Abstract
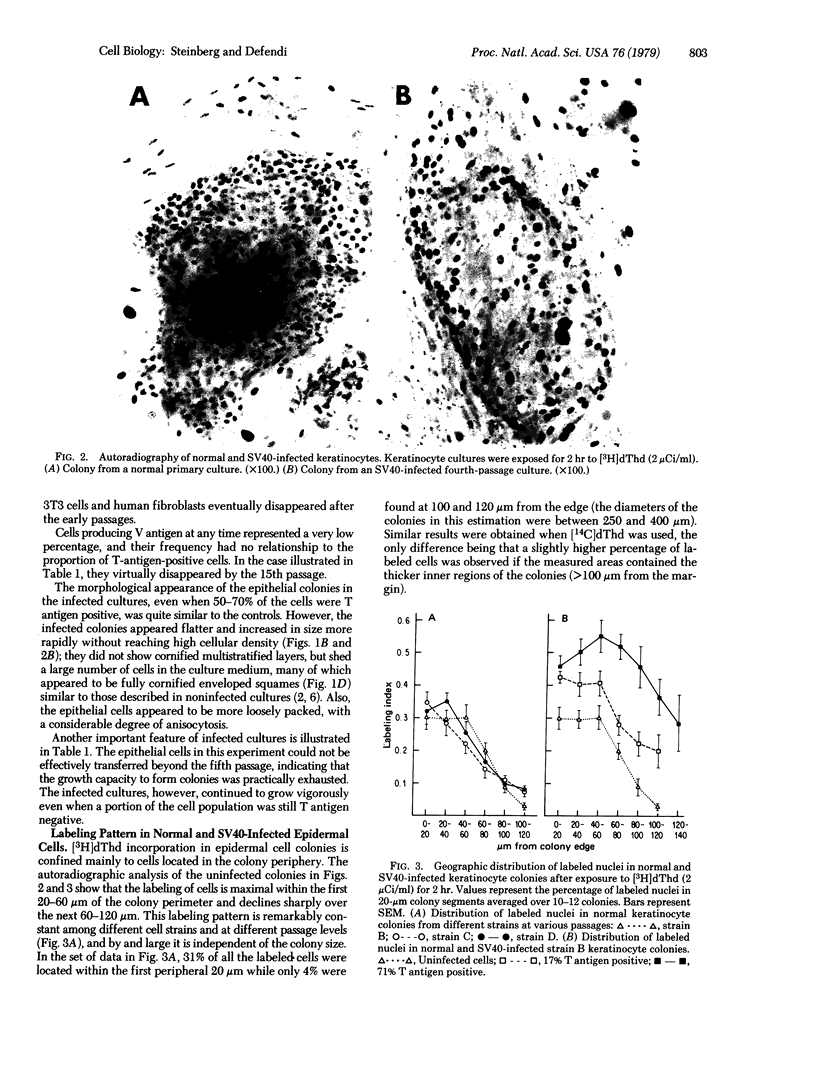
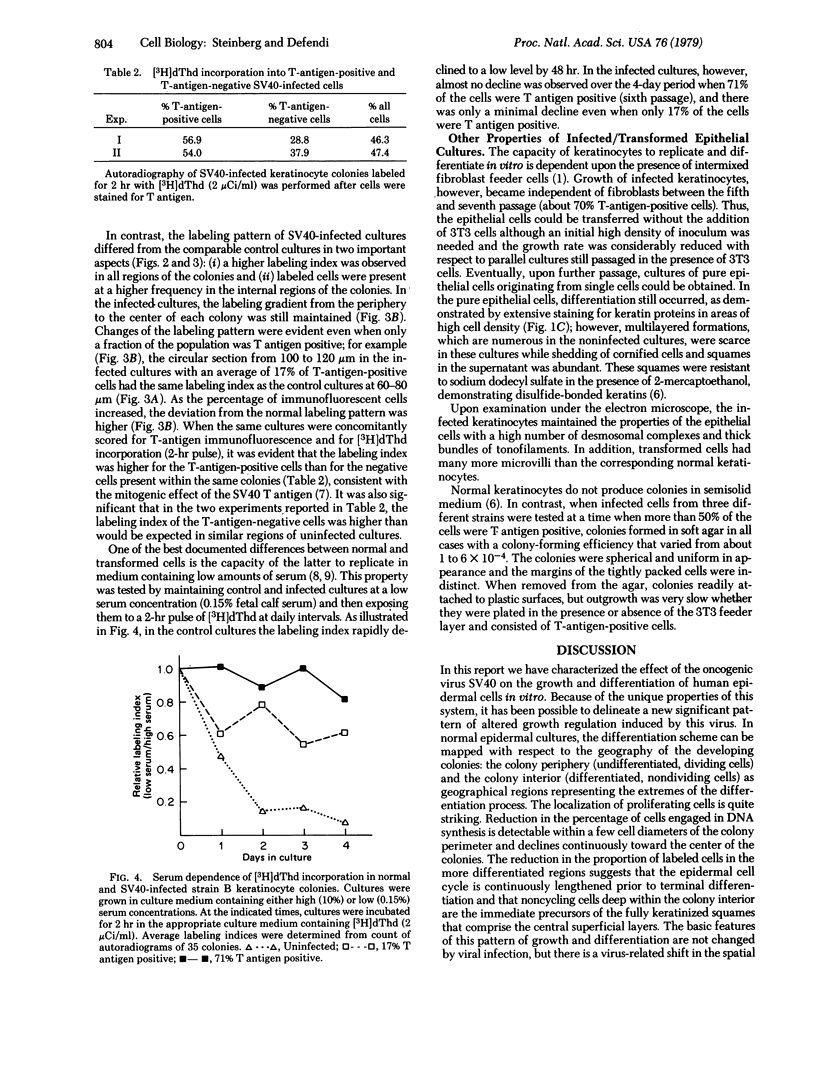
Human epidermal keratinocytes were infected by simian virus 40 in vitro. The structure of the developing keratinocyte colony reflects the spatial separation of cell division and keratinization in intact skin; thymidine-incorporating cells were primarily localized at the colony periphery whereas nondividing, histologically differentiated cells accumulated in the interior. Viral infection produced a dramatic increase in the size of the proliferative population as, simultaneously, differentiation was reduced in the colony interior. These changes were manifest when simian virus 40 T-antigen synthesis was detectable in only a small percentage of the cells; differentiation became increasingly density dependent as the percentage of T-antigen-positive cells rose over serial passage. The disruption of the normal pattern of growth/differentiation localization coincided with a loss of dependence on serum for growth, but preceded the appearance of other virus-induced properties associated with transformation; i.e., the ability to form colonies in soft agar and independence of growth from fibroblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYOUB P., SHKLAR G. A modification of the Mallory connective tissue stain as a stain for keratin. Oral Surg Oral Med Oral Pathol. 1963 May;16:580–581. doi: 10.1016/0030-4220(63)90148-8. [DOI] [PubMed] [Google Scholar]
- Cavoto F. V., Flaxman B. A. Communication between normal human epidermal cells in vitro. J Invest Dermatol. 1972 Nov;59(5):370–374. doi: 10.1111/1523-1747.ep12627491. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Eagle H., Foley G. E., Koprowski H., Lazarus H., Levine E. M., Adams R. A. Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med. 1970 Apr 1;131(4):863–879. doi: 10.1084/jem.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977 Jun;11(2):405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Defendi V. Changes in deoxyribonucleic acid synthesis regulation in Chinese hamster cells infected with simian virus 40. J Virol. 1970 Dec;6(6):738–749. doi: 10.1128/jvi.6.6.738-749.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rodesch F. Differentiation, contact inhibition and intercellular communication in retinal pigment cells. Exp Cell Res. 1973 Jan;76(1):55–62. doi: 10.1016/0014-4827(73)90418-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell. 1976 Dec;9(4 Pt 1):511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]