Abstract
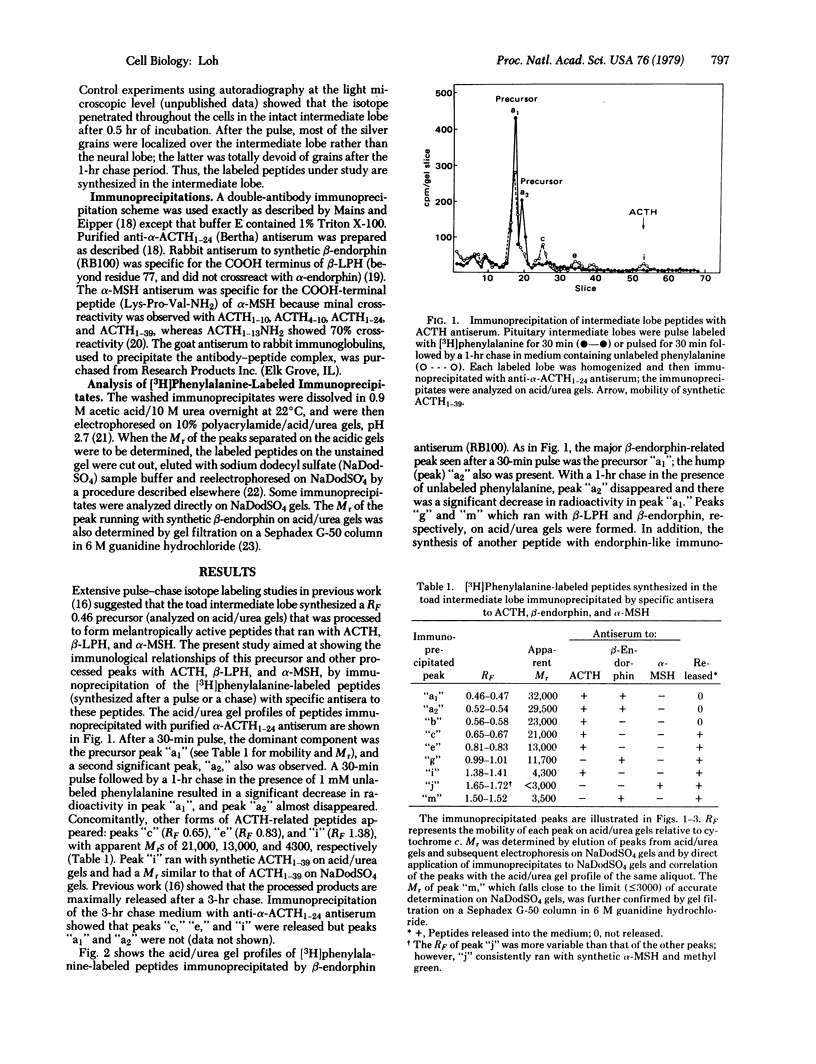
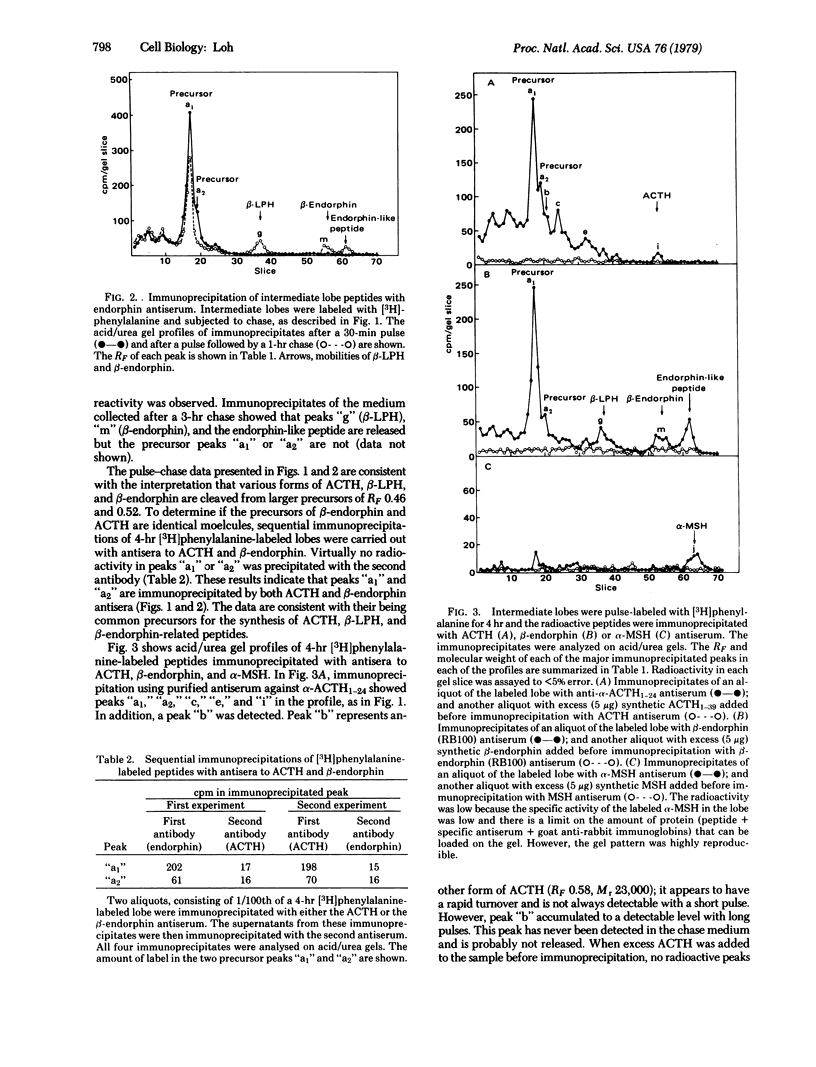
The biosynthesis of corticotropin (ACTH1--39), beta-endorphin [beta(61--91)-lipotropin] and alpha-melanotropin in the toad intermediate lobe was studied by using immunoprecipitation procedures with antisera specific for these peptides. Intermediate lobes were pulse-incubated with [3H]phenylalanine and then chase-incubated for varying periods; the radioactive proteins were immunoprecipitated. Immunoprecipitates were separated by acidic urea or sodium dodecyl sulfate polyacrylamide gel electrophoresis. Evidence from the pulse-chase and sequential immunoprecipitation studies using antisera to ACTH and beta-endorphin suggests that the toad intermediate lobe synthesizes two common precursors (apparent Mr 32,000 and 29,500) containing both the ACTH and beta-endorphin sequences. These precursors are processed to yield several forms of immunoreactive corticotropin (apparent Mr 23,000, 21,000, 13,000, and 4300), immunoreactive endorphin (apparent Mr 11,700 and 3500), and immunoreactive alpha-melanotropin. The 4300 Mr form of corticotropin and the 11,700 and 3500 Mr forms of endorphins were found to comigrate with synthetic ACTH1--39, beta-lipotropin and beta-endorphin, respectively, on both acidic urea and sodium dodecyl sulfate gels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGERS A. C. Melanophore-stimulating hormones in vertebrates. Ann N Y Acad Sci. 1963 Feb 15;100:669–677. doi: 10.1111/j.1749-6632.1963.tb42923.x. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Copenhaver J. H., Carver M. J. Characterization of acidic proteins in cell nuclei from rat brain by high-resolution acrylamide gel electrophoresis. J Neurochem. 1972 Feb;19(2):473–477. doi: 10.1111/j.1471-4159.1972.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Analysis of the common precursor to corticotropin and endorphin. J Biol Chem. 1978 Aug 25;253(16):5732–5744. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Existence of a common precursor to ACTH and endorphin in the anterior and intermediate lobes of the rat pituitary. J Supramol Struct. 1978;8(3):247–262. doi: 10.1002/jss.400080304. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Guenzi D. High molecular weight forms of adrenocorticotropic hormone are glycoproteins. J Biol Chem. 1976 Jul 10;251(13):4121–4126. [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Biosynthesis and axonal transport of rat neurohypophysial proteins and peptides. J Cell Biol. 1977 May;73(2):366–381. doi: 10.1083/jcb.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R., Ling N., Vargo T. Radioimmunoassays for alpha-endorphin and beta-endorphin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):361–366. doi: 10.1016/s0006-291x(77)80205-2. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R. Studies on secretory activity in the pars intermedia of Xenopus laevis 3. The synthesis and release of melanocyte stimulating hormone (MSH) in vitro. Tissue Cell. 1970;2(1):83–98. doi: 10.1016/s0040-8166(70)80009-x. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A., Brownstein M. J. Presence of corticotropin in brain of normal and hypophysectomized rats. Proc Natl Acad Sci U S A. 1977 Feb;74(2):648–652. doi: 10.1073/pnas.74.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H., Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Burgus R., Guillemin R. Isolation, primary structure, and synthesis of alpha-endorphin and gamma-endorphin, two peptides of hypothalamic-hypophysial origin with morphinomimetic activity. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3942–3946. doi: 10.1073/pnas.73.11.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Biosynthesis, processing, and control of release of melanotropic peptides in the neurointermediate lobe of Xenopus laevis. J Gen Physiol. 1977 Jul;70(1):37–58. doi: 10.1085/jgp.70.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Heterogeneity of melanotropic peptides in the pars intermedia and brain. Brain Res. 1977 Jul 8;130(1):169–175. doi: 10.1016/0006-8993(77)90854-x. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Low molecular weight specific proteins in identified molluscan neurons. I. Synthesis and storage. Brain Res. 1975 Jul 11;92(2):181–192. doi: 10.1016/0006-8993(75)90268-1. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. The role of glycosylation on the biosynthesis, degradation, and secretion of the ACTH-beta-lipotropin common precursor and its peptide products. FEBS Lett. 1978 Dec 15;96(2):269–272. doi: 10.1016/0014-5793(78)80415-3. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Wimersma Greidanus T. B. Effects of MSH and related peptides on avoidance behavior in rats. Front Horm Res. 1977;4:129–139. doi: 10.1159/000400358. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]


