Abstract
We describe two gene-knockout (KO) strategies in Trypanosoma brucei using Cre recombinase and loxP sites. Due to the limited number of selection markers for T. brucei, it has been difficult to generate a mutant with two genes knocked out and impractical to simultaneously knockout more than two genes, deterring detailed studies of important cellular mechanisms. The first KO strategy described can overcome the marker problem by allowing continuous re-use of drug-resistance markers. The same KO vector can be used to make a conditional KO system, when a gene of interest is essential for cell viability. As a gene of interest is removed from its original chromosomal locus by the induction of Cre recombinase, deletion is complete and instantaneous. This makes it easier to identify primary effects rather than having secondary effects obscuring phenotypic assessment, as is often the case with RNAi silencing.
Keywords: Trypanosoma brucei, Cre-recombinase, gene knockout, conditional gene knockout
Genetic manipulation of a gene of interest is essential to investigate cellular functions of its encoded protein. Because cellular processes are intricately linked with each other through interactions among various factors, often resulting in redundancy, it is also important to have a system that allows genetic manipulation of multiple genes in the same cell. Positive-selection markers have been used to generate null mutations in Trypanosoma brucei, as in other model organisms. However, because meiosis seems to occur during a specific life-cycle stage in tsetse fly [1] and has not been reproduced in culture, sequential deletion of homologous alleles using a limited number of selection markers is the only way to generate null mutations in T. brucei. Thus, it has been difficult to perform detailed genetic analyses of interactions among different pathways. RNAi knockdown has been the only technique utilized to study loss-of-function phenotypes of essential genes in T. brucei, but RNAi silencing may be incomplete or cause off-target effects. To overcome these difficulties, we developed two strategies for gene knockouts using Cre-recombinase and a series of vectors that contain loxP sites. The first system allows continuous reuse of selection markers, so that knocking out multiple genes in the same cell can be done relatively easily, and the second system allows the molecular functions of essential genes to be investigated by conditionally and instantaneously deleting wild-type alleles from their original loci.
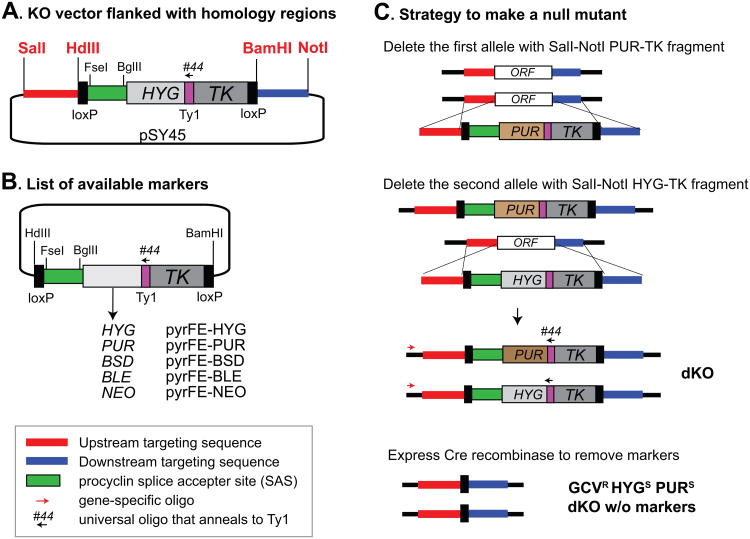
Fig. 1A shows a diagram of a knockout (KO) vector (pSY45) used to delete TbMCM-BP [2]. The plasmid contains sequences from upstream and downstream regions of the TbMCM-BP open reading frame (ORF). It has positive and negative selectable markers for hygromycin resistance (HYG) and gancyclovir (GCV) sensitivity (Herpes simplex virus thymidine kinase: HSVTK or TK in short) that are linked by a short sequence encoding the Ty1 epitope, which allows expression levels to be monitored. To maintain a reasonable level of marker expression regardless of targeting loci, a splice accepter site (SAS) from a procyclin gene, which is widely used and appears to give high levels of trans-splicing [3], was inserted upstream of the fused marker genes. The entire marker cassette is flanked with loxP sites, so it can be removed later by expressing Cre recombinase, either stably incorporated into an rDNA spacer or transiently transfected as described in detail previously [4] (Table 1). To make a KO vector for any gene of interest, upstream and downstream targeting regions can be cloned into SalI-HindIII and BamHI-NotI sites, respectively. Vectors with different selection markers fused with the TK gene are available (Fig. 1B and Table 1). Simple cloning using HindIII-BamHI or HindIII-XbaI sites can be used to swap selection markers. Oligo tb#44 anneals to the Ty1 region, which is present in all vectors containing the TK gene, and can be universally used to genotype a gene KO by PCR.
Fig. 1. Construction of a null mutant.
(A) Backbone of a knockout (KO) vector to delete the gene of interest. pSY45, which was used to knockout TbMCM-BP [2], is shown. A dual marker cassette flanked by loxP sites (loxP–HYG-TK–loxP) is located between HindIII and BamHI sites. Four unique restriction enzyme sites (red font) allow insertion of the upstream homology region (between SalI and HindIII) and the downstream homology region (between BamHI and NotI). Expression levels of targeted genes can vary, and this may affect the growth of transfectants. To maintain a consistent level of trans-splicing, a procyclin splice accepter site (SAS, green box) was inserted upstream of the marker. (B) List of available selection markers (also see Table 1 for alternatives). Five vectors containing TK-conjugated positive selection markers are available and they all have the Ty1 epitope sequence where universal genotyping oligo tb#44 anneals. Ty1 can be also used in western blots to monitor disappearance of selection markers after Cre induction. (C) Strategy to make a null T. brucei mutant without selection markers. A cassette containing PUR-TK marker flanked with upstream and downstream homology regions is recombined into a gene of interest at its original locus, generating a single KO (sKO, ΔPUR-TK/+). The remaining wild type allele can be replaced with the same cassette except the positive selection marker being HYG, resulting in a double KO (dKO, ΔPUR-TK/ΔHYG-TK). The targeting can be confirmed using oligo #44 and a gene specific oligo that anneals outside of upstream homology region used. To remove markers to reuse them later, Cre recombinase under tetracycline control (pLEW100cre or pLEW100cre-EP1) can be inserted at an rRNA array or transiently transfected [4]. Expression of Cre allows recombination between the two loxP sites. The resulting dKO will be sensitive to hygromycin and puromycin, and resistant to GCV, so PUR and HYG markers can be reused.
Table 1. Vectors and oligonucleotides to generate KO or cKO T. brucei cell lines.
| Names | Descriptions (references) |
|---|---|
| pyrFEKO-HYG | loxP–SAS–HYG-Ty1-TK–loxP flanked by TbURA3-targeting homologies |
| pyrFEKO-PUR | loxP–SAS–PUR-Ty1-TK–loxP flanked by TbURA3-targeting homologies |
| pyrFEKO-NEO | loxP–SAS–NEO-Ty1-TK–loxP flanked by TbURA3-targeting homologies |
| pyrFEKO-BLE | loxP–SAS–BLE-Ty1-TK–loxP flanked by TbURA3-targeting homologies |
| pyrFEKO-BSD | loxP–SAS–BSD-Ty1-TK–loxP flanked by TbURA3-targeting homologies |
| pHJ17* (HYG-TK) | Partial β-TUB followed by 5′ ALD UTR–loxP–SAS–HYG-Ty1-TK–loxP–3′ ALD UTR ([8]) |
| pHJ18* (PUR-TK) | partial β-TUB followed by 5′ ALD UTR–loxP–SAS–PUR-Ty1-TK–loxP–3′ ALD UTR ([8]) |
| pHJ22* (NEO-TK) | partial β-TUB followed by 5′ ALD UTR–loxP–SAS–NEO-Ty1-TK–loxP–3′ ALD UTR |
| pHJ42* (BLE-TK) | partial β-TUB followed by 5′ ALD UTR–loxP–SAS–BLE-Ty1-TK–loxP–3′ ALD UTR |
| pHJ41* (BSD-TK) | partial β-TUB followed by 5′ ALD UTR–loxP–SAS–BSD-Ty1-TK–loxP–3′ ALD UTR |
| pHJ32** (HYG) | partial β-TUB followed by 5′ ALD UTR–loxP–SAS–HYG–loxP–3′ ALD UTR |
| pHJ33** (PUR) | partial β-TUB followed by 5′ ALD UTR–loxP–SAS–PUR–loxP–3′ ALD UTR |
| pDS66 | 3xHA-intergenic region of TUB–HYG (modified from [11]) |
| pDS67 | 3xMYC-intergenic region of TUB–HYG (modified from [11]) |
| pDS68 | GFP-intergenic region of TUB–HYG (modified from [11]) |
| pDS69 | FLAG-intergenic region of TUB-HYG (modified from [11]) |
| pLEW100cre-EP1 | stable tetracycline-regulated low-level expression of cre recombinase ([4]) |
| pSY45 | loxP–SAS–HYG-Ty1-TK–loxP flanked by TbMCM-BP-targeting homologies ([2]) |
| Oligo tb44 | 5′ CTGGTTAGTATGGACTTCTCTAGA; a universal reverse oligo for genotyping; anneals to Ty1 sequences ([2,6-8]) |
| PCR-KO-forward | 5′ AAAACATAAACTCAACTGCAA; forward sequences that anneal immediate upstream of 5′ loxP in pHJ vector series; used to knockout genes by one-step PCR method ([2,6-8]) |
| PCR-KO-reverse | 5′ CAACTAACTAAATGGGCAGGA; reverse oligo that anneals immediate downstream of 3′ loxP in pHJ vector series; used to knockout genes by one-step PCR method ([2,6-8]) |
Regions containing markers and loxP sites in these pHJ vectors are equivalent to those in the pyrFEKO series. But, as ALD UTRs used in pHJ vectors are much shorter (∼500bp shorter) than ones in pyrFE, these pHJ vectors are more useful than pyrFE when PCR-targeting a location where endogenous UTRs unavailable to drive marker mRNA maturation. For example, pHJ18 was used as a template to PCR-target the 70-bp repeat with 5′ALD UTR-PUR-TK-3′ALD UTR in the active bloodstream form expression site [7,8].
These plasmids were cloned by restriction digestion with XbaI-blunted and XmaI, followed by self-ligation. This adds 27 nt to the 3′ end of HYG or PUR gene but did not interfere with the resistance to hygromycin or puromycin.
To make a null mutant (Fig.1C), a SalI-NotI-digested KO cassette containing a puromycin-resistance gene (PUR) fused with TK is used to replace the first wild-type allele. This single KO (sKO) line is then transfected with a SalI-NotI-digested HYG-TK KO cassette to delete the second allele, resulting in a null mutant (double KO, dKO). The null mutant is resistant to hygromycin and puromycin, and sensitive to gancyclovir (GCV), which counter-selects TK-expressing cells. Marker cassettes can be removed by Cre expression [4], generating a null mutant without markers, which is sensitive to hygromycin and puromycin, and resistant to GCV. As expression levels of TK may vary due to different endogenous 3′ UTR regions, the amount of GCV required for selection should generally be titrated.
Alternatively, one-step PCR, by amplifying the lox-P-flanked marker cassette with oligonucleotides containing upstream and downstream homology, can be used to delete the first allele. Efficiency of targeting by homologous recombination relies on homology length, which should be at least 50 bp [5]. We have deleted the first allele of several genes using a PCR fragment containing about 70-bp of homologous sequences [2,6-8]. However, the PCR method using short targeting sequences was not efficient for deletion of the second allele, probably due to sequences shared between the chromosomal PUR-TK region and the HYG-TK KO cassette. Plasmids and oligonucleotides used for one-step PCR KO are described in Table 1. A combination of these KO vectors and PCR oligonucleotides was used to generate several T. brucei null mutants, topo3α and rmi1 single mutants, and topo3α rmi1 double mutants. 50μg/ml GCV was used to select for the loss of TK [7,8].
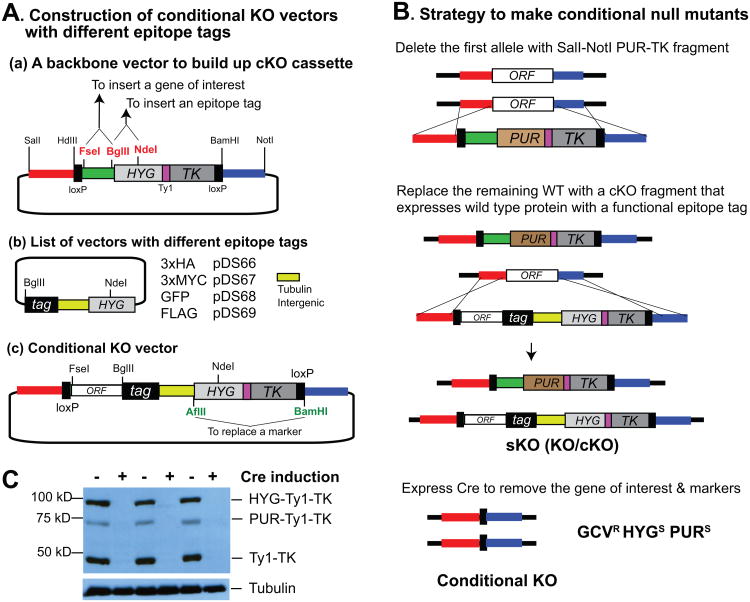
RNAi knock down is routinely used to study the roles of essential genes. However, expression of double stranded RNA may silence other genes that have similar sequences, causing off-target effects, or is sometimes not very efficient. One alternative is to use a regulated conditional expression allele [9,10]. This is not much used today because it requires several constructs, transfections, and tests of different clones to ensure tight regulation of the conditional allele. To study essential genes with less complication, we developed a conditional KO system (Fig. 2). The ORF of a gene of interest can be inserted (with or without stop codon) into the region between the FseI and BglII sites in the HYG-TK KO vector (Fig 2A(a)). A BglII-NdeI fragment (b) containing a fraction of HYG, can then be inserted to generate an epitope tagged version (c). Different epitopes are available with the HYG marker and the entire cassette is flanked with loxP sites. To generate a conditional null mutant (Fig. 2B), one allele is first deleted with the PUR-TK KO vector and the second allele is replaced with the conditional KO cassette containing the epitope-tagged gene, which allows expression (and functionality in the case of an essential gene) to be confirmed. Induction of Cre will remove both the PUR-TK cassette and the ORF–tag–HYG-TK allele, generating a null mutant.
Fig. 2. Construction of a conditional null mutant.
(A) Construction of a conditional KO vector. The coding region of a gene can be inserted into the KO vector between FseI and BglII sites without or with (if epitope tagging is not desired) a stop codon. BglII and NdeI sites can be used to add different epitope tags. Between the tag and HYG is a TUB intergenic region, to provide UTRs for the gene of interest, and HYG. The final cKO cassette will have a loxP–ORF–epitope–3′ UTR–5′ UTR–HYG-TK–loxP so the gene of interest and the marker can be removed by Cre expression. Cloning at AflII and BamHI sites can be used to replace markers. (B) Strategy to make a conditional null mutant. One allele is removed with the PUR-TK KO vector. The remaining wild-type allele can be replaced with a cKO cassette that expresses wild-type allele with various epitope tags. Targeting can be confirmed by PCR or Southern blot. Unlike with the KO strategy, recombination can also occur between ORFs resulting in loss of the upstream loxP site, in which case Cre-loxP removal will not work. Therefore, it is essential to verify that both loxP sites are present after targeting. Cre expression will remove the region between the loxP sites. (C) Loss of markers by Cre expression. Gene knockout can be confirmed by western blot using antibodies against epitopes fused to the wild-type allele and by monitoring the loss of markers, which contain the Ty1 epitope sequence. The western blot shows that marker proteins disappeared after Cre induction. Tubulin was used as a loading control.
Efficiency of Cre-loxP deletion can be assessed by western blot analysis of the tagged protein and also by western blot detection of Ty1 (loss of PUR-TK and of HYG-TK). Three independent clones are shown as examples, demonstrating the loss of selection markers after Cre induction (Fig. 2C). The loss of the gene and the markers can be confirmed by PCR analysis. Recombination can also occur between wild type alleles in the chromosome and the cKO cassette and this will result in the loss of the upstream loxP site. Therefore, it is essential to ensure that both loxP sites are present after the cKO targeting.
This conditional KO method with minor changes was used previously to generate TbMCM-BP conditional null mutant. This cKO system was indeed much more efficient than RNAi knockdown for TbMCM-BP depletion [2]. Sequences and maps of all vectors are available at http://tryps.rockefeller.edu and the vectors are available from Addgene (http://www.addgene.org/George_Cross).
Highlights.
Cre-loxP knockout (KO) system allows continuous reuse of drug selection markers.
Cre-loxP KO can be used to study genetic interaction between multiple pathways.
Conditional KO (cKO) using Cre-loxP is useful to study essential genes.
Acknowledgments
This work was supported by grant no. R01AI021729 from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
Abbreviations
- ALD
aldolase
- BLE
bleomycin-resistance gene
- BSD
blasticidin-resistance gene
- GCV
gancyclovir
- HSVTK or TK
Herpes simplex virus thymidine kinase
- HYG
hygromycin-resistance gene
- KO
knockout
- NEO
neomycin-resistance gene
- ORF
open reading frame
- PUR
puromycin-resistance gene
- SAS
splice accepter site
- UTR
untranslated region
- TUB
tubulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peacock L, Ferris V, Sharma R, Sunter J, Bailey M, Carrington M, Gibson W. Identification of the meiotic life cycle stage of Trypanosoma brucei in the tsetse fly. Proc Nat Acad Sci USA. 2011;108:3671–3676. doi: 10.1073/pnas.1019423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HS, Park SH, Günzl A, Cross GAM. MCM-BP is required for repression of life-cycle specific genes transcribed by RNA polymerase I in the mammalian infectious form of Trypanosoma brucei. PLoS One. 2013;8:e57001. doi: 10.1371/journal.pone.0057001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel TN, Tan KS, Cross GAM. A systematic study of sequence motifs for RNA trans splicing in Trypanosoma brucei. Mol Cell Biol. 2005;25:9586–9594. doi: 10.1128/MCB.25.21.9586-9594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scahill MD, Pastar I, Cross GAM. CRE recombinase-based positive-negative selection systems for genetic manipulation in Trypanosoma brucei. Mol Biochem Parasitol. 2008;157:73–82. doi: 10.1016/j.molbiopara.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes RL, McCulloch R. Trypanosoma brucei homologous recombination is dependent on substrate length and homology, though displays a differential dependence on mismatch repair as substrate length decreases. Nucl Acids Res. 2007;35:3478–3493. doi: 10.1093/nar/gkm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benmerzouga I, Concepcion-Acevedo J, Kim HS, Vandoros AV, Cross GAM, Klingbeil MM, Li B. Trypanosoma brucei Orc1 is essential for nuclear DNA replication and affects both VSG silencing and VSG switching. Mol Microbiol. 2013;87:196–210. doi: 10.1111/mmi.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS, Cross GAM. Identification of Trypanosoma brucei RMI1/BLAP75 homologue and its roles in antigenic variation. PLoS One. 2011;6:e25313. doi: 10.1371/journal.pone.0025313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Cross GAM. TOPO3alpha influences antigenic variation by monitoring expression-site-associated VSG switching in Trypanosoma brucei. PLoS Pathogens. 2010;6:e1000992. doi: 10.1371/journal.ppat.1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtz E, Leal S, Ochatt C, Cross GAM. A tightly regulated inducible expression system for dominant negative approaches in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 10.Milne KG, Guther ML, Ferguson MAJ. Acyl-CoA binding protein is essential in bloodstream form Trypanosoma brucei. Mol Biochem Parasitol. 2001;112:301–304. doi: 10.1016/s0166-6851(00)00369-8. [DOI] [PubMed] [Google Scholar]
- 11.Oberholzer M, Morand S, Kunz S, Seebeck T. A vector series for rapid PCR-mediated C-terminal in situ tagging of Trypanosoma brucei genes. Mol Biochem Parasitol. 2006;145:117–120. doi: 10.1016/j.molbiopara.2005.09.002. [DOI] [PubMed] [Google Scholar]