Abstract
Objective
To estimate the associations of change in immune response with preterm delivery, omega-3 supplementation, and fish diet.
Methods
This was an ancillary study to a randomized trial of omega-3 fatty acid supplementation for the prevention of recurrent preterm birth. In vitro maternal peripheral blood mononuclear leukocyte production of the anti-inflammatory cytokine, interleukin-10 (IL-10), and the proinflammatory cytokine, tumor necrosis factor-α (TNF-α), in response to stimulation with lipopolysaccharide, was measured at 16–22 weeks of gestation (baseline) and again at 25–28 weeks of gestation (follow-up) among women with prior spontaneous preterm birth. Changes in concentrations from baseline to follow-up (Δ) were compared separately among groups defined by gestational age category at delivery, fish diet history, and omega-3 compared with placebo treatment assignment with Kruskal-Wallis tests.
Results
IL-10 Δ differed by gestational age category among 292 women with paired assays. Concentrations increased less in women delivering between 35 and 36 6/7 weeks (48.9 pg/ml) compared with women delivering at term (159.3 pg/ml) and decreased by 65.2 pg/ml in women delivering before 35 weeks, p=.01. TNF-α Δ also differed by gestational age category among 319 women, but the pattern was inconsistent. Those delivering between 35 and 36 6/7 weeks exhibited decreased concentrations of TNF-α at follow-up compared with baseline (−356.0 pg/ml); concentrations increased among women delivering before 35 weeks, and those delivering at term, 132.1 and 86.9 pg/ml, p=.03. IL-10 Δ and TNF-α Δ were unaffected by either omega-3 supplementation or fish diet.
Conclusion
Recurrent preterm birth was associated with decreased peripheral blood mononuclear leukocyte production of IL-10 in response to a stimulus during the second trimester.
Clinical Trial Registration
Clinical Trials.gov, www.clinicaltrials.gov, NCT00135902.
Introduction
A causal link between an inflammatory response and preterm delivery is well established. (1–4) Proinflammatory cytokines and chemokines may be central in the final common pathway initiating labor due to not only infection but also decidual hemorrhage, uteroplacental ischemia, cervical disease, or immunologic phenomenon.(4–9) The Th2 anti-inflammatory cytokine, interleukin-10 (IL-10), has a significant role in the maintenance of pregnancy. (3, 10, 11) Treatment with IL-10 in animal models of intra-amniotic infection reduces IL-1β-induced uterine contractions, amniotic fluid concentrations of tumor necrosis factor α, leukocyte counts and improves pregnancy outcomes. (12–14) In human studies peripheral blood mononuclear leukocyte (PBML) production of IL-10 has been found to be higher in the first trimester but lower at term when compared with levels in non-pregnant controls suggesting that downregulation of IL-10 occurs as part of the inflammatory process necessary for term labor. (15) The role of change in regulation of anti-inflammatory or pro-inflammatory cytokine production in response to an inflammatory stimulus across gestation in preterm birth has not been examined.
The innate immune response to a stimulus, including the balance between pro-inflammatory and anti-inflammatory cytokines, may alter disease severity; this response may vary between individuals because of genetic or environmental factors including dietary exposures such as omega-3 fatty acids.. (16–20) We conducted this study to estimate the associations of change in immune response with preterm delivery, omega-3 supplementation, and fish diet. We selected a priori tumor necrosis factor-α (TNF-α) as the pro-inflammatory and IL-10 as the anti-inflammatory cytokine for study based on their functions in inflammation activation and resolution as well as preterm labor. Prior studies have demonstrated an effect of omega-3 fatty acids on PBML production of TNF-α and IL-10. (20–22)
Methods
The cohort consisted of women from 13 Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network centers enrolled in the randomized trial of omega-3 fatty acid supplementation to prevent recurrent preterm birth, registered at Clinical Trials.gov (NCT00135902), between January 2005 and October 2006. The methods and results of the trial have previously been published. (23) Inclusion criteria were a documented history of at least one prior singleton preterm delivery between 20 0/7 and 36 6/7 weeks’ gestation after spontaneous preterm labor or preterm premature rupture of the membranes, and a current singleton pregnancy. The study was approved by the institutional review board of each clinical site and of the data coordinating center. Women gave written informed consent for study participation and were enrolled between 16 and 21 6/7 weeks’ gestation. Participants were randomized to receive either a daily supplement of 2,000 mg of long chain omega-3 PUFAs or matching placebo capsules. All participants received weekly injections of 17 alpha-hydroxyprogesterone caproate (17-OHPC, 250 mg) because of their obstetric histories of spontaneous preterm delivery. (24) There was no difference in the rate of preterm birth between the omega-3 and placebo groups. Fish diet histories were assessed at baseline enrollment . The four items in the food frequency questionnaire are dark-meat fish, canned tuna, other fish and shellfish. (25)
Blood samples were collected between 16 and 22 weeks’ gestation before starting study drug and again at the follow-up visit between 25 and 28 weeks’ gestation for cytokine analysis. None of the women were in labor or prelabor when the samples were collected. Samples were shipped overnight on ice to a central laboratory (Dr. Li, Division of Inflammation Biology and Immunology, Department of Biological Sciences, Virginia Polytechnic Institute and State University, Blacksburg, VA). Peripheral blood mononuclear leukocytes were isolated from heparinized blood by Isolymph sedimentation (Gallard-Schlesinger Industries, Carle Place Inc., NY) followed by centrifugation for 5 min at 200 × g. The pellet was resuspended at a final concentration of 5×106 cells/ml in complete RPMI 1640 medium supplemented with 10% FBS. Cells were cultured at 37°C with 5% CO2 for 24 hours. TNF-α and IL-10 secretion in cell supernatants were analyzed using Enzyme-Linked ImmunoSorbent Assay (ELISA) kits according to manufacturer’s instructions. The assays were performed concomitantly in separate cell culture after incubating with 100 ng/ml lipopolysaccharide (LPS; Escherichia coli 0111:B4, Sigma Chemical Co.). Detection limits were 1 pg/ml for IL-10 and 2 pg/ml for TNF- α. (26, 27) Samples with concentrations below the limit of detection or otherwise out of range were excluded from the analysis which examined change over time.
We used Wilcoxon rank-sum and chi square tests as appropriate to compare demographic and clinical variables of women included in this ancillary study with those of women in the trial but excluded from the ancillary study because of lack of paired samples from baseline and follow-up. Change in inflammatory response to a stimulus was assessed by computing the change in concentrations of IL-10 and TNF-α in the cell supernatants (after LPS stimulation minus unstimulated) from baseline (16–22 weeks’ gestation) to follow-up (25–28 weeks’ gestation) in paired samples from the same patients. The change in concentrations of IL-10 and TNF-α were compared between groups defined by gestational age at delivery (≥ 37 weeks, 35 through 36 6/7 weeks, and < 35 weeks), fish diet history (< one fish meal per week and ≥ one fish meal per week) and treatment assignment with Kruskal-Wallis tests. (28) We chose a priori three gestational age groups because infection and inflammation are more frequently identified in early versus late preterm births. The number of extreme preterm births, <28 weeks’ gestation, with paired baseline and follow-up samples was too small for meaningful analysis as a separate group, one patient for IL-10 and two patients for TNF-α. Fish diet history categories were selected based on our previously reported analysis that showed an association between low dietary intake of fish and preterm birth in the randomized trial cohort. (29) Multivariable logistic analysis adjusting for treatment assignment, earliest gestational age of prior preterm delivery, number of prior preterm deliveries, smoking, race/ethnicity, body mass index and clinical center was conducted to test the relationship between preterm birth and fish diet history for the women with paired cytokine measurements included in this ancillary study. To adjust for multiple pair wise comparisons of cytokine concentrations between the three groups defined by gestational age at delivery, we used a p value of .017; otherwise, a p< .05 was selected as indicative of significance. All comparisons were two-sided.
Results
Among the 852 women enrolled in the trial 343 had paired cytokine measurements for either IL-10, TNF-α, or both. The median age, number of prior preterm births and distribution by race, smoking history, or fish dietary intake did not differ between those women who had paired measurements and those who did not. Women with paired measurements were more likely than women without paired measurements to be in the omega-3 treatment group. (table 1) The rate of preterm birth less than 37 weeks’ gestation for women with paired measurements was 36.7 % and was not different from the rate for women in the trial without paired measurements, 41.7%, p=.15.
Table 1.
Characteristics of Women With Paired Assays From Baseline and Follow-up for Either Interleukin-10, Tumor Necrosis Factor-α, or Both Compared With Women in the Trial for Whom Paired Assays Were Not Available
| Women With Paired Measurements for IL-10, TNF-α or Both (n =343) | Women Without Paired Cytokine Measurements (n = 509) | P * | |
|---|---|---|---|
| Median age (interquartile range) | 27 (23 – 32) | 27 (23 – 32) | .70 |
| Racial distribution | |||
| Non-Hispanic White | 178 (51.9) | 241 (47.4) | .36 |
| Non-Hispanic Black | 107 (31.2) | 181 (35.6) | |
| Other | 58 (16.9) | 87 (17.1) | |
| Median no. of prior preterm births (interquartile range) | 1 (1 – 2) | 1 (1 – 2) | .16 |
| Smoked | 57 (16.6) | 79 (15.5) | .67 |
| Assigned to omega-3 | 191 (55.7) | 243 (47.7) | .02 |
| Fewer than 1 fish meal per week | 162 (47.2) | 267 (52.5%) | .13 |
Wilcoxon rank sum or chi square tests
IL-10, interleukin-10; TNF-α, tumor necrosis factor-α.
Data are n (%) unless otherwise specified.
PBML production of both cytokines increased after LPS stimulation compared with unstimulated levels. The median (interquartile range) increase in pg/ml was 992.8 (259.9 – 1553.5) for TNF-α and 1079.5 (303.9 – 2419.6) for IL-10.
A total of 292 women had paired assays for IL-10 and 319 women had paired assays for TNF-α. Table 2 shows the median change with range and interquartile range from baseline to follow-up in concentrations (LPS stimulated minus unstimulated) for Il-10 and TNF-α for the three groups defined by gestational age at delivery. The median change in concentrations in IL-10 were different between the three groups, p = .01. The increase in IL-10 was less in women delivering at 35–36 weeks’ gestation (48.9 pg/ml) compared with women delivering at term (159.3 pg/ml) and decreased from baseline to follow-up among women delivering before 35 weeks (median decrease of 65.2 pg/ml). (table 2) The pair wise comparisons revealed the changes in concentrations were different between those delivering before 35 weeks compared to those delivering at term, p=.01 The change in median concentrations in TNF-α from baseline to follow-up also differed between the three groups defined by gestational age at delivery, p = .03, but the pattern was not consistent. Women delivering at 35–36 weeks’ gestation had a drop in concentrations from baseline to follow-up (median decrease of 356.0 pg/ml). The median increase from baseline to follow-up among women delivering at term was 86.9 pg/ml; women delivering before 35 weeks’ gestation also had an increase, 132.1 pg/ml. (table 2) The pair wise comparisons revealed the changes in concentrations were different between those delivering at 35–36 weeks compared to those delivery at term, p=.01.
Table 2.
Change from Baseline to Follow-up (Δ) in Peripheral Blood Mononuclear Leukocyte Production of Interleukin-10 and Tumor Necrosis Factor-α in Response to Lipopolysaccharide (Lipopolysaccharide Stimulated Minus Unstimulated)
| Gestational Age at Delivery (Weeks)
|
P* | |||
|---|---|---|---|---|
| Prior to 35 | 35–36 | 37 or After | ||
| IL-10 | ||||
| Number of paired samples† | 39 | 64 | 189 | |
| Median Δ‡ | −65.2 | 48.9 | 159.3 | .01 |
| Range‡ | −5394.5, 1932.4 | −4020.5, 3916.8 | −3868.6, 16094.4 | |
| Interquartile range‡ | −1217.4, 254.3 | −843.0, 563.1 | −356.2, 925.2 | |
| TNF-α | ||||
| Number of paired samples | 48 | 70 | 201 | |
| Median Δ‡ | 132.1 | −356.0 | 86.9 | .03 |
| Range‡ | −4753.5, 13008.9 | −13491.8, 20640.9 | −10151.3, 14578.7 | |
| Interquartile range‡ | −1218.0, 817.0 | −2307.5, 334.2 | −964.3, 1108.2 | |
IL-10, interleukin-10; TNF-α, tumor necrosis factor-α.
Kruskal-Wallis test
Paired samples from the same patients at baseline and follow-up.
Data are median, range, and interquartile range in pg/mL by gestational age at delivery.
Similar to the findings in the trial of 852 women, the rate of preterm birth in this ancillary study of 343 women varied by fish diet history. The rate of preterm birth < 37 weeks’ gestation was 33.7% among those who ate at least one fish meal per week and 44.4% among those who ate less than 1 fish meal per week, p=.004 (RR=0.76; 95% CI, 0.63–0.92). This association remained after controlling for treatment assignment, earliest gestational age of prior preterm delivery, number of prior preterm deliveries, smoking, race/ethnicity, body mass index and clinical center, p=.03 (OR=0.68, 95%CI 0.48–0.96). We hypothesized that modulation of the inflammatory response may be a mechanism by which fish diet reduces preterm birth; however, there was no difference in the change in concentrations from baseline to follow-up for IL-10 or TNF-α between the two groups defined by dietary fish intake, < 1 fish meal per week versus at least one fish meal per week. (table 3). Our previous analysis of the association between fish diet and preterm birth showed a U shaped pattern with probability of preterm birth decreasing with increasing fish intake but then increasing again. The protective effect of dietary fish was not observed in women eating four or more fish meals per week. (29) Therefore, we also estimated the association between change in IL-10 and TNF-α concentrations among three fish-diet groups: < 1 meal per week, 1–3 meals per week, and four or more meals per week. There were no differences in these cytokine measurements among these three groups (data not shown); Il-10, p=.58; TNF-α, p=.26. There were no differences in change in concentrations from baseline to follow-up for IL-10 or TNF-α between the omega-3 and placebo treatment groups. (table 3)
Table 3.
Change From Baseline to Follow-up (Δ) in Peripheral Blood Mononuclear Leukocyte Production of Interleukin-10 and Tumor Necrosis Factor-α in Response to Lipopolysaccharide (Lipopolysaccharide Stimulated Minus Unstimulated)
| Fish Diet History | Treatment Assignment | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Fewer Than 1 Meal per Week | 1 or More Meals per Week | P + | Omega-3 | Placebo | P* | |
| IL-10 | ||||||
| Number of paired samples† | 137 | 155 | 167 | 125 | ||
| Median Δ + | 72.5 | 120.6 | .36 | 120.6 | 72.5 | .91 |
| Range + | −5394.5, 16094.4 | −3100.5, 5833.8 | −5394.5, 16094.4 | −4020.5, 3916.8 | ||
| Interquartile range + | −652.4, 651.7 | −437.0, 846.3 | −542.2, 700.6 | −470.3, 836.6 | ||
| TNF-α | ||||||
| Number of paired samples† | 152 | 167 | 174 | 145 | ||
| Median Δ + | −198.1 | 95.4 | .10 | 20.9 | −3.1 | .55 |
| Range + | −13429.3, 20640.9 | −13491.8, 14578.7 | −13491.8, 14578.7 | −10151.3, 20640.9 | ||
| Interquartile range+ | −1673.4, 790.2 | −931.8, 1103.2 | −1205.5, 1158.8 | −1154.1, 762.5 | ||
IL-10, interleukin-10; TNF-α, tumor necrosis factor-α.
Kruskal-Wallis test
Paired samples from the same patients at baseline and follow-up
Data are median, range, and interquartile range in pg/mL by fish diet and treatment assignment
Discussion
In this cohort of women with a prior spontaneous preterm delivery, we observed a decrease in LPS-stimulated PBML production of IL-10 across the second trimester in women destined to deliver before 35 weeks’ gestation. Women who subsequently delivered at term demonstrated an increase in IL-10 production and, although women delivering late preterm also had increased IL-10 production, the increase was less than among those delivering at term. It is believed that IL-10 plays a role in the maintenance of pregnancy and downregulation of IL-10 favors an inflammatory state. (15, 30, 31) IL-10 can block preterm labor induced by intrauterine infusion of LPS in rodents (13) and IL-1β induced preterm labor in primates. (12) In a study of 7 women either in the first trimester or at term before labor and 7 age-matched non-pregnant controls, PBML production of IL-10 was higher than controls in the first trimester but dropped to non-pregnant levels at term leading the investigators to hypothesize that withdrawal of anti-inflammatory agents, including anti-inflammatory cytokines, occurs to accelerate an inflammatory process necessary for term labor. (15) Our data suggest this process may occur prematurely in women destined to deliver before term.
The study results did not support our hypothesis that the different effects of fish diet and omega-3 supplementation on preterm birth may be due to differences in modulating the immune response. Our results are in agreement with those from a randomized clinical trial in pregnant women for primary allergy prevention conducted in Sweden. Among 145 women, LPS-induced TNF-α and IL-10 secretion from whole blood cultures were not different between those receiving 2.7 grams of omega-3 PUFA supplementation daily and those receiving placebo. (32) Although some studies in non-pregnant individuals, cell cultures, and animal models have reported a suppressive effect of omega-3 fatty acid supplementation on PBML production of TNF-α and a stimulatory effect on production of IL-10 (20, 23, 24), the majority of intervention studies in humans have found no effect of omega-3 supplementation or fish consumption on cytokines or other biomarkers of inflammation. (33–39)
Strengths of our study include a well-characterized, large cohort of women at high risk for preterm birth. We examined the response to an inflammatory stimulus with measurement of both an anti-inflammatory and a pro-inflammatory cytokine over time in the second trimester with paired samples.
The weaknesses of the study must be acknowledged. The study included women with a prior preterm delivery; therefore, the results may not be generalizable to other obstetric patients. All women received 17 OHPC and it is unclear what effect this may have had on our study results. Progesterone is an immunomodulator at the maternal-fetal surface, affecting production of pro-inflammatory cytokines by macrophages and altering T-cell clone cytokine secretion in favor of IL-10. (402–42) The role of activation of the maternal inflammatory response in the preterm parturition syndrome continues to be investigated. Our data suggest a role of premature downregulation of IL-10 production and may have implications for treatment; however, other studies across a wider gestational age range are needed to confirm these results. Study of subgroups such as women with a shortened cervical length in the mid trimester, women with chronic bleeding in pregnancy, and women with multi-fetal pregnancies might offer new insight about the preterm parturition syndrome in different clinical situations.
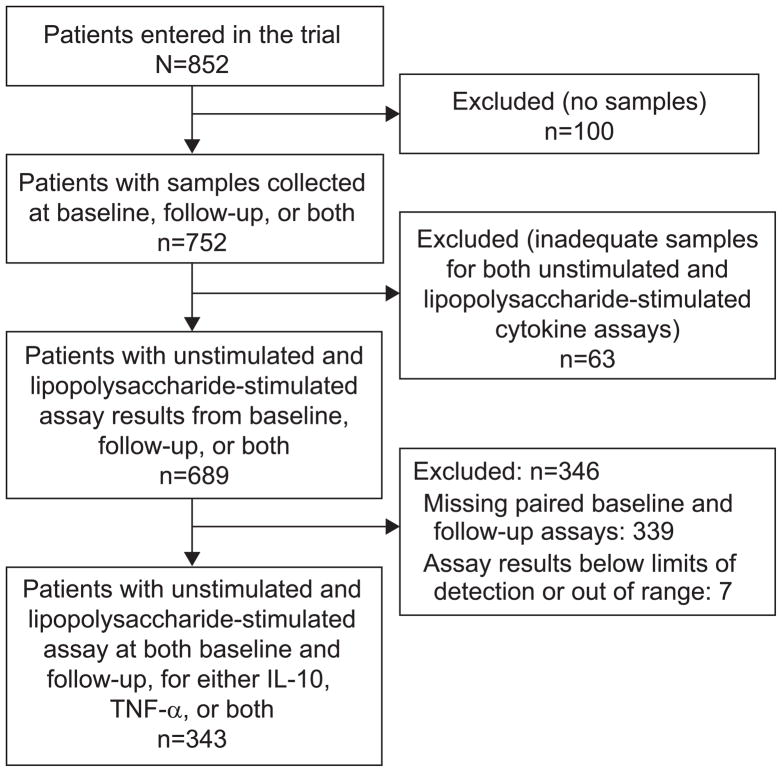
Figure 1.
Subgroup of patients entered into the randomized trial and included in this ancillary study. IL-10, interleukin-10; TNF-α, tumor necrosis factor α.
Acknowledgments
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD27860, HD27917, HD40560, HD34208, HD40485, HD21410, HD27915, HD40500, HD40512, HD40544, MO1-RR-000080, HD34136, HD27869, HD40545, HD36801, HD19897] and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
The authors thank Karen Dorman, RN, MS, for protocol development and coordination between clinical research centers, Elizabeth Thom, PhD, for protocol and data management and statistical analysis, and Catherine Y. Spong, MD, for protocol development and oversight.
Appendix
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network are as follows:
Wake Forest University Health Sciences, Winston-Salem, NC– P. Meis, M. Swain, B. Scott, C. Leftwich
Wayne State University, Detroit, MI – G. Norman, D. Driscoll, C. Sudz, L. Wynn, S. Blackwell
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Dorman, E. Prata, K. Hamden
University of Utah Health Sciences Center, Salt Lake City, UT –K. Anderson (University of Utah Health Sciences Center), S. Bonnemort (McKay-Dee Hospital), D. Lund (University of Utah Health Sciences Center), J. Russell (LDS Hospital), J. Parsons (Utah Valley Regional Medical Center)
Columbia University, New York, NY – S. Bousleiman, S. South, V. Carmona, H. Husami, C. Lankford, C. Perez
University of Pittsburgh, Pittsburgh, PA– M. Luce, M. Cotroneo
The Ohio State University, Columbus, OH – F. Johnson, M. Landon, D. Cline, H. Walker
Women and Infants Hospital, Brown University, Providence, RI – D. Allard, J. Tillinghast
Northwestern University, Chicago, IL – M. Dinsmoor (NorthShore University HealthSystem), P.J. Simon, M. Huntley, C. Whitaker-Carr, M. Ramos-Brinson, G. Mallett
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – C. Milluzzi, J. Hunter, W. Dalton, H. Ehrenberg, B. Stetzer
Drexel University College of Medicine – M. Hoffman, M. Talucci, C. Tocci, S. Wilson, M. Lake
University of Alabama at Birmingham, Birmingham, AL – W.W. Andrews, A. Northen, M. Parks, P. Blake Files
The University of Texas Health Science Center at Houston, Houston, TX – L.C. Gilstrap, B. Glenn-Cole, K. Cannon
The George Washington University Biostatistics Center – E. Thom, J. Zachary, R. Palugod, L. Leuchtenburg
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
Footnotes
Presented at the 31th Annual Meeting of the Society for Maternal Fetal Medicine, San Francisco, CA, February 11, 2011.
Dr. Spong, Associate Editor of Obstetrics & Gynecology, was not involved in the review or decision to publish this article.
References
- 1.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 2.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cyokines, prostaglandins and parturition-a review. Placenta. 2003;24 (Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastek JA, Gómez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol. 2011;38:385–406. doi: 10.1016/j.clp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Darby MI, Caritis SN, Shen-Schwartz S. Placental abruption in the preterm gestation: An association with chorioamnionitis. Obstet Gynecol. 1989;74:88–92. [PubMed] [Google Scholar]
- 6.Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikum, et al. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol. 2005;167:1443–9. doi: 10.1016/S0002-9440(10)61230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor: VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: Prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 8.Bytautiene E, Romero R, Vedernikoy YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–61. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 9.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. 2003;1:122–33. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krasnow JS, Tollerud DJ, Naus G, DeLoia JA. Endometrial Th2 cytokine expression throughout the menstrual cycle and early pregnancy. Hum Reprod. 1996;11:1747–54. doi: 10.1093/oxfordjournals.humrep.a019480. [DOI] [PubMed] [Google Scholar]
- 11.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 12.Sadowsky DW, Novy MF, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1 beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–63. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 13.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN, Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol. 2001;98:476–80. doi: 10.1016/s0029-7844(01)01424-7. [DOI] [PubMed] [Google Scholar]
- 14.Rodts-Palenik S, Wyatt-Ashmead J, Pang Y, Thigpen B, Cal Z, Rhodes P, et al. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol. 2004;191:1387–92. doi: 10.1016/j.ajog.2004.06.093. [DOI] [PubMed] [Google Scholar]
- 15.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, et al. Gestational age-dependent expression of Il-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–8. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 16.Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: A HuGE review. Genet Med. 2005;7:593–604. doi: 10.1097/01.gim.0000187223.69947.db. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya S, Raja EA, Mirazo ER, Campbell DM, Lee AJ, Norman JE, et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol. 2010;115:1125–33. doi: 10.1097/AOG.0b013e3181dffcdb. [DOI] [PubMed] [Google Scholar]
- 18.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–33. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 19.Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980–90. doi: 10.1007/s00134-008-1213-6. [DOI] [PubMed] [Google Scholar]
- 20.Hao W, Wong OY, Liu X, Lee P, Chen Y, Wong KK. ω-3 fatty acids suppress inflammatory cytokine production by macrophages and hepatocytes. J Pediatr Surg. 2010;45:2412–8. doi: 10.1016/j.jpedsurg.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–55. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 22.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JWM, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 23.Harper M, Thom E, Klebanoff M, Thorp J, Jr, Sorokin Y, Varner M, et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth. Obstet Gynecol. 2010;115:234–42. doi: 10.1097/AOG.0b013e3181cbd60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 25.Willet WC, Sampson L, Stamper MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Cousart S, Hu J, McCall CE. Characterization of interleukin-1 receptor associated kinase in normal and endotox intolerant cells. J Biol Chem. 2000;275:23340–5. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Hu J, Li L. Characterization of Tollip protein upon lipopolysaccharide challenge. Mol Immunol. 2004;41:85–92. doi: 10.1016/j.molimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman DW. A note on consistency of non-parametric rank tests and related rank transformations. Br J Math Stat Psychol. 2012;65:122–44. doi: 10.1111/j.2044-8317.2011.02017.x. [DOI] [PubMed] [Google Scholar]
- 29.Klebanoff MA, Harper M, Lai Y, Thorp J, Jr, Sorokin Y, Varner MW, et al. Fish consumption, erythrocyte fatty acids, and preterm birth. Obstet Gynecol. 2011;117:1071–7. doi: 10.1097/AOG.0b013e31821645dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, et al. Evidence for interleukin-10 mediated inhibition of cyclo-oxygenage-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warstedt K, Furuhjelm C, Duchén K, Fälth-Magnusson K, Fageräs M. The effects of omega-3 fatty acid supplementation in pregnancy on maternal eicosanoid, cytokine and chemokine secretion. Pediatr Res. 2009;66:212–7. doi: 10.1203/PDR.0b013e3181aabd1c. [DOI] [PubMed] [Google Scholar]
- 33.Vega-Lopez S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega3 polyunsaturated fatty acids and allrac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–240. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Fujioka S, Hamazaki K, Itomura M, Huan M, Nishizawa H, Sawazaki S, et al. The effects of eicosapentaenoic acid-fortified food on inflammatory markers in healthy subjects – a randomized, placebo controlled, double-blind study. J Nutr Sci Vitaminol. 2006;52:261–5. doi: 10.3177/jnsv.52.261. [DOI] [PubMed] [Google Scholar]
- 35.Lee KW, Blann AD, Lip GY. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb Res. 2006;118:305–12. doi: 10.1016/j.thromres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Browning LM, Krebs JD, Moore CS, Mishra GD, O’Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9:70–80. doi: 10.1111/j.1463-1326.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 37.Schiano V, Laurenzano E, Brevetti G, De Maio JI, Lanero S, Scopacasa F, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27:241–7. doi: 10.1016/j.clnu.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Yusof HM, Miles EA, Calder PC. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:219–28. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 39.de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158:413–28. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stites DP, Siiteri PK. Steroids as immunosuppressants in pregnancy. Immunol Rev. 1983;75:117–38. doi: 10.1111/j.1600-065x.1983.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 41.Siiteri PK, Stites DP. Immunologic and endocrine interrelationships in pregnancy. Biol Reprod. 1982;26:1–14. doi: 10.1095/biolreprod26.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Hansen PJ. Regulation of uterine immune function by progesterone--lessons from the sheep. J Reprod Immunol. 1998;40:63–79. doi: 10.1016/s0165-0378(98)00035-7. [DOI] [PubMed] [Google Scholar]