Abstract
Purpose
In light of the increased incidence of contact lens associated Acanthamoeba keratitis in recent years, this study analyzed longitudinal trends of its incidence among predominantly non-contact lens wearers in a high-volume referral center in South India.
Methods
A retrospective analysis of microbiology laboratory records at the Aravind Eye Hospital from 1988–2009 was performed. The Maximum Excess Events Test (MEET) was used to identify epidemics of Acanthamoeba keratitis.
Results
There were a total of 38,529 unique cases of infectious keratitis evaluated over this time period, of which 372 were culture-positive for Acanthamoeba. Only three cases (0.9%) of Acanthamoeba keratitis occurred among contact lens wearers. MEET identified unique Acanthamoeba keratitis epidemics in 1993 and 2002.
Conclusion
Discrete epidemics of Acanthamoeba keratitis occurred among a rural, non-contact lens wearing, population in South India in 1993 and 2002.
Keywords: Acanthamoeba, Cornea, Epidemic, India, Keratitis
INTRODUCTION
Increased incidence of Acanthamoeba keratitis in recent years has garnered much attention, with reports emerging first from the United States,1–4 then subsequently from other countries including Australia,5 New Zealand,6 and Singapore.7 Ensuing research explored various hypotheses, including type of contact lens, contact lens hygiene practices, and exposure to contaminated source water. Numerous studies eventually associated a certain contact lens solution with the increased incidence, which subsequently led to the recall of this product.1,8
While this public health concern was characterized in developed countries among contact lens wearers, a dearth of knowledge exists for Acanthamoeba keratitis trends in emerging nations, where contact lens use is less common. In these areas trauma or exposure to contaminated water is often associated with Acanthamoeba keratitis.9 In recent years, anecdotal evidence from South India hints at an increasing incidence of Acanthamoeba keratitis among a predominantly rural population.
The visual morbidity that often results from Acanthamoeba keratitis underscores the importance of studying its epidemiology. Afflicted patients are commonly left with debilitating corneal scarring and face a prolonged recovery often requiring penetrating keratoplasty. In developing nations, prevention of this rare but devastating infection is even more important as limited resources prevent many patients from obtaining sufficient treatment.
This study analyzed longitudinal trends in Acanthamoeba keratitis incidence over the last two decades among patients presenting to an eye care referral center in South India. Time series statistical methods were used to identify epidemics over this time period.
MATERIALS AND METHODS
A retrospective analysis of the microbiology laboratory database at the Aravind Eye Hospital, was conducted on all specimens evaluated from January 1, 1988 to December 31, 2009. Aravind Eye Hospital comprises several centers in various cities but for this study, only cases from the Madurai, India site were included. Acanthamoeba species were not cultured routinely prior to 1988, and laboratory diagnostic techniques have remained the same since then. All cases with a clinical diagnosis of infectious keratitis that had a smear and/or culture performed were included. Culture-positive cases of Acanthamoeba were enumerated and included in the analyses. Duplicate samples from the same patient were excluded so that each positive case corresponded to a unique individual.
A detailed description of smear and culture technique at the Aravind microbiology laboratory has been reported previously.10 In brief, after instillation of topical preservative-free lidocaine, a flame sterilized Kimura spatula was used to perform a corneal scraping, the material of which was inoculated onto sheep's blood agar, chocolate agar, potato dextrose agar, and brain heart infusion broth without gentamicin. Material from the scrapings was also smeared onto three separate glass slides for Gram stain, Giemsa stain, and KOH wet mount. When smears were positive for amoebic cysts, a further corneal scraping was performed and the material was inoculated onto non-nutrient agar overlaid with Escherichia coli to isolate Acanthamoeba spp. Microbial cultures were considered positive only if growth of the same organism was demonstrated on two or more solid media or there was semiconfluent growth at the site of inoculation on one solid medium associated with the identification of the organism on Gram or Giemsa stained corneal smears. Staphylococcus epidermidis and diphtheroids were considered positive only if there was moderate growth on at least two solid media.
For statistical detection of outbreaks, we used the Maximum Excess Events Test (MEET), which detects clustering within years and between years.11 The total number of infectious keratitis cases, regardless of smear and/or culture results, was used as a denominator. As a sensitivity test to control for biases such as changing referral patterns that may have influenced the annual number of infectious keratitis cases, MEET was performed with the total number of cornea clinic visits as the denominator. Seasonality of culture counts was tested using the Edwards test for an annual cycle.12 To assess for changes in the seasonal peak, we used circular regression using time series bootstrap (with a fixed width of 2, with 3 used as a sensitivity analysis).13 All computations were done in R version 2.10 for MacIntosh (R Foundation for Statistical Computing, Vienna, Austria).
This study adheres to the guidelines of the Declaration of Helsinki and ethical approval was obtained from the University of California, San Francisco Committee on Human Research, and the Aravind Eye Care System.
RESULTS
A total of 38,529 unique cases of infectious keratitis treated at the Madurai site of the Aravind Eye Hospital were evaluated by the microbiology laboratory from 1988 to 2009 (Table 1). An average of 1751 cases was processed annually with a range of 430–2806 cases. Half of all cases had only a smear performed while the other half underwent both a smear and culture. There were a total of 13,389 culture-positive specimens with an average of 609 per year. Of the culture-positive specimens, bacterial and fungal isolates were recovered in 5542 (41.4%) and 7475 (55.8%), respectively.
TABLE 1.
Categorization of infectious keratitis cases from 1988–2009
| Year | Total unique infectious keratitis cases | Total cultures performed | Bacteria (%)* | Fungi (%)* | Acanthamoeba species (%)* |
|---|---|---|---|---|---|
| 1988 | 430 | 430 | 138 (32%) | 211 (49%) | 4 (1%) |
| 1989 | 394 | 394 | 164 (42%) | 160 (41%) | 4 (1%) |
| 1990 | 421 | 317 | 160 (50%) | 102 (32%) | 6 (2%) |
| 1991 | 559 | 405 | 154 (38%) | 184 (45%) | 4 (1%) |
| 1992 | 903 | 732 | 218 (30%) | 351 (48%) | 13 (2%) |
| 1993 | 1161 | 1020 | 319 (31%) | 417 (41%) | 20 (2%) |
| 1994 | 1831 | 1617 | 434 (27%) | 656 (41%) | 15 (1%) |
| 1995 | 1654 | 1097 | 256 (23%) | 395 (36%) | 16 (1%) |
| 1996 | 2158 | 1048 | 335 (32%) | 299 (29%) | 10 (1%) |
| 1997 | 1950 | 557 | 132 (24%) | 201 (36%) | 20 (4%) |
| 1998 | 1934 | 778 | 225 (29%) | 271 (35%) | 12 (2%) |
| 1999 | 2239 | 737 | 281 (38%) | 196 (27%) | 21 (3%) |
| 2000 | 2383 | 751 | 244 (32%) | 289 (38%) | 19 (3%) |
| 2001 | 2131 | 660 | 168 (25%) | 200 (30%) | 24 (4%) |
| 2002 | 2057 | 657 | 167 (25%) | 200 (30%) | 40 (6%) |
| 2003 | 1871 | 617 | 183 (30%) | 177 (29%) | 12 (2%) |
| 2004 | 1782 | 1015 | 244 (24%) | 329 (32%) | 19 (2%) |
| 2005 | 2098 | 907 | 225 (25%) | 279 (31%) | 26 (3%) |
| 2006 | 2633 | 1493 | 336 (23%) | 557 (37%) | 30 (2%) |
| 2007 | 2547 | 1978 | 401 (20%) | 657 (33%) | 19 (1%) |
| 2008 | 2587 | 1901 | 402 (21%) | 659 (35%) | 12 (1%) |
| 2009 | 2806 | 1881 | 356 (19%) | 685 (36%) | 26 (1%) |
| Total | 38529 | 20992 | 5542 (26%) | 7475 (36%) | 372 (2%) |
In parentheses are the percentages of total cultures performed that were positive for bacteria, fungi, and Acanthamoeba spp., respectively, in a given year.
Over the 22-year time period, there were 372 total cases of culture-positive Acanthamoeba keratitis, averaging 17 cases per year with a range from 4–40. An additional 19 cases were smear-positive but culture-negative, amounting to 5% of the 391 total cases for which Acanthamoeba cultures were performed. Acanthamoeba comprised 1% of all infectious keratitis cases evaluated and 2.8% of all culture-positive cases. Only three cases (0.9%) occurred among contact lens wearers. All cases of Acanthamoeba keratitis were identified on smear and isolated on culture.
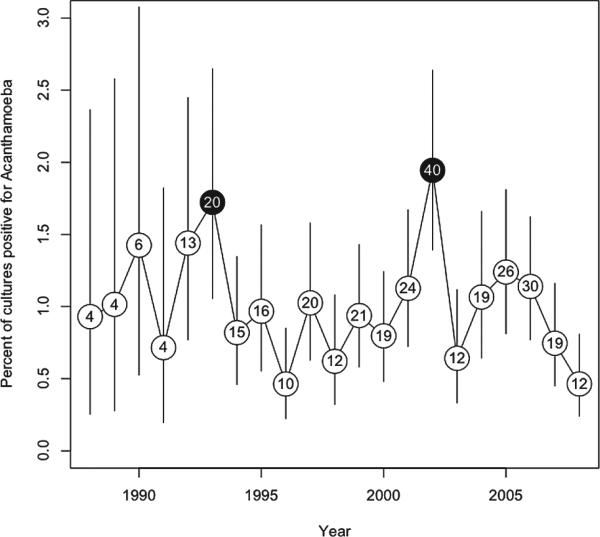
Using the total number of cases of infectious keratitis as the denominator, analyses with MEET revealed evidence of temporal clustering of Acanthamoeba isolates, with unique epidemics in 1993 and 2002 (P = 0.0015, Fig. 1). Similar results were obtained when analyses were performed using the total number of cornea clinic visits as the denominator. Using the Edwards test,12 we found evidence of seasonality (P < 0.001), with a relatively higher number of cases from January to September and a reduction in cases from October to December. This seasonal pattern persisted over time (Fisher's exact text R × C, P = 0.07), including during the two epidemic years (P = 0.33, chi-square).
FIGURE 1.
Percent of total infectious keratitis cases due to Acanthamoeba spp. The circled numbers represent the total cases of Acanthamoeba keratitis for that year with black circles indicating epidemic years (P < 0.001). Vertical lines represent 95% confidence intervals.
DISCUSSION
The upsurge in incidence of Acanthamoeba keratitis that occurred internationally in recent years has been widely reported, but to the best of our knowledge, this is the first study detailing epidemics among a predominantly non-contact lens wearing population. Unlike in developed countries, where contact lens wearers constitute more than 90% of patients with Acanthamoeba keratitis,14,15 the majority of cases in emerging nations occur in non-contact lens wearers. Studies from southern India report contact lens wear to be a risk factor in only 0–2% of cases of corneal ulcers.16–18
In light of the poor prognosis of Acanthamoeba keratitis among non-contact lens wearers, a surprisingly small body of literature exists for this group. These studies report that pathognomonic clinical features such as radial keratoneuritis are frequently absent, thus complicating and delaying clinical diagnosis.9 This delay may contribute to more advanced disease at presentation, rapid progression of corneal pathology, and worse visual outcomes.9,15,19 These characteristics highlight the importance of understanding the epidemiology of Acanthamoeba keratitis to aid preventive efforts in developing nations.
In the present study, over the last two decades discrete epidemics of Acanthamoeba keratitis were identified in 1993 and 2002 among patients seen at Aravind Eye Hospital. These epidemics occurred in non-contact lens wearers, as established by a previously published large consecutive case series of 3183 infectious keratitis cases treated at Aravind, in which only 1% of cases were associated with contact lens wear, none of which grew Acanthamoeba isolates.16 Rather, in that study, a history of corneal trauma preceded all 33 cases of Acanthamoeba keratitis,16 making it the most common risk factor among non-contact lens wearers. In the present study, only three cases (0.9%) of Acanthamoeba keratitis were associated with contact lens wear.
Environmental conditions that predispose to corneal trauma, such as severe windy seasons or extended agricultural harvests, may result in sequelae in the form of infectious keratitis outbreaks. Exposure to contaminated water sources has been reported to be a significant risk factor for Acanthamoeba keratitis. While the parasite is a ubiquitous waterborne pathogen, various studies have associated Acanthamoeba keratitis with domestic hard water supply,20 disinfection practices of water treatment plants,14 and the use of water storage tanks.21 Meier reported an Acanthamoeba keratitis epidemic in Iowa following regional flooding, hypothesizing that water sources were contaminated during the flooding.22 A review of flood records in Tamil Nadu reveal a particularly severe and prolonged flood lasting 70 days at the end of 1992.23 The delay to symptom development and clinical diagnosis could result in the increase in cases observed the following year.
Further investigation into environmental associations would shed light on the etiologies of the observed epidemics. However, this was outside the scope of our study design as data collection over the 20-year time period was not uniform with regards to demographic, environmental, and geographic characteristics of each infectious keratitis case. Potential prospective studies could incorporate demographic, environmental, and geographic data collection and spatial analyses.
There are a few limitations to this study. First, deriving data from the microbiology laboratory database may introduce selection bias as it does not capture all patients with infectious keratitis. In addition, evolving institutional and individual practice patterns influence whether corneal scrapings are routinely performed and sent to the lab for microbiological evaluation. However, prior quality assessment studies at Aravind indicate that >98% of infectious keratitis presenting to the clinic are evaluated by the microbiology laboratory. Corneal scrapes are performed in the vast majority of infectious keratitis cases. Second, the variability in the proportion of infectious keratitis cases for which cultures were performed is a potential source of bias that could have underestimated cases. Had cultures been performed on all infectious keratitis cases, additional cases of Acanthamoeba may have been uncovered. However, we do not believe we missed a significant number of cases as the prolonged clinical course of Acanthamoeba keratitis lends itself to extended follow-up, improving identification of cases. Third, there may be bias from changing referral patterns over time. In recent years, more patients have sought care at Aravind Eye Hospital, as suggested by the impressive growth in infectious keratitis cases treated over the last 20 years. Whereas patients with infectious keratitis may have suffered without treatment 20 years ago, as access to Aravind Eye Hospital has improved and the hospital itself has become more renowned, patient volume has steadily increased.
To address these concerns, MEET was performed using the number of Acanthamoeba keratitis cases as a fraction of both total infectious keratitis cases and total cornea clinic visits. These tests yielded similar results. The total number of cultures performed was not used as a denominator in MEET analyses because the proportion of infectious keratitis cases that had cultures performed varied significantly over this time period. This observed fluctuation is reflective of changing diagnostic protocols. In some years, the vast majority of infectious keratitis cases were cultured while in other years, for reasons such as cost-effectiveness, cultures were mainly performed if initial smears were positive.
Despite these limitations, this study shows that epidemics of Acanthamoeba keratitis have also occurred among predominantly non-contact lens wearers. Additional research dedicated to understanding the epidemiology of Acanthamoeba keratitis in developing nations will help clarify its associations with environmental risk factors.
ACKNOWLEDGMENTS
Financial Support: This study was supported in part by an unrestricted grant from That Man May See, Inc (San Francisco, CA), an unrestricted grant from Research to Prevent Blindness (New York, NY), and NIH-NEI 1U10EY018573.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Joslin CE, Tu EY, Shoff ME, et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol. 2007;144(2):169–180. doi: 10.1016/j.ajo.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acanthamoeba keratitis multiple states, 2005–2007. MMWR Morb Mortal Wkly Rep. 2007;56(21):532–534. [PubMed] [Google Scholar]
- 3.Thebpatiphat N, Hammersmith KM, Rocha FN, et al. Acanthamoeba keratitis: a parasite on the rise. Cornea. 2007;26(6):701–706. doi: 10.1097/ICO.0b013e31805b7e63. [DOI] [PubMed] [Google Scholar]
- 4.Sansanayudh W, Cevallos V, Porco TC, et al. Fusarium and Acanthamoeba keratitis: can a single centre detect outbreaks? Br J Ophthalmol. 2008;92(5):720–721. doi: 10.1136/bjo.2007.134171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku JY, Chan FM, Beckingsale P. Acanthamoeba keratitis cluster: an increase in Acanthamoeba keratitis in Australia. Clin Experiment Ophthalmol. 2009;37(2):181–90. doi: 10.1111/j.1442-9071.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- 6.Patel DV, Rayner S, McGhee CN. Resurgence of Acanthamoeba keratitis in Auckland, New Zealand: a 7-year review of presentation and outcomes. Clin Experiment Ophthalmol. 2010;38(1):15–20. doi: 10.1111/j.1442-9071.2009.02182.x. quiz 87. [DOI] [PubMed] [Google Scholar]
- 7.Por YM, Mehta JS, Chua JL, et al. Acanthamoeba keratitis associated with contact lens wear in Singapore. Am J Ophthalmol. 2009;148(1):7–12. e2. doi: 10.1016/j.ajo.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Verani JR, Lorick SA, Yoder JS, et al. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis. 2009;15(8):1236–1242. doi: 10.3201/eid1508.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol. 2000;84(10):1103–1108. doi: 10.1136/bjo.84.10.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan MGC, George C, Cevallos V, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tango T. A test for spatial disease clustering adjusted for multiple testing. Stat Med. 2000;19:191–204. doi: 10.1002/(sici)1097-0258(20000130)19:2<191::aid-sim281>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JH. The recognition and estimation of cyclic trends. Ann Human Genetics. 1961;25:83–87. doi: 10.1111/j.1469-1809.1961.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 13.Fisher NI, Lee AJ. Regression models for an angular response. Biometrics. 1992;48(3):665–677. [Google Scholar]
- 14.Joslin CE, Tu EY, McMahon TT, et al. Epidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreak. Am J Ophthalmol. 2006;142(2):212–217. doi: 10.1016/j.ajo.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Radford CF, Lehmann OJ, Dart JK. Acanthamoeba keratitis: multicentre survey in England 1992–6. National Acanthamoeba Keratitis Study Group. Br J Ophthalmol. 1998;82(12):1387–1392. doi: 10.1136/bjo.82.12.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharathi JM, Srinivasan M, Ramakrishnan R, et al. A study of the spectrum of Acanthamoeba keratitis: a three-year study at a tertiary eye care referral center in South India. Indian J Ophthalmol. 2007;55(1):37–42. doi: 10.4103/0301-4738.29493. [DOI] [PubMed] [Google Scholar]
- 17.Kunimoto DY, Sharma S, Garg P, et al. Corneal ulceration in the elderly in Hyderabad, South India. Br J Ophthalmol. 2000;84(1):54–59. doi: 10.1136/bjo.84.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Gopalakrishnan S, Aasuri MK, et al. Trends in contact lens-associated microbial keratitis in Southern India. Ophthalmology. 2003;110(1):138–43. doi: 10.1016/s0161-6420(02)01283-6. [DOI] [PubMed] [Google Scholar]
- 19.Chynn EW, Talamo JH, Seligman MS. Acanthamoeba keratitis: is water exposure a true risk factor? Clao J. 1997;23(1):55–56. [PubMed] [Google Scholar]
- 20.Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86(5):536–542. doi: 10.1136/bjo.86.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilvington S, Gray T, Dart J, et al. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci. 2004;45(1):165–169. doi: 10.1167/iovs.03-0559. [DOI] [PubMed] [Google Scholar]
- 22.Meier PA, Mathers WD, Sutphin JE, et al. An epidemic of presumed Acanthamoeba keratitis that followed regional flooding. Results of a case-control investigation. Arch Ophthalmol. 1998;116(8):1090–1094. doi: 10.1001/archopht.116.8.1090. [DOI] [PubMed] [Google Scholar]
- 23. [November 12, 2010];Darmouth Flood Observatory. from: http://www.dartmouth.edu/~floods/Archives/