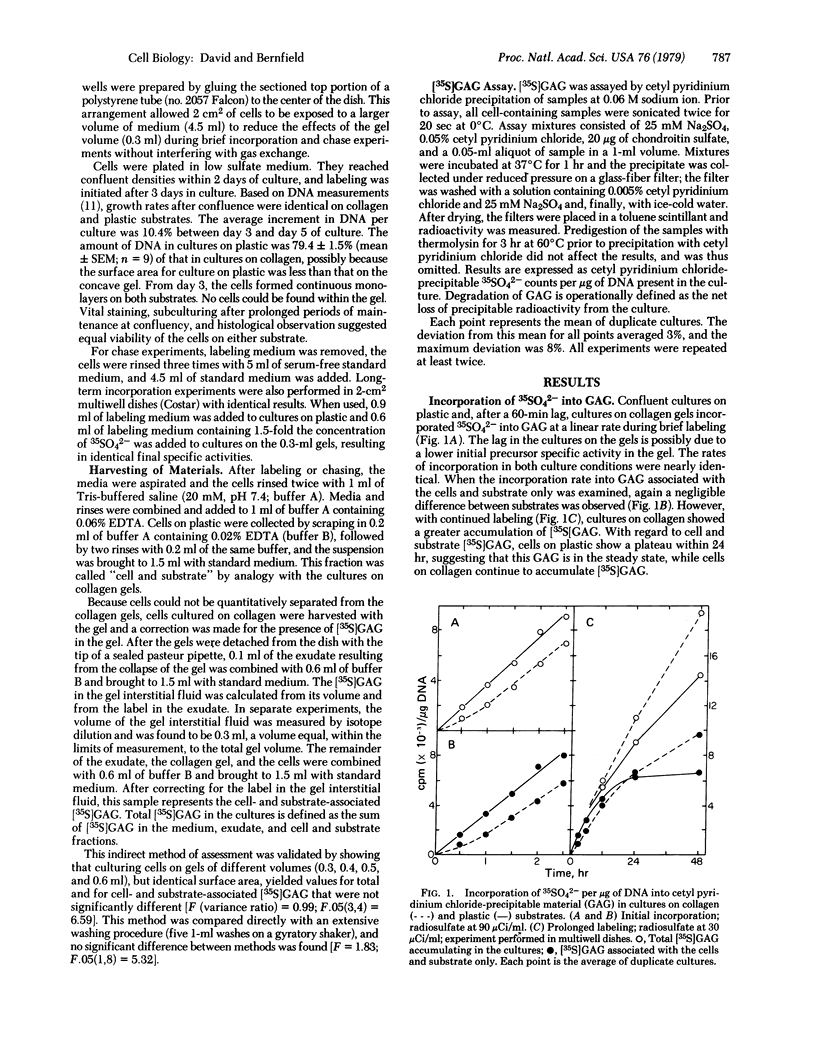
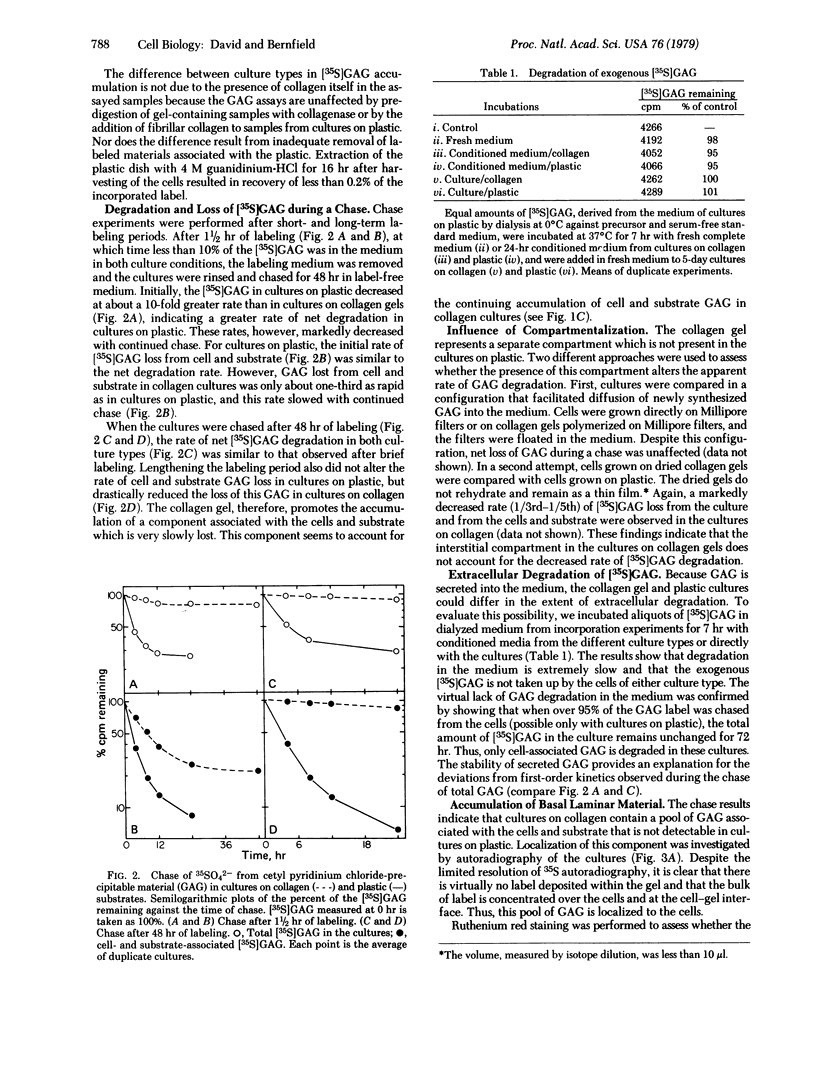
Abstract
Collagenous substrates are reported to promote the accumulation of extracellular matrix materials by epithelia in culture. Glycosaminoglycan (GAG) metabolism is compared in secondary cultures of mouse mammary epithelial cells maintained on plastic or type I collagen gel substrates. The incorporation of 35SO42- into GAG during brief labeling indicates no difference between substrates in the rate of GAG synthesis. During prolonged labeling, however, accumulation of [35S]GAG in cultures on colllagen exceeds that of cultures on plastic. This increased accumulation is due to a markedly reduced rate of GAG degradation. GAG degradation does not occur in the medium, indicating that degradation is localized to the cells. The cultures on collagen contain a slowly degrading cell-associated [35S]GAG pool and a ruthenium red-stained basal lamina, neither of which is present in cultures on plastic. The cell-associated [35S]GAG in cultures on collagen is, in part, localized to the site of the ultrastructurally identified basal lamina. Formation of the basal lamina, therefore, may result from collagen-mediated reduction in the degradation of GAG-containing molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Graham J. M., Hynes R. O., Davidson E. A., Bainton D. F. The location of proteins labeled by the 125I-lactoperoxidase system in the NIL 8 hamster fibroblast. Cell. 1975 Apr;4(4):353–365. doi: 10.1016/0092-8674(75)90156-7. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Kosher R. A., Church R. L. Stimulation of in vitro somite chondrogenesis by procollagen and collagen. Nature. 1975 Nov 27;258(5533):327–330. doi: 10.1038/258327a0. [DOI] [PubMed] [Google Scholar]
- Linder E., Vaheri A., Ruoslahti E., Wartiovaara J. Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med. 1975 Jul 1;142(1):41–49. doi: 10.1084/jem.142.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Meier L., Hay E. D. Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. I. Morphometric analysis of transfilter "induction". J Cell Biol. 1975 Aug;66(2):275–291. doi: 10.1083/jcb.66.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]