Abstract
Coronary artery perforation is a known complication of percutaneous coronary intervention and potentially life threatening. Normally, these perforations are small and localized. We report the successful surgical management of a coronary artery perforation following stent insertion with extrusion of an 8-cm endarterectomy length of the circumflex coronary artery with a brief review of the recent literature.
Keywords: coronary artery perforation, percutaneous coronary intervention, endarterectomy, coronary artery, cardiac surgery
Coronary artery perforation or rupture is an uncommon complication (0.1 to 3%) of percutaneous coronary intervention (PCI). The management depends on the complexity of the procedure and severity of the coronary injury, and mortality can be high (0 to 19%).1 2 3 Risk factors that have been reported to increase the incidence of coronary artery perforations during PCI include old age, female gender, and calcified, tortuous coronary arteries.4
In this case report, we describe the management of a complication following PCI of the circumflex coronary artery resulting in its direct dissection and endarterectomy and extrusion of an 8-cm length of the artery into the pericardial space.
Case Report
A 36-year-old male was admitted to the Coronary Care Unit with the diagnosis of acute myocardial infarction. His past medical history included hypercholesterolemia, hypertension, ex-smoking, and positive family history for ischemic heart disease. He underwent coronary angiography with angioplasty and stent insertion to the first obtuse marginal. During the procedure, a perforation of the distal circumflex coronary artery was noticed, which resulted in bleeding and pericardial collection (Fig. 1a, b).
Fig. 1.
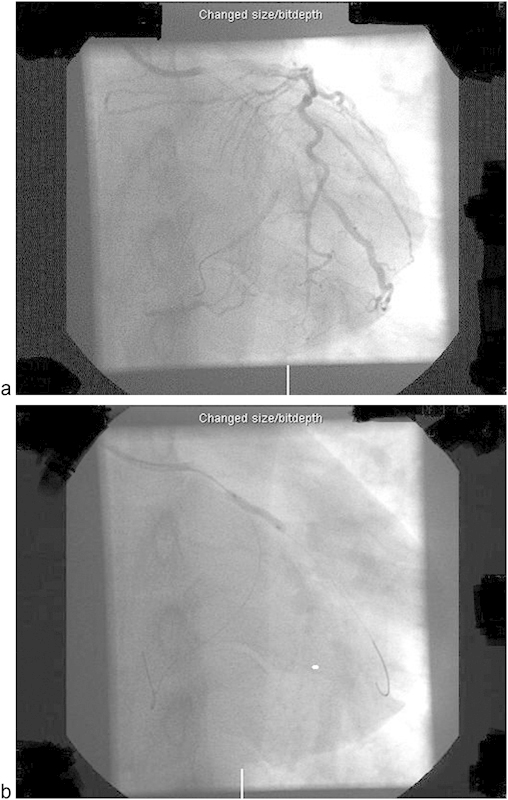
(a) Angiogram at the initiation of percutaneous coronary intervention. (b) Angiogram showing stent insertion in OM1 and kinking of distal part of the wire in the distal circumflex.
The cardiologists elected to treat the patient conservatively and a pigtail catheter was inserted with echocardiography guidance for pericardial drainage. Blood products were transfused and subsequently, within the next 8 hours, he became progressively more hemodynamically unstable due to intermittent cardiac tamponade. Inotropic support was commenced and the total pericardial drainage reached 2 L.
The patient was then referred for urgent surgical intervention and underwent an emergency exploratory median sternotomy. Once the cardiac tamponade had been relieved and hemodynamics stabilized, a 1- to 2-mm perforation on the distal branch of circumflex artery was identified and oversewn with a figure of eight 4/0 polypropylene suture. No further sutures were required and there was no evidence of any underlying intramyocardial hematoma.
Thereafter, in the posterior inferior pericardial space, a tubular piece of tissue of 8 cm in length, resembling a “blood vessel” with a diameter of approximately 3 mm was discovered (Fig. 2).
Fig. 2.
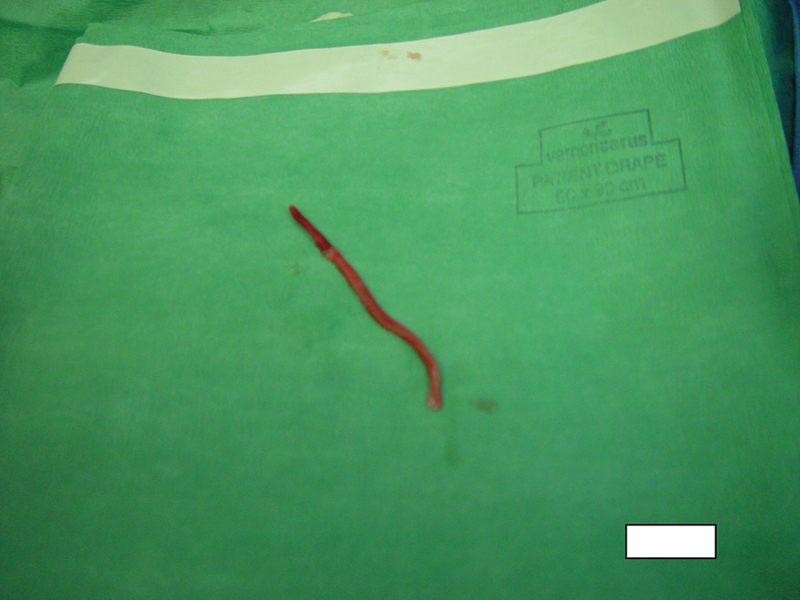
Surprising finding in the posterior inferior pericardial space.
To our judgment, this finding represents a “blood vessel” and is the result of a direct dissection and endarterectomy of the coronary artery with extravasation of its intimal and part of its medial layer through the distal perforation of the circumflex coronary artery.
Subsequently, histology showed this to be a vessel with transmural necrosis, intramural organizing thrombosis and partially calcified atherosclerotic changes, compatible with an 8-cm endarterectomy of the circumflex coronary artery.
The preoperative and poststent implantation coronary angiogram had shown stent patency, and we elected not to do any further surgical intervention. The patient made an uneventful recovery and was discharged from the hospital on the fourth postoperative day.
Three months later, a repeat coronary angiogram was done, which confirmed the patency of the circumflex stent and showed no further circumflex restenosis. The patient remained free of symptoms for 2 years following this intervention.
Discussion
According to reports in the literature, 0.1 to 3.0% of PCI procedures are complicated by coronary artery perforation or rupture often associated with pericardial effusion, cardiac tamponade, or myocardial infarction. Subepicardial bleeding has also been reported to lead to the compression of major coronary vessels resulting in myocardial ischemia. These perforations can have serious consequences and may result in death.3
Ellis et al have suggested an angiographic classification scheme of PCI perforations, into three types, which is widely accepted and used.1 These include type I extraluminal crater without any contrast extravasation, type II contrast extravasation limited to “blushing” in the myocardial or epicardial fat, and type III contrast extravasation through frank (>1 mm) perforations or type III “cavity spilling” extravasation into either the left ventricle, coronary sinus, any of the cardiac chambers or the pericardium.
According to the above classification, our case can be classified as type III coronary perforation. In addition, a direct dissection and endarterectomy of the circumflex coronary artery also occurred, and an 8-cm length of the intima and part of media layer was extravasated through the perforation site into the pericardial space.1
To the best of our knowledge, this is the first report of such a finding in the literature.
In terms of outcome, type I perforations are associated with the lowest incidence of tamponade (8%) with no reported incidence of myocardial infarction or mortality. Hence, the vast majority (85%) of this type are treated conservatively. With regards to type II perforations, they show a higher incidence of tamponade and myocardial infarction (i.e., 13 and 14%, respectively) with no reported mortality. Similarly, as in type I perforations, conservative treatment is successful in 90% of cases. However, type III perforations have a high morbidity with a much higher incidence of tamponade and myocardial infarction (i.e., 63 and 50%, respectively). Conservative treatment has been reported successful in only 44% of cases of type III perforations with mortality independent of treatment in 19% of the cases.
In principal, conservative treatment includes reversal of heparin anticoagulation, transfusion of blood products, close monitoring with echocardiography, and percutaneous pericardial drainage if indicated. Additional cardiological interventions also include prolonged intracoronary balloon inflation to occlude the perforation, the use of a perfusion balloon, insertion of stents (open or covered), local injection of thrombogenic molecules, placement of microcoils, and transcatheter embolization with autologous blood clot or glue.5 6 7 8
Surgical management following failure of conservative treatment is not standardized and depends on the surgical anatomy, patient comorbidities, and clinical condition of the patient. The presence of a stent also increases the possibility of early perioperative stent thrombosis. Surgical reports include simple suturing of the perforation, ligation of bleeding vessels, pericardial patch applications to obtain hemostasis, use of surgical glues, coronary artery bypass grafting with and without endarterectomy, stent removal, or vein patch.9 10
In the retrospective study by Fasseas et al involving 16,298 PCI procedures, only 0.58% (n = 95) presented with coronary artery perforations, the majority of which were treated by balloon inflation (66.3%, n = 63) and only 10.5% (n = 10) being elected for surgical management. Of the patients presenting with coronary artery perforation, 12.6% (n = 12) suffered from an acute myocardial infarction, 11.6% (n = 11) developed tamponade, and 7.4% (n = 7) died. Female gender and extensive use of atheroablative devices have been reported as significant risk factors for coronary perforations. A key to the reduction of the mortality rate to < 10% in this category of patients is early recognition of the coronary perforations followed by immediate treatment of the lesion.11
Shimony et al in their recent systematic review and meta-analysis involving 16 studies (197,061 PCIs) found out that the incidence of PCI complications was 0.43% (95% confidence interval [CI], 0.35 to 0.52%). Furthermore, the pooled tamponade rates were 0.4% (95% CI, 0.0 to 5.7%), 3.3% (95% CI, 0.0 to 11.4%), and 45.7% (95% CI, 34.9 to 57.5%) for patients with Ellis classes I to III coronary artery perforations, respectively. It must be highlighted that currently, no established protocol guidelines exist regarding the management strategies for PCI complications.12 However, the authors have suggested an algorithm which is consistent with our institution's practice policy. According to this algorithm, all the patients with PCI complications should be under continuous monitoring and assessment (sequential echocardiography studies). In the case of a hemodynamically unstable patient (mainly Ellis class III), the patient is either initially treated conservatively under the care of the cardiologists (pericardiocentesis, heparin reversal with protamine if there is reversal of flow into the pericardium, discontinuation of IIb/IIIa inhibitors or bivalirudin, prolonged balloon inflation for 5 to 15 minutes, gel foam embolization, and polytetrafluoroethylene-covered stent) or surgically by the cardiothoracic surgeons.12
Pericardiocentesis can be performed safely under echocardiography guidance or fluoroscopy visualization in the catheterization laboratory or the intensive cardiac care unit and can be considered as either a definitive treatment or a bridging treatment for the stabilization of the patient and for referral for open surgical intervention.12 In the retrospective study by Fejka et al involving 31 tamponade cases with PCI complications, 61% were solely treated with pericardiocentesis and 39% required further surgical intervention.13
In conclusion, early detection of a coronary artery perforation is important. Angiographic classification provides a useful tool for the algorithm of treatment. Immediate occlusion of the perforation and relief of hemodynamic compromise plays a key role in the management of these patients. Conservative treatment must include mandatory observation for at least 24 hours in an intensive care unit to detect delayed cardiac tamponade which is associated with high mortality.2 10 Cardiac surgeons should be involved early in the decision making, as surgical intervention may be necessary as in this reported case and can be lifesaving.
References
- 1.Ellis S G, Ajluni S, Arnold A Z. et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90(6):2725–2730. doi: 10.1161/01.cir.90.6.2725. [DOI] [PubMed] [Google Scholar]
- 2.Barbeau G R, Sénéchal M, Voisine P. Delayed abrupt tamponade by isolated left atrial compression following coronary artery perforation during coronary angioplasty. Catheter Cardiovasc Interv. 2005;66(4):562–565. doi: 10.1002/ccd.20472. [DOI] [PubMed] [Google Scholar]
- 3.Krabatsch T, Becher D, Schweiger M, Hetzer R. Severe left atrium compression after percutaneous coronary intervention with perforation of a circumflex branch of the left coronary artery. Interact Cardiovasc Thorac Surg. 2010;11(6):811–813. doi: 10.1510/icvts.2010.246017. [DOI] [PubMed] [Google Scholar]
- 4.Fukutomi T, Suzuki T, Popma J J. et al. Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J. 2002;66(4):349–356. doi: 10.1253/circj.66.349. [DOI] [PubMed] [Google Scholar]
- 5.Demin V V. [Successful correction with stent-graft of coronary artery rupture after angioplasty] Angiol Sosud Khir. 2003;9(2):118–121. [PubMed] [Google Scholar]
- 6.Witzke C F, Martin-Herrero F, Clarke S C, Pomerantzev E, Palacios I F. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol. 2004;16(6):257–301. [PubMed] [Google Scholar]
- 7.Tanaka S, Nishigaki K, Ojio S. et al. Transcatheter embolization by autologous blood clot is useful management for small side branch perforation due to percutaneous coronary intervention guide wire. J Cardiol. 2008;52(3):285–289. doi: 10.1016/j.jjcc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Störger H, Ruef J. Closure of guide wire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv. 2007;70(2):237–240. doi: 10.1002/ccd.21115. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi I, Sakurada T, Sato T, Abe Y, Izumi M, Abe T. Emergency coronary artery bypass grafting after left coronary artery rupture: a case report. Ann Thorac Cardiovasc Surg. 1998;4(2):91–95. [PubMed] [Google Scholar]
- 10.Gunning M G, Williams I L, Jewitt D E, Shah A M, Wainwright R J, Thomas M R. Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart. 2002;88(5):495–498. doi: 10.1136/heart.88.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasseas P, Orford J L, Panetta C J. et al. Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J. 2004;147(1):140–145. doi: 10.1016/s0002-8703(03)00505-2. [DOI] [PubMed] [Google Scholar]
- 12.Shimony A, Joseph L, Mottillo S, Eisenberg M J. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol. 2011;27(6):843–850. doi: 10.1016/j.cjca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Fejka M, Dixon S R, Safian R D. et al. Diagnosis, management, and clinical outcome of cardiac tamponade complicating percutaneous coronary intervention. Am J Cardiol. 2002;90(11):1183–1186. doi: 10.1016/s0002-9149(02)02831-x. [DOI] [PubMed] [Google Scholar]


