physics Lasers) on a Zeiss Meta 510 Laser Scanning Confocal Microscope using an excitation spectrum of 760–900nm.
Results
Chemical disruption of microtubules induces GC endoreplication in a myb double mutant
To probe the role of microtubule (MT) function in stomatal development, MT depolymerizing drugs were applied to developing Arabidopsis cotyledons and leaves. Oryzalin is a dinitroaniline herbicide that depolymerizes microtubules, a disruption that can arrest division in the M-phase (Morejohn et al., 1987).
While many stomata appear unaffected by oryzalin treatment, a fraction formed undivided, round-to-oval-shaped single guard cells (sGCs). Thus, GMC precursor cells exposed to oryzalin eventually acquire a mature stomatal fate, as shown by the expression of the guard cell fate markers E1728 and proFAMA:GFP (Fig. 1C, D, F). This sGC phenotype closely resembles that of the cdkb1;1 cdkb1;2 double mutants (Fig. 1B).
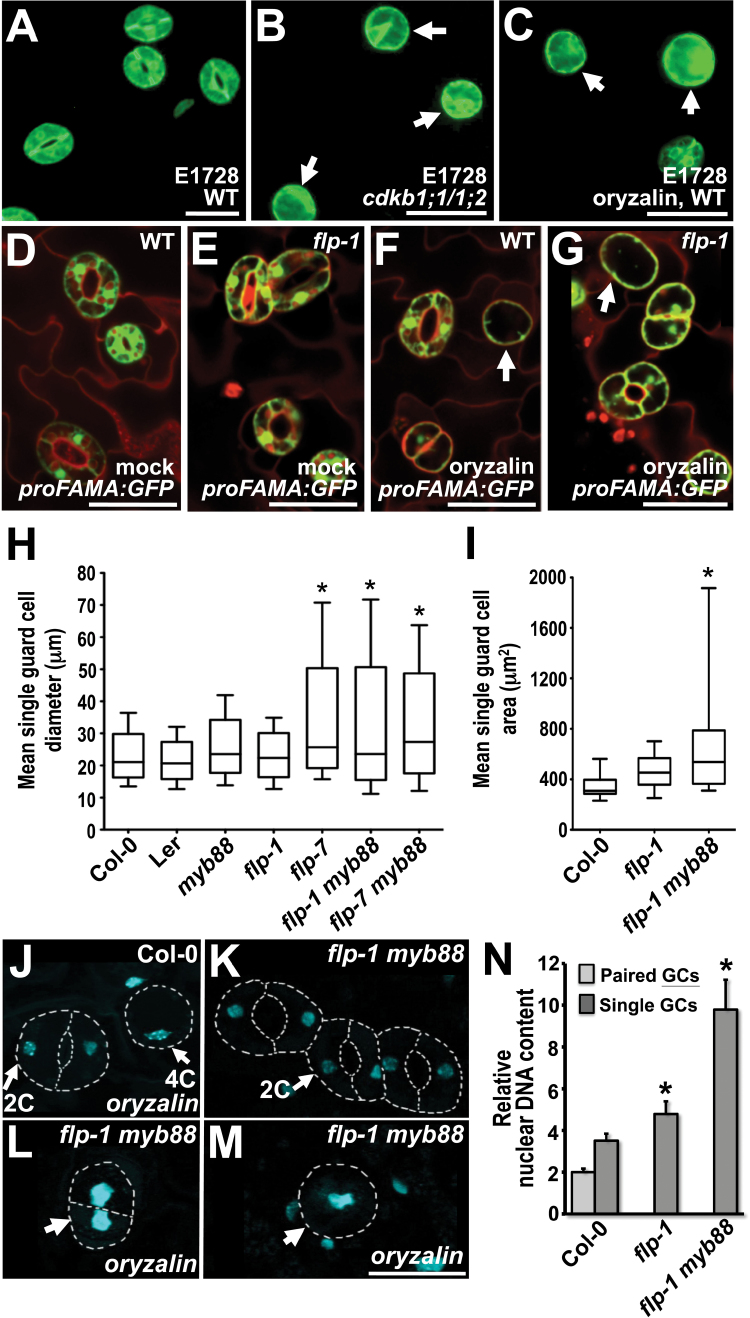
Fig. 1.
Chemical disruption of mitosis induces enlarged and highly endoreplicated single guard cells (sGCs) in flp myb88 mutants. (A) Wild-type stomata expressing the mature guard cell fate marker, E1728. (B) cdkb1;1 cdkb1;2 sGCs expressing E1728 (arrows). (C) Oryzalin-induced sGCs in wild-type also express E1728. (D–G) proFAMA:GFP expression which also marks GC fate. (D) Wild-type control (DMSO treatment). (E) flp-1 mutant background (DMSO). (F) Wild-type stomata treated with oryzalin for 24h showing an undivided sGC (arrow). (G) flp-1 treated with oryzalin showing a sGC (arrow). (H) Mean GC diameter 6 d after treatment with 25 µM oryzalin for 24h as a function of genotype. The diameters of normal GCs as well as abnormal sGCs were scored. Genotypes ranked from lowest to highest diameters (bars). (I) Mean GC area after oryzalin treatment. Three-day-old Col-0, flp-1, and flp1 myb88 seedlings treated and scored as in (H). Genotypes ranked from lowest to highest values (bars). Data in (H) and (I) presented as box plots, in which the box encompasses data for the 25th to 75th percentiles. The horizontal line within each box is the median (50%). Error bars represent the 5th (lower bar) and the 95th (upper bar) percentiles. (A–G) Scale bars=25 µm. Stars (*) denote means significantly different from the wild type (P <0.005). (J–N) Cotyledons from Col-0, flp-1, and flp-1 myb88 plants were treated with oryzalin (or DMSO control) for 24h after staining with 4′6-diamidino-2-phenylindole (DAPI). Nuclear fluorescence was imaged using wide-field microscopy. Whereas sGCs that form after oryzalin treatment display mostly 4C levels in wild-type plants, in flp1 myb88 this mean was 10C. (J–M) Micrographs showing examples of normal and enlarged guard cell nuclei. Stomatal outlines indicated by dashed lines. Arrows indicate nuclei in guard cells that are enlarged compared with other nuclei shown and that probably contain 4C DNA levels. (J–M) Bars=25 µm. (J) Stomata from wild-type (Col-0) plants treated with oryzalin. Left: probably 2C guard cells in a normal stoma. Right: a sGC with a larger nucleus likely to be 4C. (K) Stomatal cluster in a flp-1 myb88 cotyledon that was not treated with oryzalin containing 2C DNA levels in guard cells. (L, M), flp-1 myb88 cotyledons treated with oryzalin. Arrows indicate enlarged nuclei. (L) Nuclei are enlarged in both GC-like cells. (M) Enlarged nucleus in sGC. Stomatal morphogenesis appears to be blocked or delayed. (N) Graph showing nuclear sizes in Col-0, flp-1, and flp-1 myb88 plants treated with oryzalin. Relative DNA levels quantified by the areas of nuclei/gray values derived from DAPI-fluorescence. Only single guard cells were sampled in flp-1 and flp-1 myb88. Sample sizes: Col-0: 145 paired GCs and 65 sGCs; flp-1: 59 sGCs; 48 flp-1 myb88 sGCs. Bars, means ±SE. Stars (*) denote means significantly different from the wild type (P <0.001). (This figure is available in colour at JXB online.)
Since FLP and MYB88 restrict GMCs to a single mitotic cell cycle, the effects of oryzalin application were analysed in various flp and myb88 mutant backgrounds. Treatment with oryzalin of flp-1 and flp-7 (a stronger allele) single mutants and of flp-1 myb88 and flp-7 myb88 double mutant plants suppressed the excess division (cluster) phenotype and instead induced the formation of sGCs. Unlike the stomatal clusters found in mutant plants, these sGCs are correctly patterned.
Single GCs in various combinations of flp/myb88 alleles and mutants were about 50% larger compared with those in oryzalin-treated wild-type plants with respect to sGC area and diameter (Fig. 1F–I). Similarly, the largest sGCs (maximal diameter) were twice as large (72 versus 36 µm) in flp-1 myb88 compared with the wild type (Fig. 1H).
To analyse the relationship between GC size and nuclear DNA content, gray-scale values derived from DAPI (4′,6-diamidino-2-phenylindole) fluorescence were measured using wide-field microscopy. Although pavement cells in the Arabidopsis epidermis undergo varying degrees of endoreplication, stomata in the Col-0 ecotype harbour 2C levels of DNA (Melaragno et al., 1993). As expected, many single GCs induced by oryzalin in wild-type plants displayed roughly double the DNA content of normal GCs (Fig. 1J–N). This doubling is consistent with oryzalin blocking the G2-to-M transition but not S-phase progression. These 4C DNA levels after oryzalin treatment are comparable with those in sGCs induced by transformation with a CDKB1;1 N161 dominant negative construct (Boudolf et al., 2004a ) (Fig. 1N).
In contrast to treatment of N161 plants, oryzalin dramatically increased nuclear DNA levels in sGCs in a various flp myb88 mutant combinations. Whereas the Col-0 wild-type exhibited mostly 4C levels, sGCs in a flp1 myb88 double mutant harboured a mean DNA content of 10C when treated with oryzalin (Fig. 1N). By contrast, oryzalin did not affect sGC size in cdkb1;1 cdkb1;2 double mutants (see Supplementary Fig. S2 at JXB online). Thus, the substantive enlargement of drug-induced sGCs depends primarily upon the combined loss of FLP/MYB88 function.
Combined loss of FLP/CDKB1 function induces guard cell and nuclear enlargement
To explore the effects of the loss of both MYB and CDKB1 function on stomatal size and morphology, a flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant was used. In addition to exhibiting many relative large sGCs, this combined loss-of-function background also induced some stomata to enlarge considerably in mature leaves and cotyledons (Fig. 2). By contrast, guard cells in wild-type, flp-1, and in cdkb1;1 cdkb1;2 double mutant plants were much smaller than in the quadruple mutant (Fig. 2H).
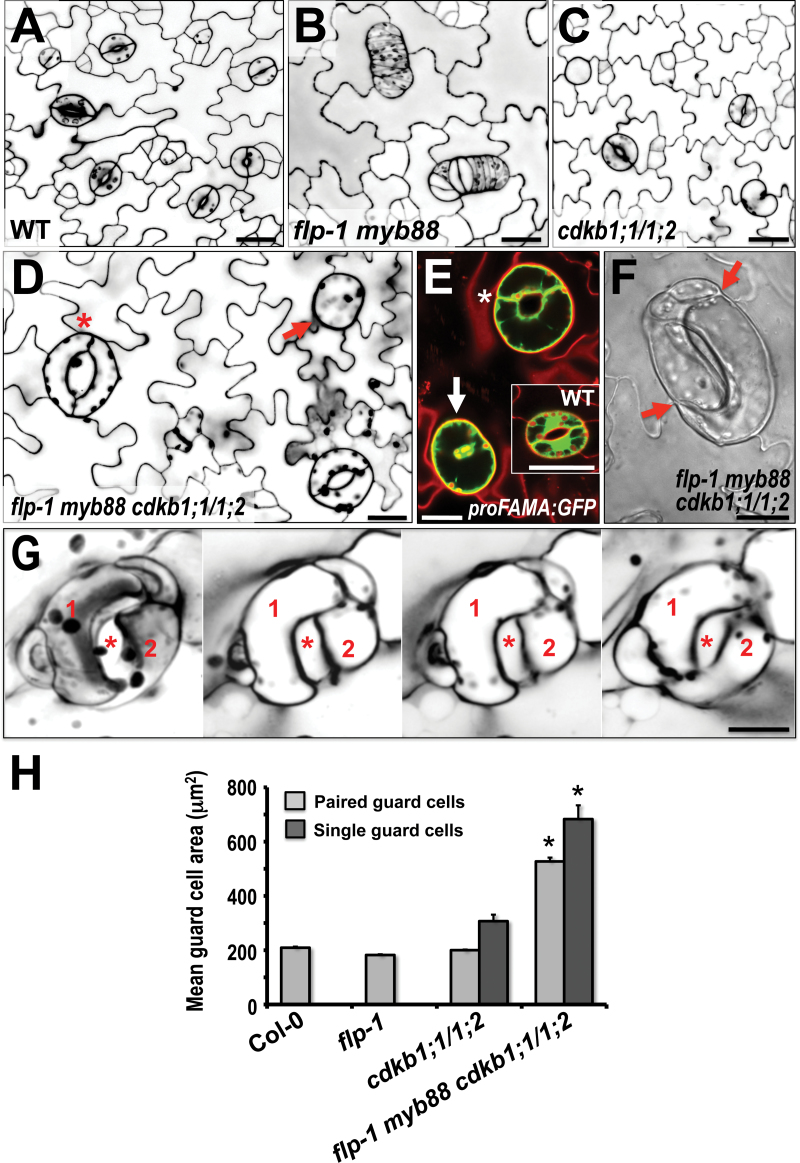
Fig. 2.
Combined loss of FLP/MYB88 and CDKB1;1/1;2 function increases stomatal size and disrupts GC and stomatal shape. (A) Wild-type (WT) stomata. (B) flp-1 myb88 stomatal clusters. (C) cdkb1;1 cdkb1;2 stomata. Single, oval-shaped GC shown at upper left. Lower right: kidney-shaped single GC. (D) flp-1 myb88 cdkb1;1 cdkb1;2 background showing enlarged GCs in an otherwise normal stoma (*) as well as an enlarged sGC upper right (red arrow). (E) proFAMA:GFP expression indicates stomatal identity in sGC (arrow, lower left) as well as in an abnormally shaped stoma (*) in flp-1 myb88 cdkb1;1 cdkb1;2. First leaf from a 15-d-old plant. Inset: normal stoma in a wild-type plant. (F) Abnormal guard cell enlargement, twisting (red arrows), and loss of kidney shape. From a 20-d-old flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant. (G) Four successive optical sections of the same abnormal stoma. Guard cells 1 and 2 are labelled in red. Star marks an abnormal and twisted stomatal pore. From a 20-d-old flp-1 myb88 cdkb1;1 cdkb1;2 cotyledon. (H) Quantification of guard cell areas measured from six, 21-d-old cotyledons. Sample sizes: Col-0 (225), flp-1 (225), cdkb1;1/1;2 (225 paired GCs and 164 sGCs), and flp-1 myb88 cdkb1;1 cdkb1;2 (225 paired GCs and 182 sGCs). Bars, means ±SE. Stars indicate values significantly different from the wild type (P <0.001). All figures in (A) to (E) are at same magnification and from 12-d-old first leaves. Cell walls in all figures (except F) were visualized with propidium iodide fluorescence. All scale bars=20 µm. (This figure is available in colour at JXB online.)
These size differences extend to nuclei. Single GCs in a flp-1 myb88 cdkb1;l cdkb1;2 quadruple mutant harbour abnormally large nuclei with a mean DNA level of 6C (Fig. 3A–E). Because high GC ploidies were only detected in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant, endoreduplicaion is only likely to ensue when both sets of genes (FLP/MYB88 and CDKB1;1/CDKB1;2) are non-functional. These data are consistent with genetically blocked mitosis (loss of CDKB1 function) inducing cell-type specific endoreplication when excess mitotic divisions (loss of MYB function) are also precluded.
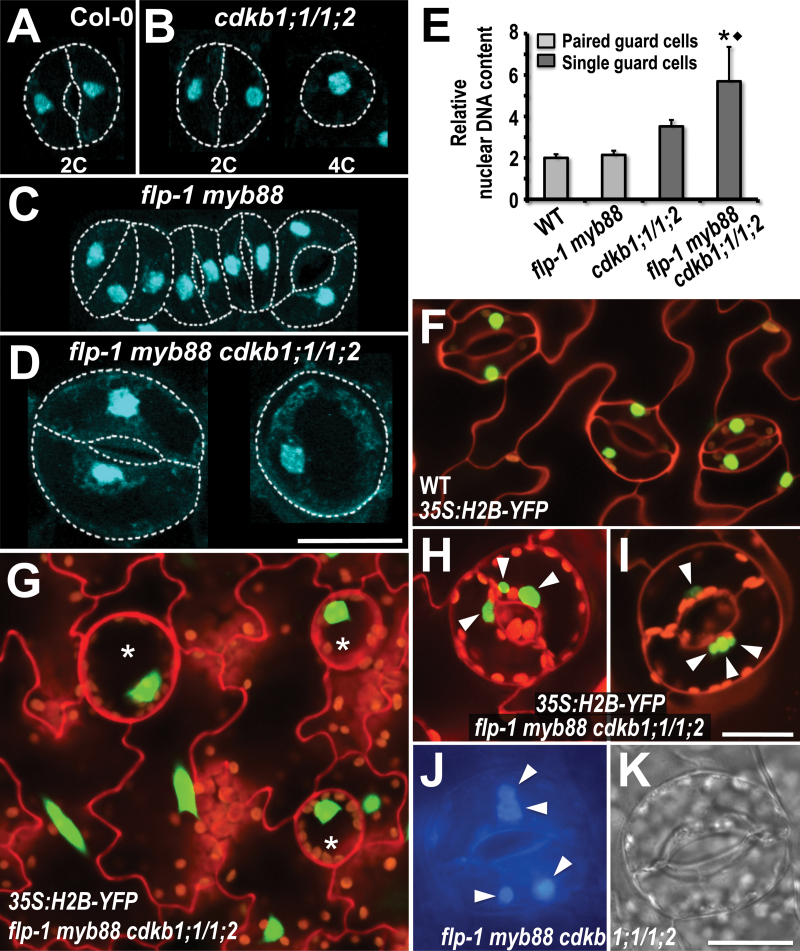
Fig. 3.
Guard Cell ploidy increases substantively in a flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant that also harbours some cells with multiple nuclei. Quantification of guard cell relative nuclear DNA content in cotyledons of Col-0, flp-1, flp-1 myb88, and flp-1 myb88 cdkb1;1 cdkb1;2 plants visualized with DAPI. White dashes indicate stomatal outline. (A) Wild-type stoma. (B) Normal stoma (left) and a single guard cell (right) in a cdkb1;1 cdkb1;2 double mutant background. (C) flp-1 myb88 stomatal cluster. Relative nuclear DNA content appears roughly similar in all cells. (D) flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant shows enlarged nuclei in a normally-shaped stoma (left) as well as in a single guard cell (right). (E) Quantification of relative nuclear DNA content in Col-0, flp-1, flp-1 myb88, and in flp-1 myb88 cdkb1;1 cdkb1;2 plants as measured by total gray values of DAPI-stained nuclei in guard cells of Col-0 (n=216), flp-1 myb88 (n=216), cdkb1;1 cdkb1;2 (n=51 single guard cells), and flp-1 myb88 cdkb1;1 cdkb1;2 (n=68 single guard cells). Bars: means ±SE. The symbols * and ♦ denote values significantly different from the wild type at P <0.001, and from the cdkb1;1 cdkb1;2 double mutant at P <0.005, respectively. Single GCs in the quadruple mutant display roughly 6C levels (mean). (F–I) Nuclear fluorescence from 35S:Histone2B-YFP. (F) Wild-type. (G) Nuclei in single GCs (stars) in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant are much larger than in those of the wild-type. (H, I) Some GCs display multiple nuclei of different sizes (arrowheads) in quadruple mutant. (J, K) multiple nuclei (arrowheads) in GCs of quadruple mutant stained with DAPI (J). (K) is a DIC image of cells shown in (J). All figures within the same group are at the same magnification, i.e. in (A–D), (F–I), and (J, K), respectively. (A) Bar =25 µm; (J, K)=20 µm. (This figure is available in colour at JXB online.)
This increase in nuclear size in large GCs of the quadruple mutant was also visualized using histone H2B fused to fluorescent GFP, which marks nuclei (Fig. 3F–I). Surprisingly, fluorescence from this construct revealed the occasional presence of multiple (2–3) nuclei in large but normally shaped guard cells (Fig. 3H–K). This finding is consistent with the presence of endomitosis, as well as endocycling, during GC enlargement in flp-1 myb88 cdkb1;1 cdkb1;2.
Reduced stomatal production in the quadruple mutant
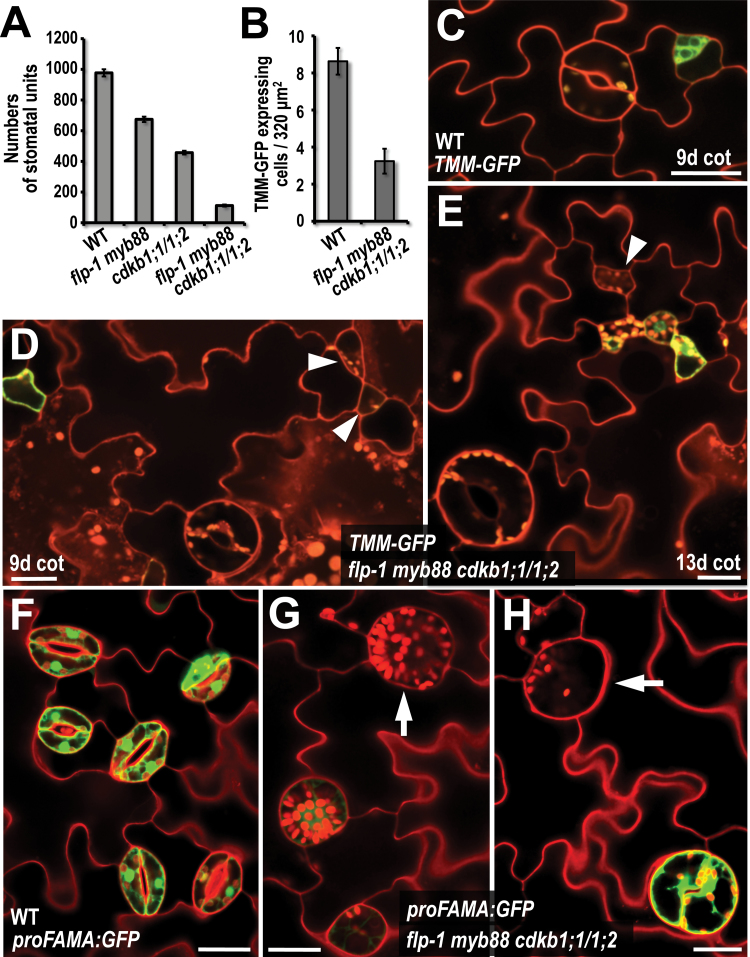
The total number of stomata or stomatal ‘units’, which here refers to normal stomata as well as sGCs, was significantly lower in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant than in the wild type (Fig. 4A). Stomatal number in Arabidopsis correlates closely with the extent of expression of the TOO MANY MOUTHS (TMM) gene. TMM encodes a receptor-like protein, and its expression specifically marks the stomatal stem cell compartment which includes meristemoids and their sister cells produced by divisions that are asymmetric in size and cell fate (Fig. 4B; Nadeau and Sack, 2002). Consistent with a decreased number of mature stomata formed in the quadruple mutant, the number of stem cells, as marked by TMM expression, was considerably reduced (Fig. 4A, B; see Supplementary Fig. S3 at JXB online). Thus the combined loss of FLP/MYB88 and CDKB1;1/CDKB1;2 function inhibits the number of stomatal lineage-specific stem cells that form.
Fig. 4.
Cell fate is disrupted in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant. (A) Numbers of stomata or sGCs (both referred to as ‘stomatal units’) in wild type, quadruple mutant, flp-1 myb88, and cdkb1;1 cdkb1;2. Bars, mean ±SE. Scored from six independent samples per genotype, 15-d-old cotyledons. (B) Number of cells expressing proTMM:TMM-GFP (TMM-GFP) scored from a 320 µm2 region of the wild type (n=97) and the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant (n=234). Bars, means ±SE. Scored from 9-d-old cotyledons, six different samples per genotype. (C) TMM-GFP in the wild type showing normal expression in an asymmetric division (upper right). (D, E) Cells that are comparable in size to a meristemoid and that appear to have formed in an asymmetric division vary in whether they express TMM-GFP. Arrowheads, meristemoid-like cells that lack GFP signal. Other small cells in the same fields display fluorescence. However, few meristemoid-like cells are normal in shape. TMM-GFP in 9 and 13-d-old cotyledons in the quadruple mutant. Note the abnormal shape and enlargement of stoma at the lower left in (E). (F) Wild type showing proFAMA:GFP fluorescence in mature stomata from a 14-d-old cotyledon. (G, H) As in (F), except in a quadruple mutant. Arrows indicate large cells that likely developed from single guard cells but that mostly (except, for example, H lower right) do not express proFAMA:GFP. All scale bars=20 µm. (This figure is available in colour at JXB online.)
The loss of MYB/CDKB1 function also disrupts GC shape and fate
Strikingly, guard cell shape and size were severely disrupted in this quadruple mutant (Fig. 2E–G). Many normally shaped guard cells later lost their shape and grew over the original edges of the GCs. No such shape changes were found in flp my88 or in ckdb1;1 ckdb1;2 double mutants (Fig. 2A–C), or following oryzalin treatment (Fig. 1). These abnormally shaped and enlarged cells still maintained a guard cell fate as shown by their expression of stomatal fate markers (Fig. 2E). However, this distortion of guard cell morphology probably impairs stomatal function since overall bilateral symmetry is compromised, and pore apertures and gaseous diffusion pathways become contorted, restricted or blocked. Consistent with these defects, shoot growth slowed and seed production was reduced during ageing (see Supplementary Fig. S4 at JXB online). Root growth was also severely reduced in the quadruple mutant (see Supplementary Fig. S4 at JXB online).
Cell fate and gene expression were also at least partially altered in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant (Fig. 4). The majority (60.7±7.08% SE, n=150) of smaller daughter cells produced after asymmetric division failed to express TMM in 9-d-old cotyledons (Fig. 4C–E), consistent with a loss in meristemoid cell fate. In addition, expression from a proFAMA:GFP transcriptional fusion was absent from some mature single guard cells in the quadruple mutant (Fig. 4F–H), indicating that some sGCs lose their fate. These findings suggest that abnormal endoreplication is associated with a disruption in cell fate as well as a decrease in epidermal cell number.
Increased GC size correlates with extensive chloroplast replication
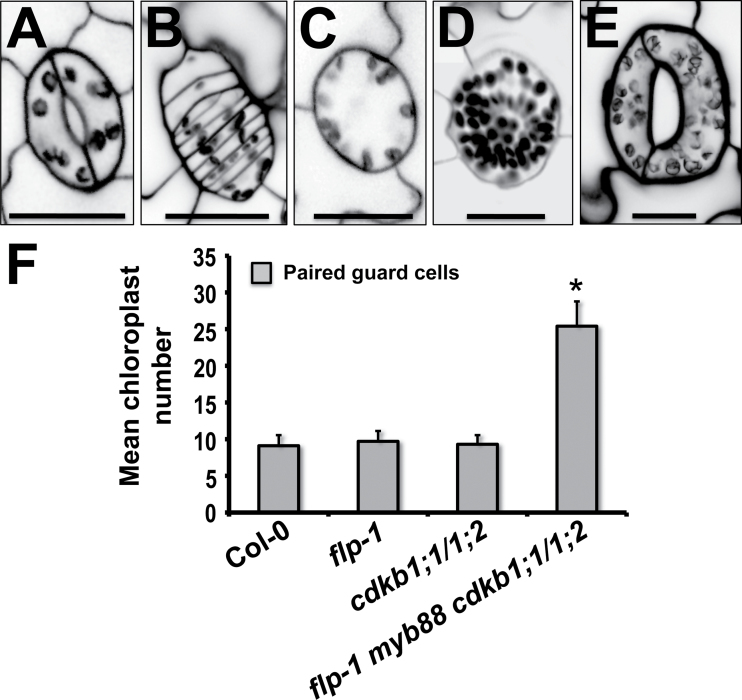
In addition to the larger size and abnormal shape of guard cells in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant, this genotype also displayed high numbers of chloroplasts in stomata (Fig. 5). Arabidopsis stomata normally contain about 10 chloroplasts per guard cell (Pyke and Leech, 1994). Comparable numbers were present in the flp-1, flp-1 myb88, and cdkb1;1 cdkb1;2 mutant backgrounds (Fig. 5A–C, F). By contrast, mean chloroplast number in stomata with paired guard cells in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant was more than double that of the wild type (25.4±3.37 SD) (Fig. 5D–F). Single GCs in the quadruple mutant also contained many chloroplasts (30.2±7.97 SD, n=25) (Fig. 5D). Thus abnormal guard cell endoreplication increases plastid number.
Fig. 5.
Combined loss of FLP/MYB88 and CDKB1;1/1;2 function increases chloroplast number in GCs. (A–E) Comparison of chloroplast number in guard cells as a function of genotype. Propidium iodide staining, confocal microscopy. (A) Wild type. (B) flp-1 myb88 double mutant. (C) cdkb1;1 cdkb1;2 double mutant. (D) Single guard cell of the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant. (E) Abnormally-shaped stoma in the quadruple mutant. (F) Mean chloroplast number in paired guard cells of different genotypes (n=10 guard cells per genotype). Bars, means ±SD. Star shows value significantly different from the wild type (P <0.001). 20-d-old cotyledons. (A–E) Scale bars=20 µm.
FLP and MYB88 do not affect leaf endoreduplication overall
The CDKB1;1 dominant negative mutant N161 (pro35S: CDKB1;1 N161) promotes endoreduplication in pavement cells as well as in other cells in the cotyledon (Boudolf et al., 2004b ). Because the loss of FLP/MYB88 function promotes endoreduplication in the stomatal lineage in a sensitized background, it was probed whether various mutant combinations also affect the degree of endoreduplication at the level of the whole organ. Using nuclei isolated from first leaves harvested 21 d after germination, ploidy distributions were analysed by flow cytometry in control (Col-0) as well as in different mutant backgrounds, i.e. in the flp-1 myb88 and cdkb1;1 cdkb1;2 double mutants, and in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant. Ploidy levels and their distributions were mostly similar between all four genotypes (see Supplementary Fig. S5 at JXB online). However, genotypes lacking CDKB1 function (i.e. cdkb1 double mutants as well as the quadruple mutant), showed 32C peaks, albeit small, and also had 16C peaks (see Supplementary Fig. S5C, D at JXB online).
Although it is likely that endoreplicated nuclei from single guard cells were present in selected flow cytometry samples, presumably this fraction was small compared with the number of nuclei present throughout the leaf (including pavement cells which are highly endoreplicated). Thus FLP and MYB88 do not normally influence endoreduplication in whole leaves, a result consistent with the prominent expression of both MYBs only in the stomatal cell lineage in the cotyledon epidermis, as well as with the lack of any obvious increase in plastid number in other epidermal cells.
Variable expression of sets of G1-to-S phase genes in the quadruple mutant
Since the quadruple mutant blocks mitosis, the expression of Arabidopsis genes known to act in the G1-to-S phase and during endoreplication were analysed in this loss-of-function background. Many G1-to-S phase genes in the quadruple mutant showed no difference in expression levels compared with the wild type. These included CELL DIVISION CYCLE6 genes (CDC6s), CDC10 TARGET1 genes (CDT1s), CDKA;1, as well as the CCS52A2/FZR1 and CCS52A1/FZR2 genes which are required for endoreplication as activators of the Anaphase-Promoting Complex/Cyclosome (APC/C) (see Supplementary Fig. S6 at JXB online; Lammens et al., 2008; Larson-Rabin et al., 2009). However, another set of genes was much more highly expressed in the quadruple mutant compared with the wild type. This set included six G1-to-S phase entry genes: (i) MINI -CHROMOSOME MAINTENANCE 2 (MCM2), (ii) MCM5, (iii–v) ORIGIN RECOGNITION COMPLEX 1a (ORC1a), ORC2, ORC3, and (vi) PROLIFERATING CELL NUCLEAR ANTIGEN1 (PCNA1) (see Supplementary Fig. S6 at JXB online).
Endoreplication initiation is also regulated by atypical E2F repressors, which are characterized by two DNA binding domains, a lack of binding to DIMERIZATION PARTNER (DP) proteins, and the absence of a RETINOBLASTOMA (RB) protein-binding domain (Lammens et al., 2009). Gene expression levels corresponding to a set of E2F related proteins that included typical E2Fs (E2Fa, E2Fb, E2Fc) as well as atypical E2Fs (E2Fd/DEL2, E2Fe/DEL1, E2Ff/DEL3) were therefore quantified in 15-d-old wild-type, flp-1 myb88, cdkb1;1 cdkb1;2 double mutants, and in the quadruple mutant cotyledons. While five of these E2Fs showed no significant differences in expression in the four genotypes, the atypical E2Ff/DEL3 was strongly expressed in the quadruple mutant (see Supplementary Fig. S7 at JXB online). Thus E2Ff probably restricts endoreplication, at least in some cell types in the epidermis of wild-type cotyledons.
Discussion
FLP conditionally limits guard cell size
The relatively small size of stomata is likely to be adaptive in regulating shoot–atmosphere gas exchange by allowing rapid pore opening and closing. This facilitates rapid stomatal responses to environmental parameters such as local and transient fluctuations in light intensity.
Stomatal size directly correlates with ploidy levels of whole plants. For example, mean stomatal length in natural hexaploid and octoploid Coffea plants were more than 50% longer than in diploids (Mishra, 1997). Stomatal size also increases with genome dosage in diploid versus triploid and tetraploid lines in Arabidopsis Col and C24 ecotypes (Miller et al., 2012). However, stomata do not normally endoreplicate, consistent with their substantively reduced size compared with other cell types (Melaragno et al., 1993).
FLP restricts divisions and enforces patterning in Arabidopsis stomatal development by ensuring that only one symmetric division occurs before stomata form (Lai et al., 2005). It is shown here that the paralogous FLP and MYB88 transcription factors can also limit guard cell endoreplication, and thus size but only when the ability of GMCs to divide is blocked.
Whereas oryzalin only arrests GMCs in G2/M in wild-type plants, in flp mutants, oryzalin switches GMCs from mitotic cycling to endocycling. These data indicate that GC endoreduplication results from the disruption of microtubule arrays that control both nuclear and cell division.
A similar phenotype arises when CDKB1;1 function is lost. Normally, in addition to promoting the symmetric division of guard mother cells, CDKB1;1 negatively regulates when endocycling starts (Boudolf et al., 2009). While endocycling is pervasive in many tissues and organs in cdkb1 mutants, it is notably absent from guard cells, even though CDKB1;1 is expressed late in the stomatal cell lineage (Boudolf et al., 2004b ). Thus, the lack of endoreplication in cdkb1;1 cdkb1;2 guard cells, but its presence when FLP function is lost, shows that FLP can conditionally restrict stomatal endocycling and enlargement.
Direct versus indirect regulation of endoreplication
Mutations in mitotic CYCLINA2 (CYCA2) genes precociously induce and extend the duration of endocycling throughout the shoot (Imai et al., 2006; Vanneste et al., 2011). Endocycling also occurs in Arabidopsis cotyledons when the CDKB1;1-CYCA2;3 mitotic complex is inactivated by the APC/C complex (Boudolf et al., 2009). In addition to CDKB1, CYCA2 genes are also expressed late in stomatal development. CYCA2:3 can also promote the last mitosis in stomatal development and its loss of function also induces the formation of single guard cells, but not guard cell endoreplication (Boudolf et al., 2004a ; Vanneste et al., 2011). Both the CDKB1;1 and CYCA2;3 genes are direct transcriptional targets of FLP/MYB88 (Xie et al., 2010; Vanneste et al., 2011). It remains to be seen whether the loss of CYCA2 function might also ectopically activate endoreplication, such as in a flp myb88 double mutant background.
Unlike FLP/MYB88, some transcription factors directly regulate endocycling. For example, the loss of function of GTL1, which encodes an Arabidopsis trihelix transcription factor, induces extra rounds of endocycling that prolong trichome growth (Breuer et al., 2009). In Drosophila, the ESCARGOT gene, which encodes a zinc finger transcription factor, induces diploid larval histoblasts to undergo endoreplication at the appropriate developmental stage (Hayashi, 1996). This comparison highlights the relatively indirect role that FLP plays in only conditionally restricting endoreplication.
FLP and broader regulation of balance between mitosis and endocycling
Although FLP directly targets the DNA replication licensing factor genes, CDC6a and CDC6b, no changes in their expression levels were detected in flp myb88, a result that might reflect the ubiquitous functions of CDC6 genes. However, the expression of other genes that regulate replication, such as PCNA1 and some ORCs and MCMs, was significantly up-regulated in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant, but not in either double mutant (flp-1 myb88 or cdkb1;1 cdkb1;2), profiles consistent with guard cell endoreplication only occurring in flp myb88 after a mitotic block.
Notably, FLP/MYB88 bind specifically to a cis-element present in the promoter regions of some potential target genes that overlaps with E2F. For example, FLP/MYB88 directly target and regulate the expression of G1-to-S phase genes which are themselves E2F targets (including CDC6a, CDC6b, CYCA2;3, and CDKB1;1) (Xie et al., 2010; Vanneste et al., 2011). Moreover, all G1-to-S phase genes found to be highly expressed in a flp myb88 cdkb1;1 cdkb1;2 background, such as MCM2, MCM5, ORC1a, ORC2, ORC3, and PCNA1, are known targets of E2F. Thus FLP/MYB88 and E2F probably co-ordinately regulate the expression of cell cycle genes that function in G1-to-S phase, especially those that act later in stomatal development. However, it cannot be ruled out that high expression levels of some G1-to-S phase genes might only be an indirect effect with respect to FLP/MYB88. In addition, FLP/MYB88 might only act conditionally in limiting cell cycling when mitosis itself is restricted.
The Drosophila MYB protein, DmMYB, is a component of a multisubunit transcriptional repressive complex termed dREAM/MMB that includes the E2F and RETINOBLASTOMA proteins (Korenjak et al., 2004). The dREAM complex targets E2F promoter binding sites and represses the transcription of genes regulated by E2F (Georlette et al., 2007; van den Heuvel and Dyson, 2008). Thus, FLP/MYB88 and E2F might act in a similar complex in Arabidopsis or might regulate common cell cycle target genes. These relationships might also explain why transcription levels of S-phase entry genes increase in a flp myb88 cdkb1;1 cdkb1;2 background even though many of these genes are direct targets of E2F but not FLP.
The high expression of E2Ff (an ‘atypical’ E2F) in the quadruple mutant is interesting since the mammalian atypical E2Fs, 7 and 8, promote endoreplication, whereas the Arabidopsis E2Fe inhibits endocycling (Lammens et al., 2008; Chen et al., 2012; Pandit et al., 2012). This raises the possibility that E2Ff might contribute to abnormally high levels of endoreplication when both FLP and CDKB1 functions are lost. To date, E2Ff has only been shown to function in restricting cell expansion in Arabidopsis by directly targeting cell wall biosynthesis genes, a role that might be consistent with E2Ff contributing to normal guard cell formation (Ramirez-Parra et al., 2004).
A well-studied endoreplication pathway involves the Anaphase-Promoting Complex/Cyclosome (APC/C). Constitutive expression of CCS52A1/FZR2, an APC/C activator, is known to result in abnormally large stomata, as does perturbation of APC/C activity (Larson-Rabin et al., 2009; Iwata et al., 2011; Komaki and Sugimoto, 2012).
APC/C activity is required to initiate endoreduplication. APC/C activator genes, such as CDH1 (also referred to as FZR or CCS52A) promote endocycle onset and progression (Larson-Rabin et al., 2009). The initiation of endocycling can also be induced when mitotic cyclins are degraded (Shcherbata et al., 2004; Narbonne-Reveau et al., 2008; Boudolf et al., 2009; Eloy et al., 2012). The activation of APC/C from late G2 to early mitosis requires its interaction with CELL DIVISION CYCLE 20 (CDC20) (also known as FIZZY (FZY)). Later during anaphase to S phase, APC/C remains active by interacting with CELL CYCLE SWITCH 52 (CCS52) (also known as CDC20 HOMOLOG 1 (CDH1)/FIZZY-RELATED (FZR)) (Pesin and Orr-Weaver, 2008).
The APC/C activator CCS52A1 has been shown to regulate the stability of the CYCA2;3 protein which restricts leaf endoreplication (Boudolf et al., 2009). Since FLP/MYB88 also directly limit the expression of CYCA2;3 and CDKB1;1, their combined control is also likely to restrict endoreplication in the stomatal pathway (Boudolf et al., 2009; Xie et al., 2010; Vanneste et al., 2011).
CDKB1 promotes pavement cell formation but restricts their size
The combined loss of CDKB1 and FLP functions leads to smaller leaves and cotyledons as well as an increase in epidermal cell size, especially in non-stomatal epidermal cells, (NSECs), which collectively occupy the greatest share of leaf area. However, the loss of just CDKB1;1 and CDKB1;2 function, whether in a double mutant or in a CDKB1;1.N161 dominant negative construct, almost halves the number of pavement cells/NSECs (although the N161 construct does not affect the size of cotyledons, as opposed to leaves) (Boudolf et al., 2004b ). By contrast, the size of leaves and cotyledons appears to be unaffected in flp myb88. Our results are consistent with previous findings that the loss of CDKB1 function is largely responsible for increased epidermal cell size. However, mechanisms underlying the relationship between increased pavement cell size and a reduction in the number of stomatal lineage stem cells formed in cdkb1 mutants remains to be determined.
Endoreplication, cell shape, and cell fate
Stomatal endoreplication in a flp myb88 cdkb1;1 cdkb1;2 quadruple mutant background is accompanied by abnormal changes in guard cell shape as well as size. Pavement cells appear to maintain a normal morphology consistent with the confinement of FLP/MYB88 expression to developing stomata in the leaf epidermis. However, many guard cells in this quadruple mutant expand out of the plane of the epidermis and grow and twist over each other resulting in the loss of stomatal bilateral symmetry.
Abnormal and excessive endoreplication has been shown to be associated with the formation of exceptionally large and irregularly shaped cells that harbour mixed guard cell and pavement cell identity but that bear little resemblance to stomata (Iwata et al., 2011). These cells arise when the function of the OMISSION OF SECOND DIVISION1 (OSD1) gene, also known as GIGAS CELL1, is also lost (Iwata et al., 2011). OSD1, which encodes a novel protein, as well as related genes, probably inhibit APC/C function. Thus while both the osd1 and the flp quadruple mutant phenotypes involve endoreplication, the former induces a much more severe shape defect. In the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant, cells that resemble mature single GCs later fail to express stomatal fate markers. In addition, many meristemoids, which are the smaller daughter cells of asymmetric division in the lineage and whose expression is normally marked by TMM, later lose TMM expression. In turn, this reduces the total number of stomata that form in the quadruple mutant (compared with the wild-type).
Cell fate disruption in the quadruple mutant is most prominent in mature cotyledons when endoreplication is more extensive. The close relationship between abnormal endoreplication, altered cell shape, and cell fate disruption, highlights the importance of co-ordinating DNA levels during normal cell morphogenesis and fate determination.
Guard cell chloroplast number and endoreplication
Whereas diploid Arabidopsis Col-0 plants have 2C guard cells that exhibit a relatively small range of sizes and number of chloroplasts (Melaragno et al., 1993; Pyke and Leech, 1994), endoreplicated guard cells in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant display more than twice as many chloroplasts compared with the wild-type or to flp-1 myb88 or cdkb1;1 cdkb1;2 double mutants. Thus, in addition to restricting GMC symmetric division, FLP and MYB88 also conditionally limit plastid replication.
While a relationship between cell size and plastid number has been questioned based upon comparisons of mesophyll and bundle sheath cells in different taxa (Olszewska et al., 1983), numerous data show that chloroplast number increases relative to nuclear DNA levels (Butterfass, 1973). This relationship has been especially well studied in guard cells in plants that have undergone polyploidization (Butterfass,1973). Our results show that local and cell-type-specific endoreplication can also induce excessive but proportional chloroplast replication. The abundance of chloroplasts in guard cells in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant is likely to be a direct consequence of endoreplication since the combined loss of FLP and MYB88 function on its own does not increase plastid number. However, it cannot be ruled out that the enhanced number of chloroplasts in the quadruple mutant might result from unknown factors in addition to or instead of endoreplication.
Overall, this work has shown that two MYB transcription factors that enforce a single symmetric division essential for normal stomatal formation can also conditionally restrict endoreplication and chloroplast and nuclear number, as well normally maintain fate and developmental progression throughout the stomatal cell lineage.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Stomatal development in the wild type and selected mutants as well as Histone 2B-YFP expression.
Supplementary Fig. S2. Oryzalin treatment does not appear to affect sGC diameter in cdkb1;1 cdkb1;2 double mutants.
Supplementary Fig. S3. Expression of TOO MANY MOUTHS during stomatal and cotyledon differentiation.
Supplementary Fig. S4. Seedling growth defects in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant compared with the wild-type as well as to the flp-1 myb88 and cdkb1;1 ckdb1;2 double mutants.
Supplementary Fig. S5. Flow cytometry profiles from PI-stained leaves.
Supplementary Fig. S6. Expression levels of cell cycle-related genes in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant as well as other mutants.
Supplementary Fig. S7. Expression levels of E2F genes in the flp-1 myb88 cdkb1;1 cdkb1;2 quadruple mutant as well as in other mutants.
Supplementary Table S1. Primers used.
Acknowledgement
This work was supported by Canadian NSERC Grant 22R92904.
References
- Bergmann DC, Sack FD. 2007. Stomatal development. Annual Review of Plant Biology 58, 163–181 [DOI] [PubMed] [Google Scholar]
- Boudolf V, Barroco R, Engler J, Verkest A, Beeckman T, Naudts M, Inzé D, De Veylder L. 2004a. B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana . The Plant Cell 16, 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Lammens T, Boruc J, et al. 2009. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiology 150, 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Vlieghe K, Beemster GTS, Magyar Z, Torres Acosta JA, Maes S, Van Der, Schueren E, Inzé D, De Veylder L. 2004b. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. The Plant Cell 16, 2683–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramsiepe J, Wester K, Weinl C, Roodbarkelari F, Kasili R, Larkin JC, Hülskamp M, Schnittger A. 2010. Endoreplication controls cell fate maintenance. PLoS Genetics 6, e1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer C, Kawamura A, Ichikawa T, Tominaga-Wada R, Wada T, Kondou Y, Muto S, Matsui M, Sugimoto K. 2009. The trihelix transcription factor GTL1 regulates ploidy-dependent cell growth in the Arabidopsis trichome. The Plant Cell 21, 2307–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfass T. 1973. Control of plastid division by means of nuclear DNA amount. Protoplasma 76, 167–195 [Google Scholar]
- Chen H-Z, Ouseph MM, Li J, et al. 2012. Canonical and atypical E2Fs regulate the mammalian endocycle. Nature 14, 1192–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Larkin JC, Schnittger A. 2011. Molecular control and function of endoreplication in development and physiology. Trends in Plant Science 16, 624–634 [DOI] [PubMed] [Google Scholar]
- Eloy NB, Gonzalez N, Van Leene J, et al. 2012. SAMBA, a plant-specific anaphase-promoting complex/cyclosome regulator is involved in early development and A-type cyclin stabilization. Proceedings of the National Academy of Sciences, USA 109, 13853–13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Knapp S. 1991. Systemic endopolyploidy in Arabidopsis thaliana . Plant Physiology 96, 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georlette D, Ahn S, MacAlpine DM, Cheung E, Lewis PW, Beall EL, Bell SP, Speed T, Manak JR, Botchan MR. 2007. Genomic profiling and expression studies reveal both positive and negative activities for the Drosophila Myb MuvB/dREAM complex in proliferating cells. Genes and Development 21, 2880–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. 1996. A Cdc2 dependent checkpoint maintains diploidy in Drosophila . Development 122, 1051–1058 [DOI] [PubMed] [Google Scholar]
- Imai K, Ohashi Y, Tsuge T, Yoshizumi T. 2006. The A-type cyclin CYCA2;3 is a key regulator of ploidy levels in Arabidopsis endoreduplication. The Plant Cell 18, 382–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata E, Ikeda S, Matsunaga S, Kurata M, Yoshioka Y, Criqui MC, Genschik P, Ito M. 2011. GIGAS CELL1, a novel negative regulator of the anaphase-promoting complex/cyclosome, is required for proper mitotic progression and cell fate determination in Arabidopsis. The Plant Cell 23, 4382–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki S, Sugimoto K. 2012. Control of the plant cell cycle by developmental and environmental cues. Plant and Cell Physiology 53, 953–964 [DOI] [PubMed] [Google Scholar]
- Kondorosi E, Roudier F, Gendreau E. 2000. Plant cell-size control: growing by ploidy? Current Opinion in Plant Biology 3, 488–492 [DOI] [PubMed] [Google Scholar]
- Korenjak M, Taylor-Harding B, Binné UK, Satterlee JS, Stevaux O, Aasland R, White-Cooper H, Dyson N, Brehm A. 2004. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell 119, 181–193 [DOI] [PubMed] [Google Scholar]
- Lai LB, Nadeau JA, Lucas J, Lee EK, Nakagawa T, Zhao L, Geisler M, Sack FD. 2005. The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. The Plant Cell 17, 2754–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens T, Boudolf V, Kheibarshekan L, et al. 2008. Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset. Proceedings of the National Academy of Sciences, USA 105, 14721–14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens T, Li J, Leone G, De Veylder L. 2009. Atypical E2Fs: new players in the E2F transcription factor family. Trends in Cell Biology 19, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Rabin Z, Li Z, Masson PH, Day CD. 2009. FZR2/CCS52A1 expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiology 149, 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HO, Davidson JM, Duronio RJ. 2009. Endoreplication: polyploidy with purpose. Genes and Developement 23, 2461–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B, Coleman AW. 1993. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. The Plant Cell 5, 1661–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meserve JH, Duronio RJ. 2012. Atypical E2Fs drive atypical cell cycles. Nature 14, 1124–1125 [DOI] [PubMed] [Google Scholar]
- Miller M, Zhang C, Chen ZJ. 2012. Ploidy and hybridity effects on growth vigor and gene expression in Arabidopsis thaliana hybrids and their parents. G3 (Bethesda) 2, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra MK. 1997. Stomatal characteristics at different ploidy levels in Coffea L. Annals of Botany 80, 689–692 [Google Scholar]
- Morejohn LC, Bureau TE, Mol Bajer J, Bajer AS, Fosket DE. 1987, Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro . Planta 172, 252–264 [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. 2002. Control of stomatal distribution on the Arabidopsis leaf surface. Science 296, 1697–1700 [DOI] [PubMed] [Google Scholar]
- Nagl W. 1976, DNA endoreduplication and polyteny understood as evolutionary strategies. Nature 261, 614–615 [DOI] [PubMed] [Google Scholar]
- Narbonne-Reveau K, Senger S, Pal M, Herr A, Richardson HE, Asano M, Deak P, Lilly MA. 2008. APC/CFzr/Cdh1 promotes cell cycle progression during the Drosophila endocycle. Development 135, 1451–1461 [DOI] [PubMed] [Google Scholar]
- Olszewska MJ, Damsz B, Rabeda E. 1983. DNA endoreplication and increase in number of chloroplast during leaf differetniation in five monocotyledonous species with different 2C DNA contents. Protoplasma 116, 41–50 [Google Scholar]
- Pandit SK, Westendorp B, Nantasanti S, van Liere E, Tooten PCJ, Cornelissen PWA, Toussaint MJM, Lamers WH, de Bruin A. 2012. E2F8 is essential for polyploidization in mammalian cells. Nature 14, 1181–1191 [DOI] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL. 2008. Regulation of APC/C activators in mitosis and meiosis. Annual Review of Cell and Developmental Biology 24, 475–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech LM. 1994. A genetic analysis of chloroplast division and expansion in Arabidopsis thaliana . Plant Physiology 104, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Parra E, López-Matas MA, Fründt C, Gutierrez C. 2004. Role of an atypical E2F transcription factor in the control of Arabidopsis cell growth and differentiation. The Plant Cell 16, 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder AHK, Chickarmane V, Cunha A, Obara B, Manjunath BS, Meyerowitz EM. 2010. Variability in the control of cell division underlies sepal epidermal patterning in Arabidopsis thaliana . Plos Biology 8, e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbata HR, Althauser C, Findley SD, Ruohola-Baker H. 2004. The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development 131, 3169–3181 [DOI] [PubMed] [Google Scholar]
- Suda J, Trávnícek P. 2006. Reliable DNA ploidy determination in dehydrated tissues of vascular plants by DAPI flow cytometry: new prospects for plant research. Cytometry A 69, 273–280 [DOI] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Roberts K. 2003. ‘Big it up’: endoreduplication and cell-size control in plants. Current Opinion in Plant Biology 6, 544–553 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakamura S, Kawamukai M, Koizumi N, Nakagawa T. 2011. Development of a series of gateway binary vectors possessing a tunicamycin resistance gene as a marker for the transformation of Arabidopsis thaliana . Bioscience, Biotechnology, Biochemistry 75, 804–807 [DOI] [PubMed] [Google Scholar]
- Van den Heuvel S, Dyson NJ. 2008. Conserved functions of the pRB and E2F families. Nature Reviews Molecular Cell Biology 9, 713–724 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Coppens F, Lee EK, et al. 2011. Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis. EMBO Journal 30, 3430–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lee EK, Lucas JR, Morohashi K, Li D, Murray JAH, Sack FD, Grotewold E. 2010. Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB FOUR LIPS via direct targeting of core cell cycle genes. The Plant Cell 22, 2306–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.