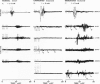
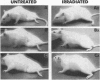
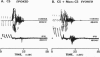Abstract
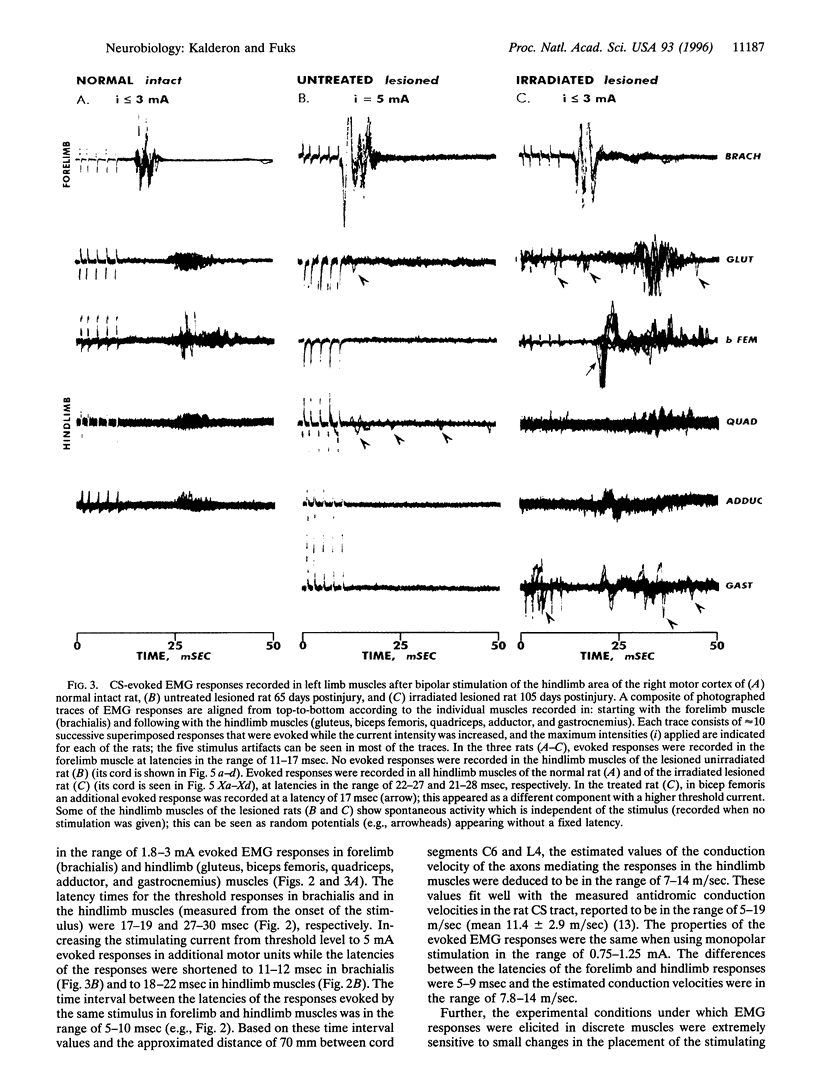
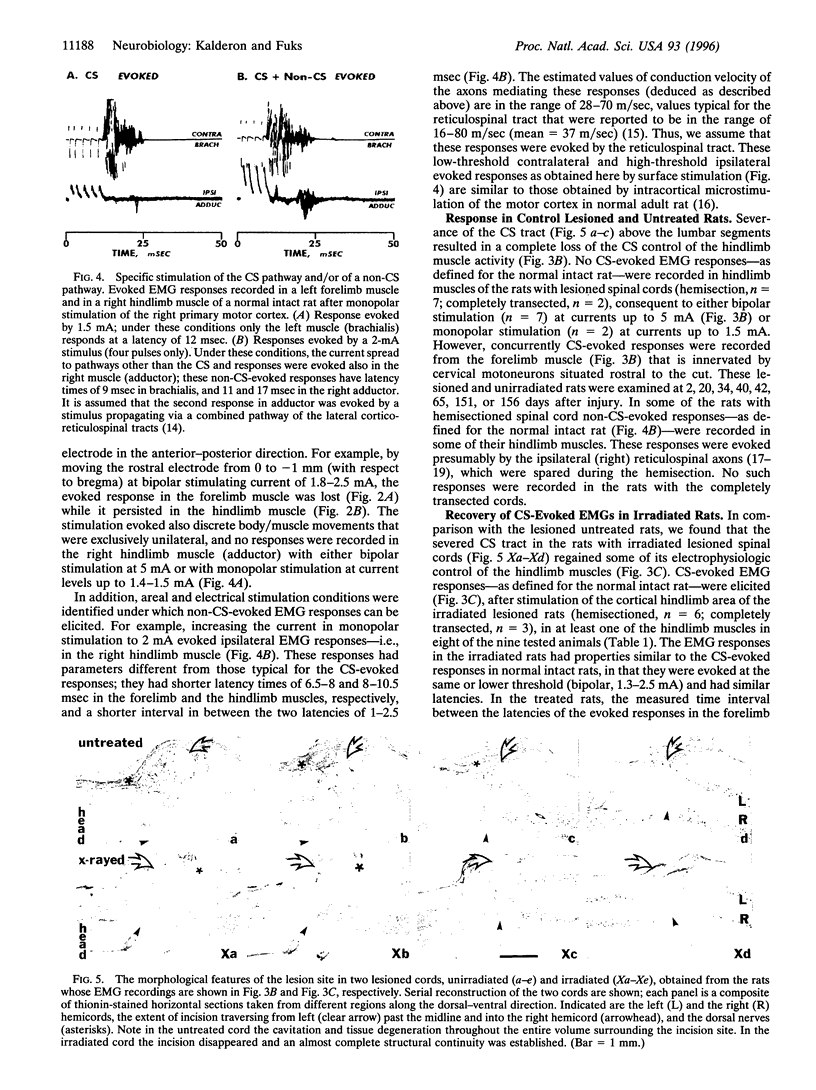
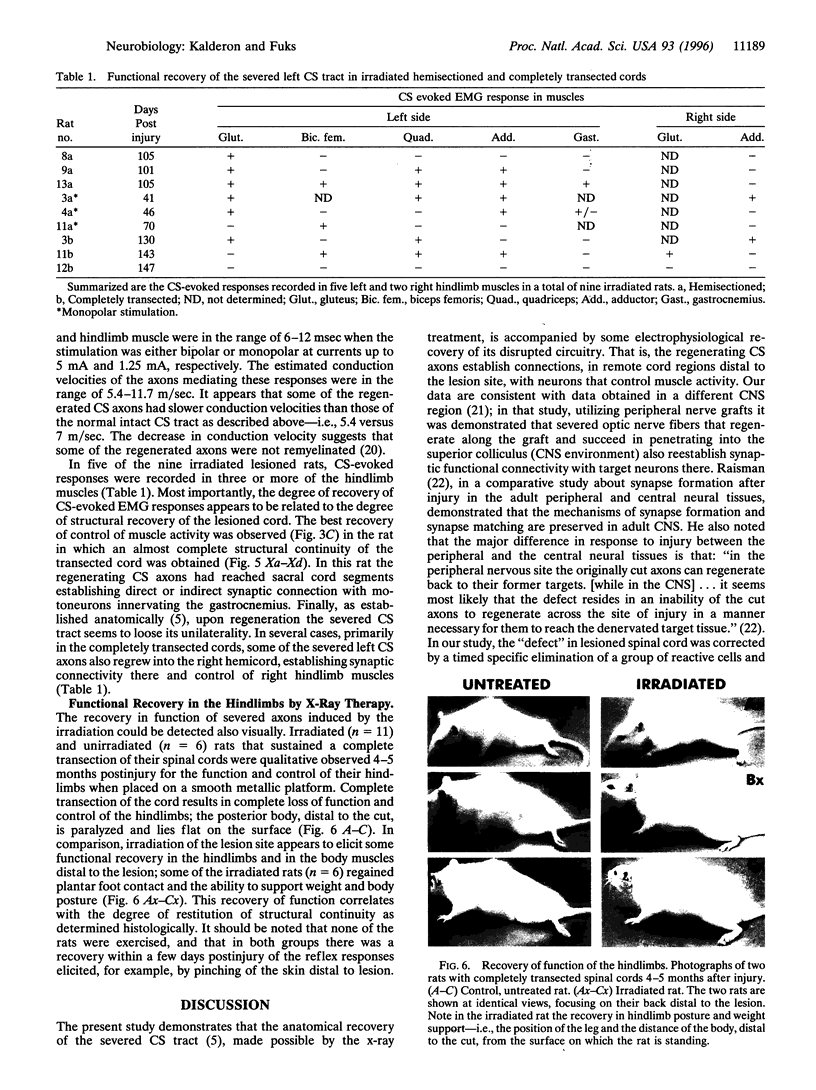
Mechanical injury to the adult mammalian spinal cord results in permanent loss of structural integrity at the lesion site and of the brain-controlled function distal to the lesion. Some of these consequences were permanently averted by altering the cellular constituents at the lesion site with x-irradiation delivered within a critical time window after injury. We have reported in a separate article that x-irradiation of sectioned adult rat spinal cord resulted in restitution of structural continuity and regrowth of severed corticospinal axons across and deep into the distal stump. Here, we report that after x-ray therapy of the lesion site severed corticospinal axons of transected adult rat spinal cord recover electrophysiologic control of activity of hindlimb muscles innervated by motoneurons distal to the lesion. The degree of recovery of control of muscle activity was directly related to the degree of restitution of structural integrity. This restitution of electrophysiologic function implies that the regenerating corticospinal axons reestablish connectivity with neurons within the target field in the distal stump. Our data suggest that recovery of structural continuity is a sufficient condition for the axotomized corticospinal neurons to regain some of their disrupted function in cord regions distal to the lesion site.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbeau H., Rossignol S. Enhancement of locomotor recovery following spinal cord injury. Curr Opin Neurol. 1994 Dec;7(6):517–524. doi: 10.1097/00019052-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Bresnahan J. C. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J Neurol Sci. 1978 Jun;37(1-2):59–82. doi: 10.1016/0022-510x(78)90228-9. [DOI] [PubMed] [Google Scholar]
- Brown L. T., Jr Projections and termination of the corticospinal tract in rodents. Exp Brain Res. 1971 Oct 25;13(4):432–450. doi: 10.1007/BF00234340. [DOI] [PubMed] [Google Scholar]
- Cottingham S. L., Femano P. A., Pfaff D. W. Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp Neurol. 1987 Sep;97(3):704–724. doi: 10.1016/0014-4886(87)90127-0. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Wise S. P. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982 Nov 20;212(1):76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Fox J. E. Reticulospinal neurones in the rat. Brain Res. 1970 Sep 29;23(1):35–40. doi: 10.1016/0006-8993(70)90347-1. [DOI] [PubMed] [Google Scholar]
- Kakulas B. A. The clinical neuropathology of spinal cord injury. A guide to the future. Paraplegia. 1987 Jun;25(3):212–216. doi: 10.1038/sc.1987.37. [DOI] [PubMed] [Google Scholar]
- Kalderon N., Alfieri A. A., Fuks Z. Beneficial effects of x-irradiation on recovery of lesioned mammalian central nervous tissue. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10058–10062. doi: 10.1073/pnas.87.24.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon N., Fuks Z. Structural recovery in lesioned adult mammalian spinal cord by x-irradiation of the lesion site. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11179–11184. doi: 10.1073/pnas.93.20.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartje-Tillotson G., Neafsey E. J., Castro A. J. Electrophysiological analysis of motor cortical plasticity after cortical lesions in newborn rats. Brain Res. 1985 Apr 15;332(1):103–111. doi: 10.1016/0006-8993(85)90393-2. [DOI] [PubMed] [Google Scholar]
- Keirstead S. A., Rasminsky M., Fukuda Y., Carter D. A., Aguayo A. J., Vidal-Sanz M. Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science. 1989 Oct 13;246(4927):255–257. doi: 10.1126/science.2799387. [DOI] [PubMed] [Google Scholar]
- Maffei L., Berardi N., Domenici L., Parisi V., Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci. 1992 Dec;12(12):4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. F., Vertes R. P., Waltzer R. Spinal projections of the gigantocellular reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res. 1985;58(1):154–162. doi: 10.1007/BF00238963. [DOI] [PubMed] [Google Scholar]
- Mediratta N. K., Nicoll J. A. Conduction velocities of corticospinal axons in the rat studied by recording cortical antidromic responses. J Physiol. 1983 Mar;336:545–561. doi: 10.1113/jphysiol.1983.sp014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. Formation of synapses in the adult rat after injury: similarities and differences between a peripheral and a central nervous site. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):349–359. doi: 10.1098/rstb.1977.0047. [DOI] [PubMed] [Google Scholar]
- Robbins A., Schwartz-Giblin S., Pfaff D. W. Ascending and descending projections to medullary reticular formation sites which activate deep lumbar back muscles in the rat. Exp Brain Res. 1990;80(3):463–474. doi: 10.1007/BF00227988. [DOI] [PubMed] [Google Scholar]
- Sanes J. N., Suner S., Donoghue J. P. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res. 1990;79(3):479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Simon D. K., Prusky G. T., O'Leary D. D., Constantine-Paton M. N-methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlsing H. L., Feringa E. R. A ventral uncrossed corticospinal tract in the rat. Exp Neurol. 1980 Nov;70(2):282–287. doi: 10.1016/0014-4886(80)90027-8. [DOI] [PubMed] [Google Scholar]
- Waldron H. A., Gwyn D. G. Descending nerve tracts in the spinal cord of the rat. I. Fibers from the midbrain. J Comp Neurol. 1969 Oct;137(2):143–153. doi: 10.1002/cne.901370203. [DOI] [PubMed] [Google Scholar]
- Waxman S. G. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977 Oct;34(10):585–589. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Wrathall J. R., Pettegrew R. K., Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985 Apr;88(1):108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- Zemlan F. P., Pfaff D. W. Topographical organization in medullary reticulospinal systems as demonstrated by the horseradish peroxidase technique. Brain Res. 1979 Sep 28;174(1):161–166. doi: 10.1016/0006-8993(79)90811-4. [DOI] [PubMed] [Google Scholar]