Abstract
In peripheral nerves, Schwann cells form myelin, which facilitates the rapid conduction of action potentials along axons in the vertebrate nervous system. Myelinating Schwann cells are derived from neural crest progenitors in a step-wise process that is regulated by extracellular signals and transcription factors. In addition to forming the myelin sheath, Schwann cells orchestrate much of the regenerative response that occurs after injury to peripheral nerves. In response to injury, myelinating Schwann cells dedifferentiate into repair cells that are essential for axonal regeneration, and then redifferentiate into myelinating Schwann cells to restore nerve function. Although this remarkable plasticity has long been recognized, many questions remain unanswered regarding the signaling pathways regulating both myelination and the Schwann cell response to injury.
Introduction
The scope and complexity of the vertebrate nervous system requires the rapid transmission of action potentials over long distances. The myelin sheath is an evolutionary adaptation that allows axons to rapidly propagate action potentials [1]. Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS) form myelin by wrapping their cell membranes around axons to form a multilayered membranous sheath that insulates and supports axons [2]. Voltage gated sodium channels cluster at the unmyelinated gaps between myelin segments—the nodes of Ranvier [1]. Depolarization of the axonal membrane at the nodes allows action potentials to propagate in a saltatory manner. Diseases of myelin, including multiple sclerosis in the CNS [3], and Charcot-Marie-Tooth disease in the PNS [4], underscore its clinical importance.
Schwann cells arise from the neural crest in a series of developmental stages [5]. Schwann cell precursors comigrate with growing axons in peripheral nerves, and depend upon axonal signals, such as Neuregulin 1 (Nrg1), for their survival and differentiation into myelinating Schwann cells [5,6]. Myelinating Schwann cells are post-mitotic, but in response to injury, they lose contact with axons and undergo a process of dedifferentiation [7,8]. The dedifferentiation of Schwann cells following injury is essential for successful regeneration in the PNS, and recent studies have greatly expanded our knowledge of the signals and transcriptional programs that regulate the Schwann cell injury response and remyelination [9–12]. Comprehensive reviews of the signaling pathways, transcription factors, and cell biological processes involved in myelination in the PNS have recently been published elsewhere [13,14]. Here, we highlight recent work on the mechanisms controlling the initiation and maintenance of myelin in the PNS, as well as the response of Schwann cells to peripheral nerve injury.
Schwann cell development and Neuregulin 1 signaling
The transcription factor Sox10 plays an essential role in the specification of Schwann cells from the neural crest and their progression past the immature stage [5,15]. Immature Schwann cells associate with multiple axons, but Schwann cells that have progressed to the promyelinating stage associate with only a single axon [5]. The transcription factors Oct6 (Scip/Pou3f1), Brn2 (Pou3f2), and Krox20 (Egr2) are important for the transition from the promyelinating to the myelinating stage, in which a Schwann cell begins to wrap its cell membrane many times around a single axon, forming the myelin sheath [16–19]. Some Schwann cells do not form myelin and remain associated with multiple small caliber axons, such as nociceptive fibers [20]. The Nrg1 signaling pathway controls nearly all aspects of Schwann cell development from specification to myelination [6]. Nrg1 signals, predominantly the axonal Nrg1 type III isoform (Nrg1-III), are transduced through the ErbB2/ErbB3 heterodimeric receptor in Schwann cells. Nrg1-III signaling activates many downstream pathways in Schwann cells including the phosphatidylinositol-3-kinase (PI3K) pathway, the phospholipase C-γ (PLC-γ) Ca2+ signaling pathway, and the MEK/ERK pathway [6,13]. The level of Nrg1-III expression in axons regulates the initiation of myelination as well as myelin sheath thickness [21,22]. Several studies have demonstrated that signaling downstream of PI3K positively regulates both of these events in vivo [23–25]. Similarly, other in vivo studies have shown that MEK/ERK signaling regulates Schwann cell differentiation and myelin sheath thickness [26–28]. Additional work is needed to understand how the multiple pathways activated downstream of ErbB2 are coordinated to regulate myelination.
Recent studies have demonstrated that Nrg1 signaling is modulated by a number of different proteases, including the β-secretase (BACE1) and the tumor necrosis factor-α-converting enzyme (TACE/ADAM17) [29–31]. BACE1 promotes myelination, and mice lacking BACE1 have abnormally thin myelin sheaths [29,31], suggesting that BACE1-processed forms of Nrg1 are active ErbB ligands. The overexpression of Nrg1-III in neurons induces hypermyelination in the PNS of BACE1 mutant mice, indicating that BACE activity is apparently not required to generate active Nrg1 signals in some experimental contexts [32]. A careful study of TACE mutant mice demonstrated that TACE represses myelination—the mutants exhibit hypermyelination in peripheral nerves [30]. There are conflicting reports about the sites of TACE-mediated proteolytic cleavage in Nrg1-III, as well as the activity of TACE-cleaved products [30,33,34]. Future studies will be required to determine the precise activity of differentially processed forms of Nrg1 in vivo. Interestingly, mice lacking a functional copy of the intermediate filament protein vimentin exhibit increased levels of Nrg1 signaling and hypermyelination in the PNS, phenotypes similar to the overexpression of Nrg1-III or the genetic ablation of TACE in neurons [35]. Although the mechanism remains unclear, it is possible that directed cytoskeletal transport is important for proper presentation of Nrg1 ligands.
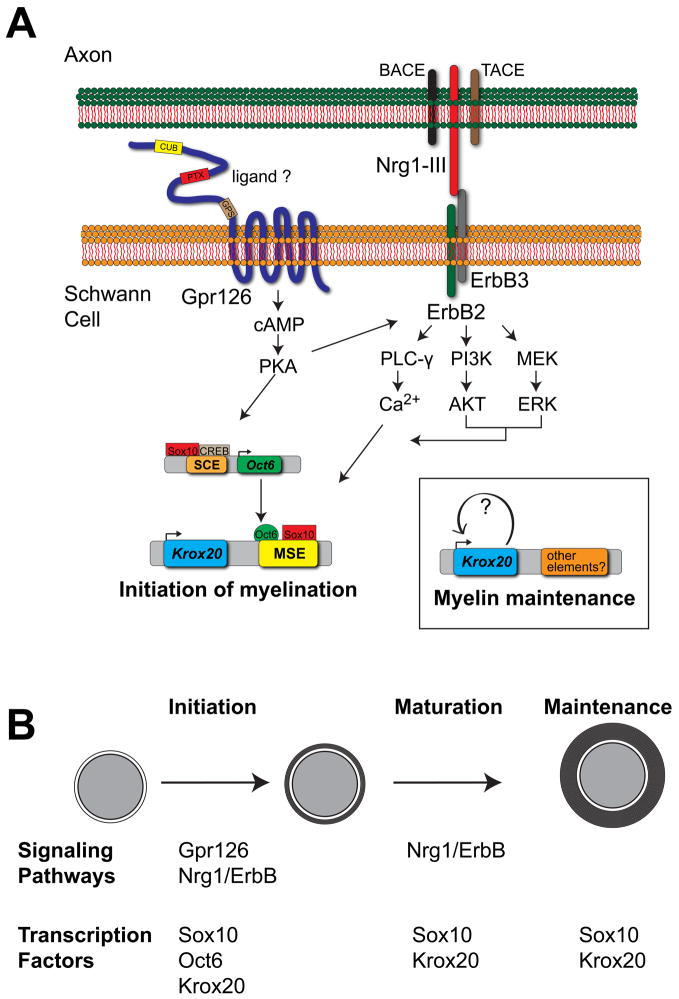
In addition to controlling the initiation of myelination, Nrg1-III signals also control Schwann cell specification, survival, proliferation, directed migration, radial sorting, and myelin sheath thickness [6]. How Nrg1-III signals can generate such a wide array of Schwann cell responses through the same ErbB2/ErbB3 receptor complex is unclear, but it has been proposed that axonal Nrg1-III acts in a concentration dependent manner, with Schwann cells exhibiting distinct responses to different levels of Nrg1-III [6,21,22]. Additionally, other signaling pathways may intersect with the Nrg1-ErbB pathway at different stages of Schwann cell development, so that combinatorial actions of multiple pathways could affect the Schwann cell response to Nrg1-III (Figure 1).
Figure 1.
A simplified model depicting the signaling pathways and transcription factors controlling the initiation, maturation, and maintenance of myelin. (A) Gpr126 and Nrg1-III/ErbB coordinately activate Oct6 and Krox20 expression to initiate myelination. PKA functions downstream of Gpr126 [43], and Sox10 and CREB binding sites are present in the SCE [39,71]. Sox10 and Oct6 binding sites are present in the Krox20 myelinating Schwann cell element (MSE) [72]. Krox20 is maintained independently of Gpr126 after initiation, which likely depends on elements other than the MSE [43]. Downstream of ErbB2, ERK may have context dependent roles that determine whether it synergizes or opposes the effects of Akt. PLC-γ signaling results in Ca2+ increases that are reported to activate the MSE via an NFAT dependent mechanism [73] (B) A representation of the stages of myelination during which the pathways and transcription factors shown in A are known to function. Of the factors shown, only Sox10 and Krox20 are required continuously in mature myelinating Schwann cells in the absence of injury [54,74].
Gpr126 signaling and the initiation of myelination
Signaling mediated by the second messenger cAMP was first implicated in the regulation of Schwann cell differentiation years ago [36,37]. The promyelinating transcription factor Oct6 is strongly induced in cultured Schwann cells in response to cAMP [38], and binding sites for the cAMP response element binding protein (CREB) are present in the Oct6 cis-regulatory Schwann cell enhancer (SCE) [39]. Genetic analysis in zebrafish identified the orphan adhesion G protein-coupled receptor (GPCR) Gpr126 as an essential regulator of myelination in the PNS and suggested that Gpr126 triggers myelination by elevating cAMP levels [40,41]. Schwann cells of Gpr126 mutants in fish and mouse fail to express Oct6 or Krox20, arrest at the promyelinating stage, and do not generate myelin [40,42]. The function of Gpr126 in myelination is autonomous to Schwann cells [40]. Myelination can be restored in zebrafish gpr126 mutants in vivo by the application of forskolin, which directly activates adenylate cyclase to increase cAMP [40], and by the expression of activated PKA in Schwann cells [43]. These results provide strong evidence that Gpr126 is the receptor that activates cAMP signaling to initiate myelination in Schwann cells in vivo (Figure 1). Several in vitro studies have suggested that cAMP may amplify the strength of Nrg1 signals [44–46], while others have demonstrated that cAMP changes a Schwann cell’s response to Nrg1-III from proliferation to myelination [47]. A recent in vivo study demonstrated that increased levels of Nrg1-III are not sufficient to rescue myelination in gpr126 mutant zebrafish [43]. In combination, these studies suggest that cAMP may not simply amplify Nrg1-III signals, but rather qualitatively alters a Schwann cell’s response to Nrg1-III.
The ligand that activates Gpr126 remains unknown. Because axonal signals regulate the expression of myelin genes [36,48], an axonal ligand may activate Gpr126. Alternatively, the Schwann cell extracellular matrix, long recognized to be essential for myelination [49,50], may contain a ligand that activates Gpr126. Schwann cells contribute to the formation of the extracellular matrix by secreting laminin and collagen [51,52]. Interestingly, collagen III binds and activates another adhesion GPCR, Gpr56 [53]. This raises the intriguing possibility that an autocrine signal from the Schwann cell extracellular matrix may activate Gpr126, which then works in concert with axonally derived signals (such as Nrg1-III), to initiate myelination at the appropriate developmental stage.
Different mechanisms control the initiation and maturation of myelin
Some of the critical regulators of the initiation of myelination, including Krox20, are also required to maintain mature myelin. The loss of Krox20 from mature myelinating Schwann cells leads to rapid demyelination in adult nerves [54]. Interestingly, although Gpr126 signaling is required to initiate Krox20 expression and myelination [40,42], this receptor is no longer required to maintain Krox20 expression or mature myelin at later stages [43] (Figure 1). Indeed, when the initiation defect in gpr126 mutant zebrafish was bypassed by transient elevation of cAMP, Schwann cell membrane wrapping proceeded normally and krox20 expression in peripheral nerves was maintained for months in the absence of Gpr126 signaling [43]. Previous work in vitro demonstrated that cAMP is required continuously in cultured Schwann cells to maintain a myelinating phenotype [55]. The in vivo studies described above, however, indicate that if cAMP is required continuously to maintain myelin, it must occur independently of Gpr126 signaling [43].
In addition to Gpr126, other genes appear to have different functions during the initiation and maintenance phases of myelination. The promyelinating transcription factors Oct6 and Brn2 are transiently expressed during the initiation of myelination, but expression is absent from the mature nerve [17,38,56,57]. Although Nrg1-ErbB signaling is known to regulate both the initiation of myelination and myelin sheath thickness [21,22], it is not required for myelin maintenance in the absence of injury [58,59]. Oct6, however, is expressed once again following injury [56,57], and Nrg1-ErbB signaling is required for remyelination [12,59]. It remains to be determined if Gpr126 signaling is similarly required for remyelination, but its pivotal role during development makes this a strong possibility. Conversely, mutation of the prion protein PrPC results in demyelinating polyneuropathy in adult mice, while having almost no effect on developmental myelination [60]. PrPC appears to function axonally, and independently of Nrg1 signaling, but its mechanism of action in myelin maintenance remains unclear [60]. Future work will help clarify the relationship between the regulators of myelin initiation, maintenance, and repair.
The Schwann cell response to peripheral nerve injury
One of the hallmarks of the PNS is the robust regenerative response that occurs after peripheral nerve injury [7]. Peripheral nerve injury induces a complex series of cellular events that take place as the axons distal to the injury site degenerate a—process known as Wallerian degeneration [7]. During Wallerian degeneration, the expression of Krox20 and other myelin genes is lost in Schwann cells, along with the myelin sheath itself (Figure 2). Additionally, the expression of transcription factors associated with immature Schwann cells is increased [8]. Dedifferentiated Schwann cells form unique columnar structures called Bands of Bunger, along which regenerating axons grow. Upon successful axonal regeneration, Schwann cells regain their contact with axons and begin to differentiate once again into myelinating cells. A number of signaling pathways are activated in Schwann cells following peripheral nerve injury, including Notch, JAK-STAT, and mitogen-activated protein kinases (MAPKs) [8,61,62], but the nature of the axonal injury signals remain elusive.
Figure 2.
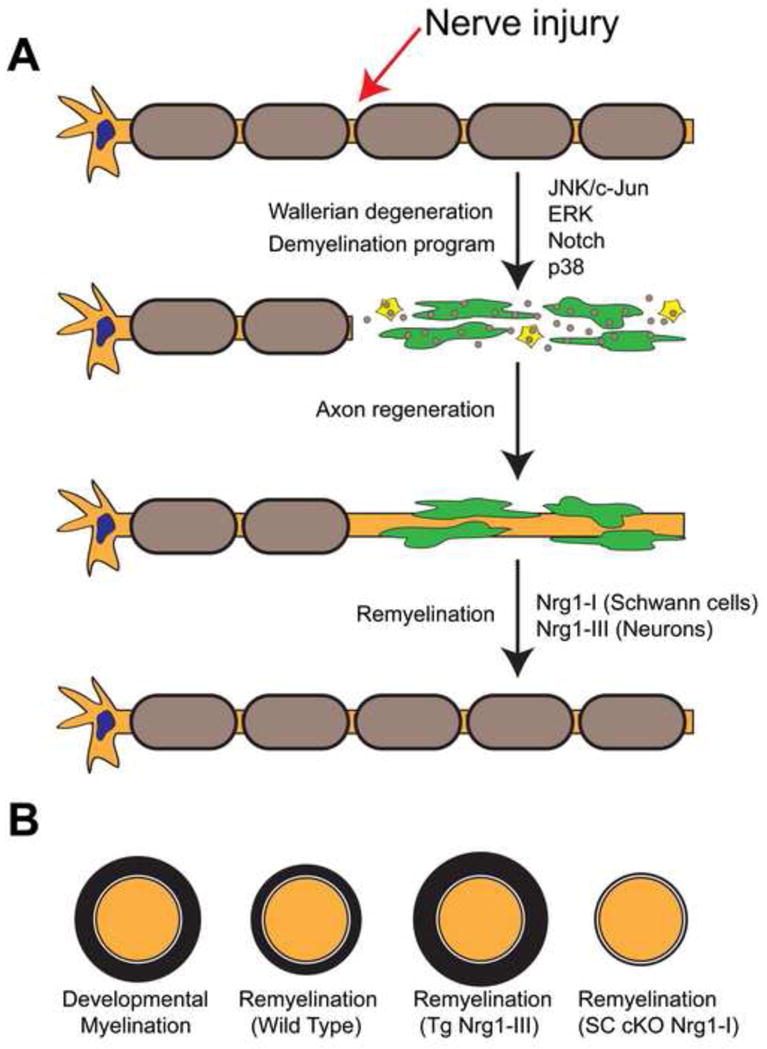
A model illustrating the Schwann cell response to peripheral nerve injury. (A) During Wallerian degeneration, Schwann cells distal to the site of injury dedifferentiate (green cells), and participate in the phagocytosis of their own myelin sheaths (small brown circles), and recruit macrophages (yellow cells) to aid in the clearance of myelin debris. Wallerian degeneration is associated with increased activity in multiple signaling pathways in Schwann cells, including JNK/c-Jun, ERK, Notch, and p38. (B) After successful axonal regeneration, remyelination occurs but Schwann cells produce myelin sheaths of reduced thickness. Overexpression of Nrg-III in neurons restores full myelin thickness during regeneration. The conditional deletion of Nrg1-I in Schwann cells results in severe defects in remyelination. See [12] for details.
c-Jun and the Schwann cell injury response
The transcription factor c-Jun is expressed in immature Schwann cells, but is downregulated in mature myelinating cells [63]. After injury, c-Jun is upregulated and phosphorylated in Schwann cells, where it has been suggested to drive a demyelination program [63]. Two recent studies demonstrated that the conditional ablation of c-Jun in Schwann cells resulted in increased neuronal death following nerve injury, in addition to defects in axonal regeneration and reduced functional recovery [9,10]. Analysis of Schwann cells after injury indicated that c-Jun is required for the downregulation of myelin structural genes such as MBP and P0, and for the upregulation of trophic factors associated with regeneration such as BDNF and GDNF, as well as markers of immature Schwann cells such as p75NTR and Krox24 [9,10]. Transcriptional analysis also identified several genes that were expressed in Schwann cells of the injured nerve but not in immature Schwann cells, providing molecular evidence that injury activated Schwann cells are distinct from immature Schwann cells [9]. GDNF and Artemin, which encode ligands for the Ret receptor tyrosine kinase, are also activated by c-Jun in Schwann cells to mediate pararcrine signaling required for neuronal survival [10].
Following injury, c-Jun mutant Schwann cells form Bands of Bunger with aberrant structure, which could in part explain the regeneration failure observed in these mutants. c-Jun mutant Schwann cells also exhibited deficiencies in the phagocytosis of myelin following injury, which contributed to delayed Wallerian degeneration [9]. Notably, mutant mice lacking c-Jun in Schwann cells undergo normal PNS myelination during development, indicating that c-Jun function appears to be specific to the injury response [9]. In conjunction with previous work [8], these studies collectively demonstrate that Schwann cells following injury are distinct from immature Schwann cells during development, and that these repair Schwann cells are essential for PNS regeneration. Although c-Jun is normally expressed at very low levels in mature peripheral nerves, it is upregulated in the nerves of patients with peripheral neuropathy, suggesting that the inappropriate activation of this repair program may underlie the pathology in these patients [64].
ERK signaling in Schwann cells
A number of studies have focused on ERK signaling in PNS development and regeneration. ERK is activated by phosphorylation shortly after peripheral nerve injury [61,65], and can induce the dedifferentiation of Schwann cells in vitro [65,66]. A recent study demonstrated that peripheral nerves undergo a process highly reminiscent of Wallerian degeneration in response to increased ERK signaling, including demyelination, Schwann cell proliferation, and the recruitment of inflammatory cells to the nerve, despite the fact that axons remained completely intact. These results differed from another study reporting that increased levels of ERK activity caused hypermyelination when assayed at P30 in mice [27], suggesting a role for ERK signaling in promoting myelination. In addition, analysis of conditional ERK knockouts have clearly demonstrated that ERK signaling is required for Schwann cell differentiation and myelination in vivo, likely by mediating the effects of Nrg1 signals [28]. Thus, different studies have concluded that ERK signaling has both positive [26–28,67], and negative [11,65,66,68] effects on myelination. Different levels or duration of ERK activity may elicit distinct responses, potentially explaining these seemingly paradoxical observations [11]. In addition it has also been suggested that other signaling pathways may interact with ERK signaling to influence the outcome [69].
c-Jun is also activated downstream of ERK [68], which prompts the question of whether ERK, JNK, or both, are primarily responsible for the Schwann cell injury response. The JNK mediated phosphorylation of c-Jun is apparently not required for the demyelinating effects of c-Jun in cell culture [63], which suggests that either JNK activates c-Jun indirectly, or perhaps JNK independent function of c-Jun could play a role in its activity. Clarifying the roles of the different MAPK pathways and how they operate with other pathways, including Notch [62], to regulate the Schwann cell injury response will require additional analyses of conditional mouse mutants, as well as investigation into the nature of the unknown axonal injury signals.
Remyelination
Despite the robust regenerative capacity of the PNS, new myelin sheaths surrounding axons after injury are significantly thinner than the sheaths that initially formed during development [70] (Figure 2). Because Nrg1-III signaling regulates myelin sheath thickness [21], it has been proposed that Schwann cells either do not receive, or cannot appropriately respond to Nrg1-III signals from regenerating axons following injury [1,12]. A recent study demonstrated that the overexpression of Nrg1-III in neurons restored not only myelin sheath thickness upon remyelination, but also caused hypermyelination relative to wild type [12] (Figure 2). Interestingly, the overexpression of Nrg1 type I (Nrg1-I) in neurons also restored normal myelin sheath thickness following injury [12], although Nrg1-I does not affect myelin sheath thickness during development [21]. The expression of endogenous Nrg1-I is very low in neurons and uninjured Schwann cells, but expression is significantly increased in Schwann cells following nerve injury [12]. Strikingly, the conditional deletion of Nrg1-I in Schwann cells resulted in severe defects in remyelination, while having no effect on developmental myelination [12] (Figure 2).
These results suggest a model in which the loss of axonal contact following injury activates Nrg1-I expression in Schwann cells, which functions as an autocrine/paracrine signal to promote remyelination [12]. As Schwann cells remain denervated while axons are regrowing into distal nerve stumps, autocrine Nrg1-I signaling could promote the timely redifferentiation of Schwann cells, thus allowing for adequate remyelination after axonally supplied Nrg1-III becomes available again. The defects in remyelination following Schwann cell-specific loss of Nrg1-I imply that there is a critical period after injury in which Schwann cells are dependent on Schwann cell-derived Nrg1-I signaling in order to fully respond to later axonal Nrg1-III signals [12]. Further analysis of this critical period during the switch from Nrg1-III to Nrg1-I signaling and back during regeneration will provide additional mechanistic insight.
Concluding Remarks
The Schwann cell is an extraordinarily plastic cell that is instrumental in both the development and the regeneration of peripheral nerves. The understanding of the signaling pathways involved in developmental myelination and the Schwann cell response to injury is progressing, but important questions remain unanswered concerning the nature of these signals and their regulation. Future studies will help to illuminate the mechanisms that regulate the remarkable diversity of Schwann cell responses to different signals during development and in regeneration.
Highlights.
Gpr126 and Neuregulin1 signaling coordinately regulate the initiation of myelination
Different mechanisms regulate the initiation, maturation, and maintenance of myelin
c-Jun regulates the Schwann cell response to axonal injury and mediates repair
MAPK signaling has dual roles in both myelination and Wallerian degeneration
Novel signaling mechanisms are emerging that mediate remyelination
Acknowledgments
The authors apologize to colleagues whose work was not referenced due to space limitations. We thank Dave Lyons, and members of the Talbot laboratory for helpful discussions and comments on the manuscript. T.D.G. was supported by the Stanford Developmental Biology and Genetics National Institutes of Health (NIH) Training Grant (5T32 GM007790). Research in the authors’ laboratory was supported by a grant from the NIH [R01 NS050223 to W.S.T.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 2.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 3.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 4.Suter U, Scherer SS. Disease mechanisms in inherited neuropathies. Nat Rev Neurosci. 2003;4:714–726. doi: 10.1038/nrn1196. [DOI] [PubMed] [Google Scholar]
- 5.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 6.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 8.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 9**.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. This paper demonstrates that c-Jun controls the molecular and structural phenotype of Schwann cells following injury to the PNS. Mice containing a Schwann cell-specific deletion of c-Jun had normal developmental myelination, but after injury to peripheral nerves exhibited slow myelin clearance, increased neuronal death, and a failure in axonal regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. The Journal of cell biology. 2012;198:127–141. doi: 10.1083/jcb.201205025. Similar to [14], this study showed that mice lacking c-Jun specifically in Schwann cells exhibited defects in axonal regeneration and increased neuronal death following PNS injury. Additionally, the authors identify GDNF and Artemin as downstream targets of c-Jun, and propse that signaling from Schwann cells to axons mediated by Ret recepeptors is required for normal regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. The authors demonstrate that increased ERK signaling in Schwann cells, caused by the activation of an inducible Raf-kinase transgene, leads to demyelination, Schwann cell dedifferentiation, and recruitment of inflammatory cells to the nerve, despite the absence of axonal injury. The authors suggest that ERK mediated signaling is required for the normal Schwann cell response following injury. [DOI] [PubMed] [Google Scholar]
- 12**.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. This paper shows that overexpression of Nrg1-III, and also Nrg1-I in neurons leads to an increase in the thickness of myelin sheaths during remyelination following injury. Surprisingly, Nrg1-I in Schwann cells is required for normal remyelination, despite having no effect on developmental myelination. [DOI] [PubMed] [Google Scholar]
- 13.Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35:123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bosl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. The Journal of cell biology. 2010;189:701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermingham JR, Jr, Scherer SS, O’Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- 17.Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, Levavasseur F, Raghoenath S, Grosveld F, Meijer D. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17:1380–1391. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, Grosveld F, Meijer D. The POU factor Oct-6 and Schwann cell differentiation. Science. 1996;273:507–510. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- 19.Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 20.Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56:1518–1531. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- 21.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 22.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotter L, Ozcelik M, Jacob C, Pereira JA, Locher V, Baumann R, Relvas JB, Suter U, Tricaud N. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science. 2010;328:1415–1418. doi: 10.1126/science.1187735. [DOI] [PubMed] [Google Scholar]
- 24.Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. The Journal of neuroscience. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman DL, Krols M, Wu LM, Grove M, Nave KA, Gangloff YG, Brophy PJ. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. The Journal of neuroscience. 2012;32:1817–1825. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Peles E, Selbach M, Birchmeier W, Birchmeier C. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci U S A. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. The Journal of neuroscience. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. The authors demonstrate that increased ERK signaling in Schwann cells, caused by the expression of a constitutively active Mek1 transgene, leads to increased myelin sheath thickness in Schwann cells. This result suggests that ERK signaling functions to promote myelination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, Landreth GE, Snider WD. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. This study convincingly shows that ERK signaling is required for normal Schwann cell development and myelination in vivo. Conditional mouse knockouts lacking ERK1/2 in neural crest cells exhibit the complete absence of Schwann cells in peripheral nerves, and mice lacking ERK1/2 in Schwann cells at later stages have abnormally thin myelin sheaths, suggesting that Nrg1-ErbB signaling is impaired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 30*.La Marca R, Cerri F, Horiuchi K, Bachi A, Feltri ML, Wrabetz L, Blobel CP, Quattrini A, Salzer JL, Taveggia C. TACE (ADAM17) inhibits Schwann cell myelination. Nat Neurosci. 2011;14:857–865. doi: 10.1038/nn.2849. The authors demonstrate that mutant mice lacking TACE in neurons exhibited precocious myelination, as well as increased myelin sheath thickess, similar to the phenotype of mice overexpressing neuronal Nrg1-III. They suggest that proteolytic processing of Nrg1-III by TACE functions as a negative regulator of myelination that opposes the positive regulation mediated by BACE1 processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 32.Velanac V, Unterbarnscheidt T, Hinrichs W, Gummert MN, Fischer TM, Rossner MJ, Trimarco A, Brivio V, Taveggia C, Willem M, Haass C, Mobius W, Nave KA, Schwab MH. Bace1 processing of NRG1 type III produces a myelin-inducing signal but is not essential for the stimulation of myelination. Glia. 2012;60:203–217. doi: 10.1002/glia.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, Kremmer E, Tahirovic S, Edbauer D, Lichtenthaler SF, Schmid B, Willem M, Haass C. Dual Cleavage of Neuregulin 1 Type III by BACE1 and ADAM17 Liberates Its EGF-Like Domain and Allows Paracrine Signaling. The Journal of neuroscience. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. The authors identify an additional cleavage site in Nrg1-III mediated by TACE, and suggest that this cleavge liberates a soluble peptide with signaling potential. They further demonstrate that the overexpression of mRNA encoding this fragment functions to positively regulate myelination in zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X, Prior M, He W, Hu X, Tang X, Shen W, Yadav S, Kiryu-Seo S, Miller R, Trapp BD, Yan R. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. The Journal of biological chemistry. 2011;286:23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triolo D, Dina G, Taveggia C, Vaccari I, Porrello E, Rivellini C, Domi T, La Marca R, Cerri F, Bolino A, Quattrini A, Previtali SC. Vimentin regulates peripheral nerve myelination. Development. 2012;139:1359–1367. doi: 10.1242/dev.072371. [DOI] [PubMed] [Google Scholar]
- 36.Lemke G, Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988;102:499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- 37.Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. The Journal of cell biology. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monuki ES, Weinmaster G, Kuhn R, Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989;3:783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- 39.Mandemakers W, Zwart R, Jaegle M, Walbeehm E, Visser P, Grosveld F, Meijer D. A distal Schwann cell-specific enhancer mediates axonal regulation of the Oct-6 transcription factor during peripheral nerve development and regeneration. The EMBO journal. 2000;19:2992–3003. doi: 10.1093/emboj/19.12.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pogoda HM, Sternheim N, Lyons DA, Diamond B, Hawkins TA, Woods IG, Bhatt DH, Franzini-Armstrong C, Dominguez C, Arana N, Jacobs J, Nix R, Fetcho JR, Talbot WS. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev Biol. 2006;298:118–131. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Glenn TD, Talbot WS. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development. 2013;140 doi: 10.1242/dev.093401. [In Press]. This paper shows that Gpr126 signaling is required for the initiation, but not the maturation, of myelin. Transient elevation of cAMP in vivo in gpr126 mutant zebrafish resulted in sustained Schwann cell membrane wrapping and compaction of mature myelin in the absence of Gpr126. The authors further show that activated PKA is sufficient to rescue myelination in gpr126 mutants, while Nrg1-III overexpression is not. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monje PV, Bartlett Bunge M, Wood PM. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia. 2006;53:649–659. doi: 10.1002/glia.20330. [DOI] [PubMed] [Google Scholar]
- 45.Monje PV, Athauda G, Wood PM. Protein kinase A-mediated gating of neuregulin-dependent ErbB2-ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. The Journal of biological chemistry. 2008;283:34087–34100. doi: 10.1074/jbc.M802318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon C, Korade Z, Carter BD. Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-kappaB promotes Schwann cell differentiation into a myelinating phenotype. The Journal of neuroscience. 2008;28:3738–3746. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arthur-Farraj P, Wanek K, Hantke J, Davis CM, Jayakar A, Parkinson DB, Mirsky R, Jessen KR. Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–733. doi: 10.1002/glia.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy P, Topilko P, Schneider-Maunoury S, Seitanidou T, Baron-Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- 49.Bunge RP, Bunge MB. Evidence that contact with connective tissue matrix is required for normal interaction between Schwann cells and nerve fibers. The Journal of cell biology. 1978;78:943–950. doi: 10.1083/jcb.78.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 51.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56:1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 52.Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. Journal of the peripheral nervous system : JPNS. 2005;10:128–143. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- 53.Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci U S A. 2011;108:12925–12930. doi: 10.1073/pnas.1104821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat J-M, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. The Journal of neuroscience. 2006;26:9771–9779. doi: 10.1523/JNEUROSCI.0716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monje PV, Soto J, Bacallao K, Wood PM. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. The Journal of biological chemistry. 2010;285:31024–31036. doi: 10.1074/jbc.M110.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scherer SS, Wang DY, Kuhn R, Lemke G, Wrabetz L, Kamholz J. Axons regulate Schwann cell expression of the POU transcription factor SCIP. The Journal of neuroscience. 1994;14:1930–1942. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The Transcription Factors SCIP and Krox-20 Mark Distinct Stages and Cell Fates in Schwann Cell Differentiation. Mol Cell Neurosci. 1996;8:129–145. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
- 58.Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. The Journal of neuroscience. 2006;26:2124–2131. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, Bennett DL. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. The Journal of neuroscience. 2011;31:3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P, Steele AD, Toyka KV, Nave KA, Weis J, Aguzzi A. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci. 2010;13:310–318. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]
- 61.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166:392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 62.Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. The Journal of cell biology. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutton EJ, Carty L, Laura M, Houlden H, Lunn MP, Brandner S, Mirsky R, Jessen K, Reilly MM. c-Jun expression in human neuropathies: a pilot study. Journal of the peripheral nervous system : JPNS. 2011;16:295–303. doi: 10.1111/j.1529-8027.2011.00360.x. [DOI] [PubMed] [Google Scholar]
- 65.Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. The EMBO journal. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. The Journal of neuroscience. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, Svaren J, Casaccia P. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci. 2010;13:1472–1480. doi: 10.1038/nn.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. The Journal of neuroscience. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newbern JM, Snider WD. Bers-ERK Schwann cells coordinate nerve regeneration. Neuron. 2012;73:623–626. doi: 10.1016/j.neuron.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Schroder JM. Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res. 1972;45:49–65. doi: 10.1016/0006-8993(72)90215-6. [DOI] [PubMed] [Google Scholar]
- 71.Jagalur NB, Ghazvini M, Mandemakers W, Driegen S, Maas A, Jones EA, Jaegle M, Grosveld F, Svaren J, Meijer D. Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. The Journal of neuroscience. 2011;31:8585–8594. doi: 10.1523/JNEUROSCI.0659-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghislain J, Charnay P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO reports. 2006;7:52–58. doi: 10.1038/sj.embor.7400573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bremer M, Frob F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022–1032. doi: 10.1002/glia.21173. [DOI] [PubMed] [Google Scholar]