Abstract
Background
While epidemiologic studies suggest that metformin use among diabetics may decrease prostate cancer (PC) incidence, the effect of metformin use on PC outcome is unclear. We investigated the association between pre-operative metformin use, dose and duration of use and biochemical recurrence (BCR) in PC patients with diabetes who underwent radical prostatectomy (RP).
Methods
We conducted a retrospective cohort analysis within the Shared Equal Access Regional Cancer Hospital (SEARCH) database of 371 PC patients with diabetes who underwent RP. Time to BCR between metformin users and non-users, and by metformin dose and duration of use was assessed using multivariable Cox proportional analysis adjusted for demographic, clinical and/or pathologic features. Time to castrate-resistant PC (CPRC), metastases and PC-specific mortality were explored as secondary outcomes using unadjusted analyses.
Results
Of 371 diabetic men, 156 (42%) were using metformin prior to RP. Metformin use was associated with more recent year of surgery (p<0.0001) but no clinical or pathologic characteristics. After adjustment for year of surgery, clinical and pathologic features, there were no associations between metformin use (HR 0.93; 95%CI 0.61–1.41), high metformin dose (HR 0.96; 95%CI 0.57–1.61) or duration of use (HR 1.00; 95%CI 0.99–1.02) and time to BCR. A total of 14 patients (3.8%) developed CRPC, 10 (2.7%) distant metastases and 8 (2.2%) died from PC. Unadjusted analysis suggested high metformin dose versus non-use was associated with increased risk of CRPC (HR 5.1; 95%CI 1.6–16.5), metastases (HR 4.8; 95%CI 1.2–18.5) and PC-specific mortality (HR 5.0; 95%CI 1.1–22.5).
Conclusions
Metformin use, dose or duration of use was not associated with BCR in this cohort of diabetic PC patients treated with RP. The suggestion that higher metformin dose was associated with increased risk of CPRC, metastases and PC-specific mortality merits testing in large prospective studies with longer follow-up.
Keywords: prostate cancer, metformin, diabetes, biochemical recurrence, outcomes
Introduction
Prostate cancer (PC) is the second most commonly diagnosed male cancer with nearly 1 000 000 new cases per year worldwide 1. Prevalence of type II diabetes is rising, and is estimated to affect ~10% of men in Westernized society 2. Meta-analyses have found diabetes to be associated with 14% – 21% decreased overall PC incidence, and subgroup analyses have demonstrated a temporal association between diabetes and decreased PC risk, with significant protective effect seen only in men with diabetes longer than 5 years 3,4. It has therefore been hypothesized that the metabolic and hormonal environment of advanced/end-stage diabetes, characterized by reduced bioavailable testosterone levels and a hypoinsulinemic state, is consistent with protection from overall PC incidence.
Metformin, a first line therapy for type II diabetes, is associated with reduced overall cancer incidence 5–8 and decreased cancer-specific mortality among individuals with diabetes, relative to either sulfonylurea or insulin use 9,10. In PC, preclinical studies have shown that metformin can exert direct anti-proliferative effects on PC cells both in vitro and in vivo via a variety of mechanisms including cell cycle arrest 11, mTOR inhibition via AMPK-independent mechanisms 12 in addition to growth inhibition via AMPK-dependent mechanisms 13. In addition, since elevated systemic insulin levels pre-PC diagnosis (using C-peptide as a surrogate) have been associated with PC mortality 14, it is possible that the systemic insulin-lowering properties of metformin may also contribute to protection against PC progression.
To date, four observational studies have specifically addressed the effect of metformin on PC risk in humans. One population-based case-control study found metformin use to be associated with a borderline significant 44% decrease in PC incidence in diabetics (OR 0.56; 95% CI 0.32–1.00) 15, while another found metformin use to reduce PC risk by 20% (OR 0.80; 95% CI 0.73–0.88) 16. On the contrary, both a cohort study 17 and a nested case-control study 18 reported a lack of association between metformin therapy and PC risk in diabetic patients. Regarding PC-specific outcomes, to our knowledge only three retrospective cohort studies have been published to date. One examined 210 diabetic patients, 112 of whom were taking metformin, and found no effect of metformin on risk of biochemical recurrence (BCR) following radical prostatectomy (RP) 19. These null findings were subsequently replicated in a larger study of 885 RP patients with diabetes, 323 of whom were taking metformin, which found no effect on BCR, metastases or overall survival 20. Another examined 319 diabetic patients who underwent external beam radiation therapy for localized PC, 157 of whom were taking metformin, and found metformin use to be associated with significantly reduced risk of BCR, castrate resistant PC (CPRC), distant metastasis, and PC-specific mortality 21. To our knowledge, no studies have examined the effect of metformin dose or duration of use on PC outcomes.
Given these conflicting results regarding the association between metformin and PC outcomes, we sought to test whether metformin use, dose and duration of use was associated with outcomes among diabetic men undergoing RP using the Shared Equal Access Regional Cancer Hospital (SEARCH) database 22. Given epidemiologic and biological evidence suggesting anti-tumorigenic properties of metformin, we hypothesized that metformin use would be associated with more favorable pathologic features and reduced risk of BCR following RP relative to diabetic patients not taking metformin.
Methods
Study population
After obtaining Institutional Review Board approval from each institution, data from patients undergoing RP between 1988 and 2010 at four VA Medical Centers (West Los Angeles, CA; Durham, NC; Asheville, NC; and Augusta, GA) were combined into Shared Equal Access Regional Cancer Hospital (SEARCH) 22. SEARCH does not include patients treated with preoperative androgen deprivation or radiation therapy. After excluding 136 men without known diabetes status, we identified 2,349 men with known diabetes status, of whom 394 (17%) were diabetic at the time of surgery. Lastly, we excluded men who underwent surgery prior to 1995, the year that metformin was introduced in the US, giving rise to a final cohort of 371 men. Although we could not definitively distinguish Type I from Type II diabetes, a chart review of patients at the Durham VA showed that 97% had Type II diabetes 23. Thus, the vast majority of our cohort is likely to have Type II diabetes.
Exposure assessment and definitions
Pre-operative metformin use was assessed by examining metformin use at time of surgery. Metformin dose at time of surgery and earliest issue date (from which duration of use in months was calculated) was ascertained by examining the pharmacy database within the VA computerized medical records, and was available for all metformin users.
Follow-up
Follow-up protocols were left to the discretion of the treating physicians. BCR was defined as a single PSA >0.2 ng/ml, two consecutive concentrations at 0.2 ng/ml, or secondary treatment for detectable postoperative PSA. Men receiving adjuvant therapy ≤6 months after surgery for an undetectable PSA were considered non-recurrent at the time of adjuvant therapy, and their follow-up was censored at that point for BCR, but continued for long-term outcomes. Distant metastases, defined as bone, visceral or distant adenopathy (not pelvic adenopathy), were determined by review of radionuclide bone scans, magnetic resonance imaging scans, computed tomography scans, plain radiograph reports, and clinical progress notes. Decision to perform radiographic imaging was at the treating physician’s discretion. Castrate resistant PC (CRPC) was defined using Prostate Cancer Working Group Two criteria: 25% PSA increase from the androgen deprivation therapy (ADT) PSA nadir and PSA increase ≥2 ng/ml 24. Prostate cancer-specific mortality was defined as death in any patient with metastases showing PC progression following ADT.
Statistical analysis
Differences in demographic, clinical and pathologic factors between metformin users and non-users were examined using t-tests and χ2 tests for continuous and categorical variables, respectively, and rank-sum tests for continuous variables not normally distributed. For examining distribution of clinicopathological features across metformin doses, we used ANOVA, χ2, and Kruskal-Wallis as appropriate.
Multivariable logistic regression analysis was used to test whether metformin use was associated with pathological Gleason score (<7 versus ≥7), extracapsular extension, seminal vesicle invasion and positive margins. There were insufficient numbers of men (n=2) with positive lymph nodes to examine this pathologic feature. The influence of metformin dose (available for all metformin users) on pathologic features and BCR was modeled using daily metformin dose as a three tier categorical variable (<2000 mg/day (low; n=83), ≥2000 mg/day (high; n=73), versus 0 mg (i.e. metformin non-use)). We examined the effect of duration of metformin use (measured in months) on pathologic features and BCR using the earliest issue date of metformin (continuous; available for all metformin users). Logistic regression models were adjusted for age at surgery (continuous), year of surgery (continuous), BMI (continuous), race (black, nonblack), preoperative PSA (continuous, natural log-transformed), surgical center (categorical), biopsy Gleason score (2–6, 7, 8–10) and clinical stage (T1 vs. T2/T3).
Time from RP to BCR (primary outcome) and time to CRPC, metastases, and PC-specific death (secondary outcomes) were compared between metformin users and non-users using Kaplan-Meier plots and the log-rank test. With a cohort of 371 patients, we had 80% power to detect a HR of <0.75 or >1.33 for metformin use and risk of BCR, using a two-sided alpha<0.05. Cox proportional hazards models were used to test whether metformin use independently predicted time to these events. For our primary outcome of BCR, we adjusted for clinical factors (age at surgery, year of surgery, BMI, race, preoperative PSA, surgical center, biopsy Gleason score and clinical stage). As secondary analysis, we adjusted for aforementioned clinical factors (dropping biopsy Gleason), in addition to pathologic factors (pathological Gleason score (2–6, 7, 8–10), extracapsular extension, seminal vesicle invasion and positive margins (all categorical)). In addition, we examined the association of metformin dose and duration of use with risk of the various outcomes, adjusting for aforementioned clinical or clinical and pathologic features. For secondary outcomes (time to CRPC, metastases and PC-specific death), the small numbers of events precluded multivariable analysis and so clinical and pathologic variables were added to our models one at a time, and results were treated as exploratory.
Statistical analysis was performed using Stata, version 11.0 (Stata Corp, College Station, TX, USA).
Results
Among all 371 diabetic men, 156 (42%) were using metformin at the time of surgery (Table 1). Metformin users were more recently treated compared to non-users (p<0.0001), resulting in significantly shorter follow up for metformin users (59 vs. 73 months, p=0.004). The number of biopsy cores sampled and duration of diabetes significantly differed between users and non-users, but there were no other significant differences in demographic or clinical characteristics between metformin users and non-users. Percentage glycosylated hemoglobin (HbA1c) in the year prior to surgery, a measure of diabetes control, was available for 293 men (79%) and did not differ between metformin users and non-users (p=0.425) (Table 1). High metformin dose was significantly associated with increased Hba1c levels (p=0.02) and increased seminal vesicle invasion (p=0.023), but was unrelated to other clinical or pathological characteristics (Table 2). On multivariable analysis, there were no associations between metformin use, dose or duration of use and adverse pathologic features in this cohort (Table 3). Adjusting our analyses for duration of diabetes and Hba1c levels did not alter our results (data not shown).
Table 1.
Demographic, clinical and pathological features of metformin users and non-users.
| Metformin use pre-surgery
|
|||
|---|---|---|---|
| No (n=215) | Yes (n=156) | P* | |
| Age at surgery, mean (SD) | 62.2 (5.8) | 61.6 (5.8) | 0.292ɷ |
|
| |||
| Months follow-up, median (IQR) | 73 (45–110) | 59 (37–81) | 0.004† |
|
| |||
| Race, n (%) | |||
| White | 98 (45.6) | 77 (49.4) | 0.475§ |
| Black | 103 (47.9) | 73 (46.8) | |
| Other | 14 (6.5) | 6 (3.9) | |
|
| |||
| Year of surgery, median (IQR) | 2002 (1998–2006) | 2005 (2003–2007) | <0.0001† |
|
| |||
| BMI (kg/m2), n (%) | |||
| ≤24.9 | 24 (12.8) | 15 (9.8) | 0.565§ |
| 25.0 – 29.9 | 76 (40.6) | 69 (45.1) | |
| 30.0–34.9 | 63 (33.7) | 45 (29.4) | |
| ≥35.0 | 24 (12.8) | 24 (15.7) | |
|
| |||
| Duration of diabetes (months), median (IQR) | 37 (10–86) | 52 (26–97) | 0.004† |
|
| |||
| Duration metformin use (months), median (IQR) | NA | 26 (11–45) | NA |
|
| |||
| % HbA1c, median (IQR) | 6.8 (6.2–8.0) | 7.1 (6.1–8.1) | 0.425† |
|
| |||
| Preoperative PSA, median (IQR) | 6.5 (4.7–10.2) | 5.7 (4.7–8.3) | 0.106† |
|
| |||
| Biopsy Gleason sum, n (%) | |||
| 2–6 | 119 (55.9) | 79 (51.3) | 0.687§ |
| 7 | 70 (32.9) | 56 (36.4) | |
| 8–10 | 24 (11.3) | 19 (12.3) | |
|
| |||
| Clinical stage, n (%) | |||
| T1 | 131 (67.2) | 104 (69.8) | 0.605§ |
| T2/T3 | 64 (32.8) | 45 (30.2) | |
|
| |||
| Total biopsy cores, n (%) | 9 (7–11) | 10 (8–12) | 0.019 |
|
| |||
| % positive cores, median (IQR) | 25 (16.7–50.0) | 33 (16.7–57.7) | 0.127† |
|
| |||
| Prostate weight, median (IQR) | 37.7 (29.4–50.0) | 39 (30.0–50.5) | 0.436† |
|
| |||
| Pathologic Gleason sum, n (%) | |||
| 2–6 | 68 (31.8) | 44 (28.2) | 0.505§ |
| 7 | 120 (56.1) | 87 (55.8) | |
| 8–10 | 26 (12.2) | 25 (16.0) | |
|
| |||
| Positive surgical margins, n (%) | 97 (45.1) | 73 (46.8) | 0.749§ |
|
| |||
| Seminal vesicle invasion, n (%) | 23 (10.7) | 13 (8.4) | 0.459§ |
|
| |||
| Extracapsular extension, n (%) | 39 (18.1) | 33 (21.7) | 0.396§ |
|
| |||
| Lymph node metastases, n (%) | 1 (0.5) | 1 (0.6) | 0.881§ |
SD = standard deviation; IQR = interquartile range
p values computed using ɷ t test, § chi-square or † rank sum
Table 2.
Demographic, clinical and pathological features of metformin users and non-users according to metformin dose.
| Metformin dose pre-surgery
|
p* | |||
|---|---|---|---|---|
| None (n=215) | Low dose (<2000mg/day; n=83) | High dose (≥2000mg/day; n=73) | ||
|
| ||||
| Age at surgery, mean (SD) | 62.2 (5.8) | 62.0 (5.3) | 61.0 (6.2) | 0.372ɷ |
|
| ||||
| Months follow-up, median (IQR) | 73 (45–110) | 60 (39–82) | 58 (31–78) | 0.013† |
|
| ||||
| Race, n (%) | ||||
| White | 98 (45.6) | 38 (45.8) | 39 (53.4) | 0.639§ |
| Black | 103 (47.9) | 42 (50.6) | 31 (42.4) | |
| Non-white-non-black | 14 (6.5) | 3 (3.6) | 3 (4.1) | |
|
| ||||
| Year of surgery, median (IQR) | 2002 (1998–2006) | 2005 (2003–2007) | 2006 (2003–2007) | 0.0001† |
|
| ||||
| BMI, n (%) | ||||
| ≤24.9 kg/m2 | 24 (12.8) | 10 (12.5) | 5 (6.9) | 0.764§ |
| 25.0 – 29.9 kg/m2 | 76 (40.6) | 36 (45.0) | 33 (45.2) | |
| 30.0–34.9 kg/m2 | 63 (33.7) | 22 (27.5) | 23 (31.5) | |
| ≥35.0 kg/m2 | 24 (12.8) | 12 (15.0) | 12 (16.4) | |
|
| ||||
| Duration of diabetes (months), median (IQR) | 37 (10–86) | 46 (21–78) | 68 (37–104) | 0.027† |
|
| ||||
| Duration metformin use (months), median (IQR) | NA | 21 (7–38) | 37 (18–52) | 0.0001† |
|
| ||||
| % HbA1c, median (IQR) | 6.8 (6.2–8.0) | 6.7 (6.0–7.6) | 7.7 (6.4–8.5) | 0.020† |
|
| ||||
| Preoperative PSA, median (IQR) | 6.5 (4.7–10.2) | 5.9 (4.8–9.4) | 5.3 (4.6–7.5) | 0.094† |
|
| ||||
| Biopsy Gleason sum, n (%) | ||||
| 2–6 | 119 (55.9) | 45 (54.9) | 34 (47.2) | 0.791§ |
| 7 | 70 (32.9) | 28 (34.2) | 28 (38.9) | |
| 8–10 | 24 (11.3) | 9 (11.0) | 10 (13.9) | |
|
| ||||
| Clinical stage, n (%) | ||||
| T1 | 131 (67.2) | 52 (67.5) | 52 (72.2) | 0.724§ |
| T2/T3 | 64 (32.8) | 25 (32.4) | 20 (27.8) | |
|
| ||||
| Total biopsy cores, n (%) | 9 (7–11) | 10 (8–12) | 10 (8–12) | 0.064 |
|
| ||||
| % positive cores, median (IQR) | 25 (16.7–50.0) | 33 (16.7–58.3) | 32 (16.7–51.8) | 0.307† |
|
| ||||
| Prostate weight, median (IQR) | 37.7 (29.4–50.0) | 39 (30.8–51.4) | 39 (29.0–50.5) | 0.727† |
|
| ||||
| Pathological Gleason Sum, n (%) | ||||
| 2–6 | 68 (31.8) | 27 (32.5) | 17 (23.3) | 0.228§ |
| 7 | 120 (56.1) | 40 (48.2) | 47 (64.4) | |
| 8–10 | 26 (12.2) | 16 (19.3) | 9 (12.3) | |
|
| ||||
| Positive surgical margins, n (%) | 97 (45.1) | 38 (45.8) | 35 (48.0) | 0.916§ |
|
| ||||
| Seminal vesicle invasion, n (%) | 23 (10.7) | 2 (2.4) | 11 (15.1) | 0.023§ |
|
| ||||
| Extracapsular extension, n (%) | 39 (18.1) | 18 (22.0) | 15 (21.4) | 0.695§ |
|
| ||||
| Lymph node metastases, n (%) | 1 (0.5) | 0 (0) | 1 (1.4) | 0.788§ |
SD = standard deviation; IQR = interquartile range
p values computed using ɷ anova, § chi-square or † Kruskal-Wallis
Table 3.
Risk of adverse pathological features by metformin use, dose and duration of use.
| Use (n=145) vs. nonuse (n=181) | Low dose (n=74) vs. nonuse (n=181) | High dose (n=43) vs. nonuse (n=181) | Months of use (among users; n=145) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Path. Gleason ≥7 vs. <7 | 1.03 (0.58–1.83) | 0.920 | 0.83 (0.42–1.64) | 0.585 | 1.32 (0.63–2.76) | 0.457 | 0.99 (0.96–1.01) | 0.167 |
| Extracapsular extension | 1.73 (0.93–3.22) | 0.082 | 1.72 (0.82–3.60) | 0.152 | 1.75 (0.82–3.75) | 0.149 | 1.00 (0.98–1.02) | 0.680 |
| Seminal vesicle invasion | 0.95 (0.40–2.30) | 0.917 | 0.13 (0.02–1.05) | 0.055 | 2.32 (0.88–6.13) | 0.088 | 0.99 (0.95–1.03) | 0.607 |
| Positive margins | 1.14 (0.70–1.87) | 0.588 | 1.11 (0.61–2.02) | 0.736 | 1.18 (0.65–2.16) | 0.586 | 1.00 (0.99–1.02) | 0.675 |
p values computed using multivariable logistic regression and adjusted for clinical features: age at surgery, year of surgery, BMI, race, preoperative PSA, surgical center, biopsy Gleason score and clinical stage.
Of 371 diabetic patients, 134 (36%) progressed to BCR. Of metformin users, 49 (31%) recurred and 85 (40%) of metformin non-users recurred. Median follow up among men who did not recur was 65 months (IQR: 40–96). We analyzed crude risk of BCR in diabetic patients, comparing metformin users and non-users. Metformin use was not significantly associated with risk of BCR in crude analysis (HR 0.86, 95% CI 0.61–1.23), nor following adjustment for clinical features (HR 1.01, 95% CI 0.67–1.52), nor following adjustment for both clinical and pathologic features (HR 0.93, 95% CI 0.61–1.41; Table 4). Furthermore, there was no association of metformin dose or months of use with risk of BCR on crude or adjusted analyses (Table 4). Adjusting our analyses for duration of diabetes and Hba1c levels did not alter our results (data not shown).
Table 4.
Risk of biochemical recurrence by metformin use, dose and duration of use.
| Use (n=155) vs. nonuse (n=214) | Low dose (n=83) vs. nonuse (n=214) | High dose (n=72) vs. nonuse (n=214) | Months of use (among users; n=155) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Crude | 0.86 (0.61–1.23) | 0.410 | 0.78 (0.49–1.22) | 0.276 | 0.96 (0.62–1.51) | 0.871 | 1.00 (0.99–1.02) | 0.391 |
| Model 1* | 1.01 (0.67–1.52) | 0.974 | 0.92 (0.56–1.54) | 0.759 | 1.11 (0.67–1.83) | 0.698 | 1.01 (0.99–1.03) | 0.053 |
| Model 2** | 0.93 (0.61–1.41) | 0.731 | 0.90 (0.53–1.53) | 0.691 | 0.96 (0.57–1.61) | 0.879 | 1.01 (0.99–1.02) | 0.489 |
adjusted for clinical features: age at surgery, year of surgery, BMI, race, pre-operative PSA, surgical center, biopsy Gleason score and clinical stage.
adjusted for clinical and pathologic features: age at surgery, year of surgery, BMI, race, pre-operative PSA, surgical center, clinical stage, pathological Gleason score, extracapsular extension, seminal vesicle invasion and positive surgical margins.
We assessed the association between metformin use, duration of use, and dose with longer-term outcomes of CRPC, distant metastasis and PC-specific mortality. A total of 14 patients (3.8%) developed CRPC, 10 (2.7%) distant metastases and 8 (2.2%) died from PC. On unadjusted analysis, metformin use was unrelated to metastases or PC-specific mortality, however metformin use was associated with borderline increased risk of CRPC (Table 5; p=0.054). While duration of metformin use did not show any association with any longer-term outcomes, there were suggestions that high metformin dose (≥2000 mg versus no metformin) was associated with increased risk of distant outcomes including CRPC (HR 5.1; 95%CI 1.6–16.5; p=0.006), distant metastases (HR 4.8; 95%CI 1.23–18.5; p=0.024) and PC-specific mortality (HR 4.97; 95%CI 1.10–22.5; p=0.037), with no significant associations at low metformin dose (Table 5). Unfortunately limited numbers of events precluded multivariable analysis. However, when clinical and pathologic features were added to the unadjusted model one at a time (data not shown), no variable markedly altered the HR for these distant outcomes.
Table 5.
Risk of long term outcomes by metformin use, dose and duration of use.
| Use (n=156) vs. nonuse (n=215) | Low dose (n=83) vs. nonuse (n=215) | High dose (n=73) vs. nonuse (n=215) | Months of use (among users; n=156) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| CPRC | 2.98 (0.98–9.05) | 0.054 | 1.22 (0.23–6.33) | 0.817 | 5.13 (1.60–16.5) | 0.006 | 1.01 (0.99–1.04) | 0.292 |
| Metastasis | 2.53 (0.70–9.22) | 0.158 | 0.78 (0.09–7.06) | 0.824 | 4.76 (1.23–18.5) | 0.024 | 1.01 (0.98–1.04) | 0.454 |
| PC-specific death | 2.89 (0.68–12.3) | 0.150 | 1.08 (0.11–10.47) | 0.948 | 4.97 (1.10–22.5) | 0.037 | 1.02 (0.98–1.06) | 0.311 |
p values computed using Cox proportional hazard analysis. Limited number of events precluded multivariable analysis; however, when clinical and pathologic features were added to the unadjusted model one at a time, no variable markedly altered the hazard ratio for these distant outcomes.
Discussion
In this cohort of diabetic PC patients there was no effect of metformin use, dose or duration of use on adverse pathologic features or time to BCR. Three retrospective cohort studies previously examined the effect of metformin use on PC-specific outcomes. Similar to our own study, Patel et al. found no associations between metformin use and BCR or pathologic outcome in RP patients 19. In a larger but otherwise similar retrospective cohort study of 323 metformin users and 562 non-users, Kaushik et al. found no associations between metformin use and BCR, adverse pathology, metastasis or overall survival 20. Our null findings regarding metformin use and BCR are in agreement with both of these prior studies. Spratt et al. examined the effect of metformin use on BCR and long-term outcomes after external beam radiation therapy for localized PC. In contrast to our analysis, and that of Kaushik et al., this study reported a significant reduction in risk of BCR, development of CPRC, distant metastases and PC-specific mortality in metformin users versus non-users 21. Of note, this study reported an extremely large HR of 5.15 (95%CI 1.53–17.35) for metformin non-use on PC-specific mortality which, viewed alternatively, corresponds to a HR of 0.19 for metformin use. For purposes of comparison, two randomized trials of RP vs. watchful waiting showed that RP reduced PC-specific mortality by ~40% 25,26. Therefore, this study suggested metformin use is approximately twice as effective as RP for reducing PC-specific mortality. As such, such a strong effect of metformin seems unlikely and the results of this prior radiation study should be interpreted with caution.
While our study was certainly limited by small numbers and short follow-up, we saw no benefit of metformin use for improving oncologic outcomes in PC patients following RP. In fact, our exploratory analysis suggested that higher metformin dose may even increase risk of CPRC, distant metastases and PC-specific mortality, although again our numbers were too small to conduct multivariable analysis or draw firm conclusions. These exploratory findings must be interpreted with caution as higher metformin dose may reflect an effort to improve poor diabetes control. Indeed, we found that Hba1c levels were significantly elevated in patients receiving higher doses of metformin. However, there is currently no evidence to suggest that poor diabetes control is associated with worse PC-specific long-term outcomes. Of note, we previously reported in this same patient cohort that while elevated Hba1c levels among patients with diabetes were associated with higher pathologic Gleason score, there was no association of Hba1c levels with BCR 27. In this analysis, adjusting our results for Hba1c levels did not alter our findings. Therefore, this suggestion of a dose-dependent effect of metformin on worsening long-term PC-specific outcomes may merit further investigation. The effect of metformin on lowering insulin levels may, to some extent, mirror the lower insulin environment of long-standing diabetes. In other words, metformin users and individuals with long-standing diabetes may have lower insulin levels than diabetic metformin non-users and diabetes-free individuals, respectively. We speculate that this lower insulin environment, while it may reduce overall PC incidence, may also select for more aggressive, growth factor-independent PC. Indeed, epidemiologic evidence suggests that, despite the protective effect of longer-term diabetes (>5 years) on overall PC risk 4, longer duration of diabetes was found to significantly increase risk of metastasis following RP 23. As such, these data support the hypothesis that PC tumors which can survive and grow in a lower insulin environment are more aggressive, though this remains speculative. Of note, metformin users, particularly those on high metformin doses, had significantly longer duration of diabetes which may present a potential source of bias in this study, although adjusting our models for duration of diabetes did not alter our results.
This study has several limitations which must be considered. We could not assess use of other diabetic medications including insulin, sulfonylureas or thiazolidinediones. We used earliest issue date for metformin to estimate duration of use, but we could not confirm whether patients took metformin continuously from earliest issue date to RP. Furthermore, it is possible that some metformin nonusers became metformin users after RP but before BCR. Given our hypothesis that metformin use delays BCR, this may bias our results towards the null. Future studies are required to assess the potential impact of postoperative metformin use on BCR. Neither could we definitively distinguish Type I from Type II diabetes, however median age of diabetes diagnosis was 57 years old, and thus the majority are likely to be Type II diabetic patients. As with all observational epidemiologic studies of drug effects, our study may be subject to confounding by indication, as allocation of treatment was not randomized and thus may differ by risk profile of the patient. However, a study strength was near complete BMI and HbA1c data for 90% and 75% of patients, respectively. We were therefore able to confirm that neither BMI nor diabetes control differed significantly when patients were stratified by metformin use, thus addressing these potentially confounding factors to the greatest extent possible in this retrospective cohort study. One final point to consider is that the median duration of metformin use in this study was 26 months, with the majority of men using metformin for less than 4 years. Whether this is sufficient time for metformin to have an impact on PC outcomes is unknown.
In conclusion, observational epidemiologic evidence supporting a role for metformin in PC prevention and treatment is weak. Our retrospective cohort analysis found no effect of metformin use on BCR in an RP cohort of PC patients with diabetes. Further exploratory analysis found a suggestion that higher metformin dose, versus metformin non-use, increased the risk of CPRC, metastases and PC-specific mortality. Future prospective studies and ultimately RCTs are required in order to establish whether there is any role for metformin in PC oncologic management.
Figure 1.
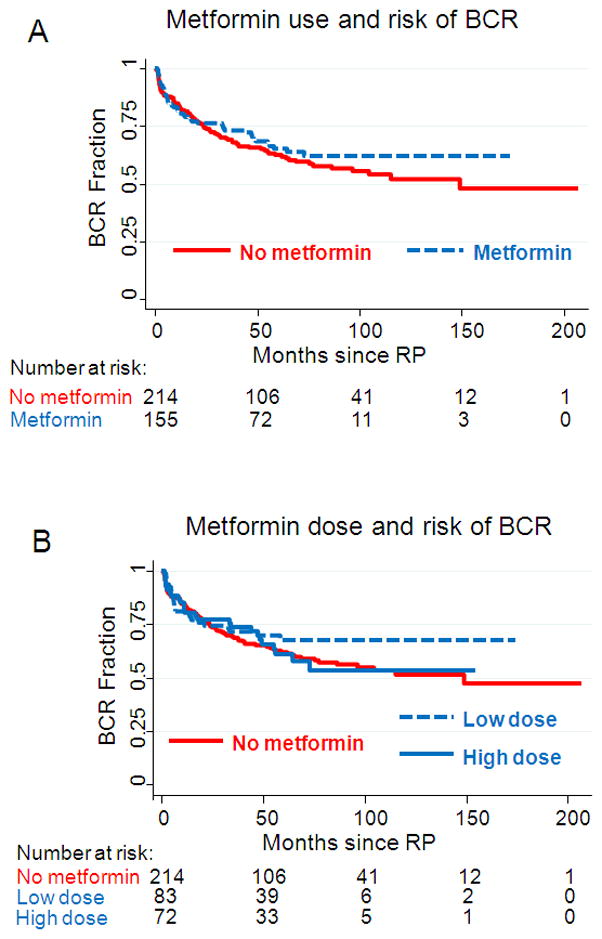
Kaplan Meier crude analysis of risk of biochemical recurrence following RP in diabetic men, stratified by A) metformin use and B) metformin dose at the time of RP.
Acknowledgments
Grant support: EHA: NCI 5R25-CA126938-03; SJF: NIH Grant 1-R01-CA131235-01A1 and NIH 1K24CA160653
Footnotes
Conflicts of interest: The authors have no competing financial conflicts of interest.
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. European urology. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2. 7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124(6):1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Yang Y, Skrip L, Hu D, Wang Y, Wong C, et al. Diabetes mellitus and risk of prostate cancer: an updated meta-analysis based on 12 case-control and 25 cohort studies. Acta diabetologica. 2012 doi: 10.1007/s00592-012-0439-5. [DOI] [PubMed] [Google Scholar]
- 5.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3(11):1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 8.Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. The oncologist. 2012;17 (6):813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 10.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27(25):3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 12.Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71(13):4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 13.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1(5):369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. The lancet oncology. 2008;9(11):1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer causes & control: CCC. 2009;20(9):1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. American journal of epidemiology. 2008;168(8):925–931. doi: 10.1093/aje/kwn190. [DOI] [PubMed] [Google Scholar]
- 17.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 18.Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev. 2011;20(2):337–344. doi: 10.1158/1055-9965.EPI-10-0940. [DOI] [PubMed] [Google Scholar]
- 19.Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology. 2010;76(5):1240–1244. doi: 10.1016/j.urology.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Kaushik D, Karnes RJ, Eisenberg MS, Rangel LJ, Carlson RE, Bergstralh EJ. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urologic oncology. 2013 doi: 10.1016/j.urolonc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and Prostate Cancer: Reduced Development of Castration-resistant Disease and Prostate Cancer Mortality. European urology. 2012 doi: 10.1016/j.eururo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitley BM, Moreira DM, Thomas JA, Aronson WJ, Terris MK, Presti JC, Jr, et al. Preoperative weight change and risk of adverse outcome following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. Prostate cancer and prostatic diseases. 2011;14(4):361–366. doi: 10.1038/pcan.2011.42. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Aronson WJ, Terris MK, Presti JC, Jr, Kane CJ, Amling CL, et al. Diabetes predicts metastasis after radical prostatectomy in obese men: results from the SEARCH database. BJU international. 2013 doi: 10.1111/j.1464-410X.2012.11687.x. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Presti JC, Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Glycemic control and prostate cancer progression: results from the SEARCH database. The Prostate. 2010;70(14):1540–1546. doi: 10.1002/pros.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]


