Abstract
Background
Type 2 diabetes may increase the risk of amnestic mild cognitive impairment (aMCI) through Alzheimer's disease (AD)-related and vascular pathology and may also increase the risk of nonamnestic MCI (naMCI) through vascular disease mechanisms. We examined the association of type 2 diabetes with mild cognitive impairment (MCI) and MCI subtype (aMCI and naMCI) overall and by sex.
Methods
Participants were Olmsted County, Minnesota residents (70 years and older) enrolled in a prospective, population-based study. At baseline and every 15 months thereafter, participants were evaluated using the Clinical Dementia Rating scale, a neurological evaluation, and neuropsychological testing for a diagnosis of normal cognition, MCI, and dementia by a consensus panel. Type 2 diabetes was ascertained from the medical records of participants at baseline.
Results
Over a median 4.0 years of follow-up, 348 of 1450 subjects developed MCI. Type 2 diabetes was associated (hazard ratio [95% confidence interval]) with MCI (1.39 [1.08–1.79]), aMCI (1.58 [1.17–2.15]; multiple domain: 1.58 [1.01–2.47]; single domain: 1.49 [1.09–2.05]), and the hazard ratio for naMCI was elevated (1.37 [0.84–2.24]). Diabetes was strongly associated with multiple-domain aMCI in men (2.42 [1.31–4.48]) and an elevated risk of multiple domain naMCI in men (2.11 [0.70–6.33]), and with single domain naMCI in women (2.32 [1.04–5.20]).
Conclusions
Diabetes was associated with an increased risk of MCI in elderly persons. The association of diabetes with MCI may vary with subtype, number of domains, and sex. Prevention and control of diabetes may reduce the risk of MCI and Alzheimer's disease.
Keywords: Mild cognitive impairment, Risk factors, Type 2 diabetes, Incidence, Cohort studies, Population-based studies, Sex differences, Diabetic retinopathy, Diabetic neuropathy
1. Introduction
Several studies have reported associations of type 2 diabetes mellitus with an increased risk of cognitive impairment and dementia [1–9], including Alzheimer's disease (AD) [8,10,11] and vascular dementia [11,12]. These studies suggest that type 2 diabetes may also be associated with mild cognitive impairment (MCI) subtypes: with amnestic MCI (aMCI) through both AD and vascular pathology, and with nonamnestic MCI (naMCI) through vascular disease mechanisms [2]. Despite this, there are few population-based studies on associations of type 2 diabetes with incident MCI subtypes.
Relationships between risk factors and cognitive impairment may differ based on study of subjects with incident versus prevalent cognitive impairment. The study of incident cases of MCI establishes a temporal association, includes a broad spectrum of disease severity, and may represent a progressive disorder. In contrast, prevalent cases may include more slowly progressive cases and may be influenced by survival bias. Furthermore, the identification of MCI subtypes on the basis of cognitive profiles may offer additional insights regarding severity because MCI subtypes reflect the extent of regional cortical involvement and the underlying etiology of the MCI. Single-domain MCI syndromes are likely to represent more circumscribed pathology, whereas multidomain MCI may represent more extensive disease. Amnestic presentations of MCI are more likely to be due to AD pathophysiology, whereas naMCI probably includes non-AD type conditions, especially cerebrovascular disease. Because of the pressing unanswered questions about the role of diabetes in dementing illness in regard to cerebrovascular versus AD pathways, the study of associations of type 2 diabetes with incident MCI subtypes and number of domains affected offers a novel approach to the mechanisms of diabetes in cognitive impairment and the impact of disease extent.
Previous studies have reported a sexual dimorphism in the occurrence of dementia, for AD in particular, with higher estimates in women than in men [13–15]. More recently, we and others have reported a sexual dimorphism in incidence and prevalence of MCI, but with higher estimates in men than in women [16–20]. Some imaging studies have also reported sex differences in brain aging that may partly explain the apparent discordance in the sexual dimorphism in the occurrence of dementia versus MCI [21–24]. Together, these studies and another that reported sex differences in inflammatory markers in men and women [25] suggest that risk factors for MCI vary in men and women and underscore the need to identify modifiable risk factors that have a differential impact on risk of MCI in men versus women. Therefore, the objective of this study was to investigate the association of type 2 diabetes mellitus with MCI and MCI subtypes overall, and by sex, in a population-based, prospective cohort enrolled in the Mayo Clinic Study of Aging.
2. Methods
2.1. Study cohort
We established the Mayo Clinic Study of Aging to estimate the incidence and identify risk factors for MCI in Olmsted County, MN. Details of the study design and participant recruitment are described in detail elsewhere [16, 17, 26]. In brief, we used the medical records-linkage system of the Rochester Epidemiology Project to construct a sampling frame of Olmsted County residents who were aged 70 to 89 years on October 1, 2004 (n = 9953) [27]. From an age- and sex-stratified random sample of 5233 subjects, 2719 (61.8%) of 4398 eligible subjects agreed to participate in the baseline assessment either in person (n = 2050; 46.6%; full participants) or by telephone (n = 669; 15.2%; telephone-only participants) [17, 26].
The institutional review boards of the Mayo Clinic and of Olmsted Medical Center approved the study. Written informed consent was obtained for all participants who were examined as part of the study.
2.2. Clinical measurements
2.2.1. In-person evaluations
Each subject was evaluated by a nurse or study coordinator as well as a physician and underwent extensive cognitive testing by a psychometrist. The nurse interview included questions about memory administered to the participant, and the Clinical Dementia Rating scale and the Functional Activities Questionnaire were administered to an informant. The neurological evaluation included the Short Test of Mental Status [28], a medical history review, and a neurological examination. The cognitive testing used nine tests to assess function in four cognitive domains: memory, executive function, language, and visuospatial skills. The raw scores on each test were transformed into age-adjusted scores using normative data from Mayo's Older Americans Normative Studies and were scaled to have a mean of 10 and a standard deviation (SD) of 3 [29]. Domain scores were computed by summing the adjusted and scaled test scores within a domain and scaling again to allow comparisons across domains [17,26].
2.2.2. Diagnostic criteria for MCI
Performance in a specific cognitive domain was assessed by comparing the domain score with the score (means and SD) for normal subjects from the Olmsted County population [29]. Cognitive impairment was considered if the score was ≥1.0 SD below the mean; however, a final decision about impairment was based on a consensus agreement among the examining physician, nurse, and neuropsychologist, taking into account education, prior occupation, visual or hearing deficits, and other information [16,17,26].
A diagnosis of MCI was based on published criteria: cognitive concern by subject, informant, nurse, or physician; impairment in one or more of the four cognitive domains (from cognitive battery); essentially normal functional activities; and absence of dementia [17,26,30]. Subjects with MCI were classified as having aMCI if the memory domain was impaired; naMCI if the memory domain was not impaired, but one or more nonmemory domains were impaired; and as having single- versus multiple-domain MCI. A diagnosis of dementia was based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria. Subjects who performed within the normative range and did not meet criteria for MCI or dementia were considered to be cognitively normal [17,26,30].
2.2.3. Longitudinal follow-up
Participants were evaluated at 15-month intervals using the same protocol for clinical and cognitive findings as was used for full participants at baseline. Information from previous evaluations was not considered in making a diagnosis during follow-up. Participants who declined in-person evaluation at follow-up were invited to participate via a telephone interview (partial participants) that included the Telephone Interview of Cognitive Statusmodified, (TICS-m), the Clinical Dementia Rating scale, and the Neuropsychiatric Inventory Questionnaire. The MCI subtype could not be determined in partial participants who developed MCI because they did not complete the extensive cognitive testing.
2.3. Assessment of diabetes and covariates
Information about type 2 diabetes was ascertained from the medical records archived by the records-linkage system of the Rochester Epidemiology Project [27]. Diabetes was defined as any of the following: treatment for diabetes (oral antidiabetic agents, insulin, or both), a fasting blood glucose ≥ 126 mg/dL reported two or more times, or a physician diagnosis of diabetes [31]. A physician diagnosis of diabetes-related complications such as diabetic neuropathy, diabetic retinopathy, or diabetic nephropathy (not attributed to hypertension) was also ascertained from the medical records at baseline.
Demographic characteristics including date of birth, number of years of education, and smoking history were assessed by interview. History of hypertension or coronary artery disease at baseline was abstracted from a review of the medical records. History of stroke was obtained by the physician and validated using the medical records when possible. Depression was assessed using the Beck Depression Inventory II. Daily medications were assessed from a review of the medications brought to each evaluation. Dyslipidemia at baseline was defined as cholesterol >200 mg/dL, low high-density lipoprotein (<40 mg/dL in men or <50 mg/dL in women), triglycerides >150 mg/dL, or treatment to lower lipid levels. Frequency of moderate exercise in the previous year was assessed by questionnaire [32]. Body mass index (BMI) was measured by direct exam, and APOE genotyping was performed at the baseline evaluation.
2.4. Statistical analyses
Persons who were cognitively normal at baseline were considered at risk for incident MCI. The onset of MCI was defined by the midpoint between the last assessment as cognitively normal and the first-ever assessment as MCI; 18 subjects who developed dementia without an intervening diagnosis of MCI are not included in these analyses. Subjects who refused participation, could not be contacted, or died during follow-up were censored at their last evaluation. We computed the person-years of follow-up as the time from the baseline evaluation to onset of MCI, censoring, or date of last follow-up. Our analyses included only first-ever MCI diagnoses and did not consider subjects who reverted to normal after an initial diagnosis of MCI.
We estimated incidence rates by history of diabetes using incidence density methods (cases per 1000 person-years). The incidence rates were directly standardized by age and sex to the Olmsted County population on October 1, 2004, and adjusted for nonparticipation at baseline using reciprocal probability weighting in Poisson regression models [33]. In our primary analyses, we used multivariable Cox proportional hazards models with age as the time variable to assess associations (hazard ratios [HR] and 95% confidence intervals [CI]) of diabetes with incident MCI and with MCI subtypes, thus taking into account differential follow-up. In the base model (Model 1), we adjusted for sex, years of education (≤12 vs. >12), and nonparticipation at baseline using reciprocal probability weighting [33]. In Model 2, we also adjusted for APOE ε4 genotype (any ε4 vs. no ε4; we excluded ε2/ε4) and for potential confounders: obesity (BMI), hypertension, coronary artery disease, stroke, dyslipidemia, use of statins, moderate exercise (≤1 vs. >1 per month), and depression. In Model 3, we included Model 2 variables, but we excluded subjects with a history of stroke because of the strong association of stroke with cognitive impairment. We examined the interaction of diabetes with age at baseline and sex in regard to MCI. To assess the impact of disease severity, we conducted stratified analyses by level of glycemic control (hemoglobin A1c [HbA1c7] <7% vs. ≥7%), type of treatment for diabetes (no treatment or diet only, oral hypoglycemic agents, insulin treatment with or without oral treatment), duration of disease (dichotomized at the median), and presence of diabetes-related complications. These analyses were restricted to in-person participants at baseline; partial participants during follow-up (n = 24) are not included in the analyses for aMCI and naMCI because their MCI subtype could not be determined.
In separate analyses, we censored partial participants from the analyses for any MCI because incident diagnoses were only assessed by telephone. We used time-dependent covariate analyses to take into account subjects with new diagnoses of type 2 diabetes since enrollment. We also compared the frequencies of subjects who died or were lost to follow-up by diabetes and by sex.
3. Results
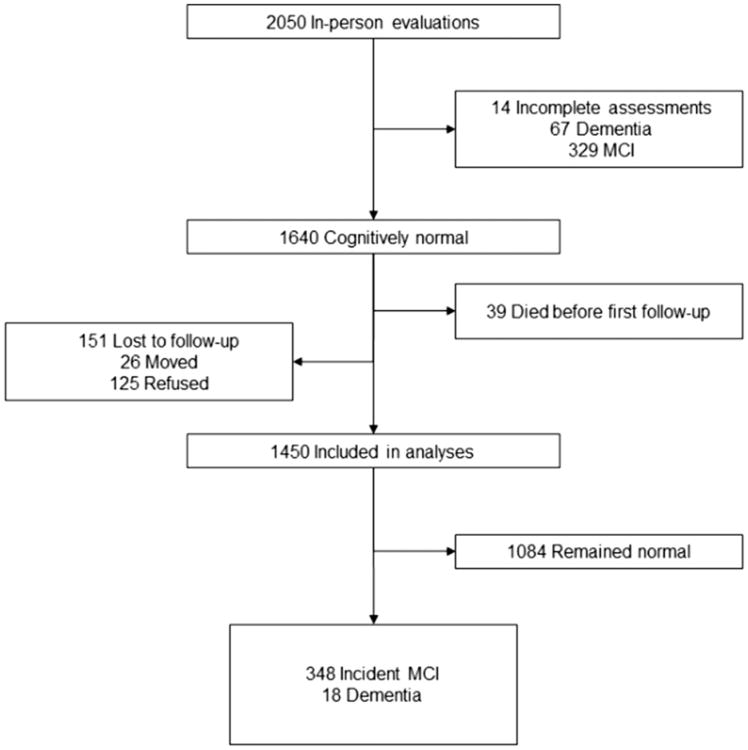
Figure 1 describes the study flow chart. Of the 1640 who were cognitively normal at baseline, 39 died and 151 were lost to follow-up (125 refused after the baseline evaluation and 26 moved away), and 1450 had at least one follow-up. Subjects lost to follow-up had lower education than subjects with one or more follow-up (55.0% vs. 43.2% had ≤ 12 years of education; P = .006); however, they were similar in sex, age, and history of stroke. The number of follow-up evaluations was 4 in 628 subjects, 3 in 441, 2 in 213, and 1 in 168 subjects.
Fig. 1.
Flow chart of study participants: 231 had aMCI, and 93 had naMCI. The clinical subtype of MCI could not be determined for 24 subjects. Subjects with incident dementia are not included in the analyses.
Table 1 describes the characteristics of subjects by diabetes at baseline. Subjects with diabetes were more often men and had higher frequencies of obesity (BMI ≥ 30 kg/m2), hypertension, dyslipidemia, use of statins, and coronary artery disease compared with subjects without diabetes, but they did not differ in age, education, APOE ε4 genotype, or history of depression.
Table 1. Characteristics of subjects with and without type 2 diabetes at baseline.
| Variable | Total N = 1450, n % | With type 2 diabetes N = 248, n % | Without type 2 diabetes N = 1202, n % | P |
|---|---|---|---|---|
| Age* | 79.3 (74.9, 83.4) | 79.3 (74.5, 83.1) | 79.4 (75.0, 83.5) | .52 |
| 70–79 | 766 (52.8) | 132 (53.2) | 634 (52.7) | .89 |
| 80–89 | 684 (47.2) | 116 (46.8) | 568 (47.3) | |
| Sex | ||||
| Women | 728 (50.2) | 109 (44.0) | 619 (51.5) | .03 |
| Men | 722 (49.8) | 139 (56.0) | 583 (48.5) | |
| Education, years† | ||||
| ≤12 | 621 (42.8) | 114 (46.0) | 507 (42.2) | .27 |
| >12 | 829 (57.2) | 134 (54.0) | 695 (57.8) | |
| BMI ≥30 kg/m2‡ | 393 (27.6) | 110 (45.1) | 283 (24.0) | <.0001 |
| Hypertension | 1100 (75.9) | 226 (91.1) | 874 (72.7) | <.0001 |
| Dyslipidemia | 1122 (77.4) | 220 (88.7) | 902 (75.0) | <.0001 |
| Use of statins | 658 (45.4) | 156 (62.9) | 502 (41.8) | <.0001 |
| Current smoking | 51 (3.5) | 4.0 (1.6) | 47 (3.9) | .07 |
| Stroke | 138 (9.5) | 26 (10.5) | 112 (9.3) | .57 |
| Coronary artery disease | 589 (40.6) | 140 (56.5) | 449 (37.4) | <.0001 |
| APOE ε4 genotype§ | ||||
| ε2/ε2, ε2/ε3, ε3/ε3 | 1089 (75.5) | 193 (78.5) | 896 (74.9) | .48 |
| ε3/ε4, ε4/ε4 | 317 (22.0) | 47 (19.1) | 270 (22.6) | |
| ε2/ε4 | 37 (2.6) | 6 (2.4) | 31 (2.6) | |
| Depression¶ | 85 (6.1) | 16 (6.7) | 69 (5.9) | .66 |
Age at baseline visit, median (25th, 75th percentiles).
Median (25th, 75th percentiles): 13 (12, 16) overall, 13 (12, 16) in men, and 13 (12,16) in women (P = .41).
26 subjects had missing data: 4 with diabetes and 22 without diabetes.
7 subjects had missing data: 2 with diabetes and 5 without diabetes.
50 subjects had missing data: 9 with diabetes and 41 without diabetes.
Over a median follow-up of 4.0 years (interquartile range 2.5–5.1; 5351.1 person-years), 348 subjects developed incident MCI (Fig. 1). The median (25th, 75th percentile) duration of follow-up was 3.8 (1.9, 5.1) years in subjects with diabetes and 4.1 (2.6, 5.2) years in subjects who did not have diabetes at baseline. The present manuscript is based on a longer duration of follow-up of the cohort, and thus on a higher number of incident events, than our recently published manuscript on MCI incidence [16]. The incidence (per 1000 person years) of MCI, standardized to the Olmsted County population in men and women combined, was higher in persons with diabetes (83.6) than in persons without diabetes (60.2). Among subjects with diabetes, the incidence was higher in men than in women (105.2 vs. 68.2 in women) for MCI and for aMCI (74.2 vs. 44.1 in women), but it was similar for naMCI (25.2 vs. 21.8 in women).
Table 2 shows cohort analyses for diabetes and MCI. Diabetes was significantly associated with an increased risk of any MCI and with aMCI in men and women combined, and in men considered separately, but it was not associated with naMCI. The HR for naMCI was nonsignificantly elevated 1.7-fold in women, but the adjusted analyses suggested confounding by stroke. There was suggestion of a stronger effect of diabetes on the risk of MCI in younger subjects (P for interaction = .10 for MCI; P for interaction = .02 for aMCI) and in men (P for interaction = .17; data not presented).
Table 2. Association of type 2 diabetes with incident MCI and with MCI subtypes by sex.
| Full sample | Restricted sample | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Number of subjects n | Number of MCI events n (%) | Model 1* | Model 2† | Model 3‡ | ||||
|
|
|
|
||||||
| MCI Outcome | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Any MCI | ||||||||
| Men | ||||||||
| Diabetes No | 583 | 134 (23.0) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 139 | 47 (33.8) | 1.64 (1.18–2.28) | .004 | 1.69 (1.16–2.46) | .007 | 2.05 (1.39–3.03) | .0003 |
| Women | ||||||||
| Diabetes No | 619 | 138 (22.3) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 109 | 29 (26.6) | 1.12 (0.75–1.69) | .58 | 1.18 (0.73–1.92) | .50 | 1.06 (0.62–1.78) | .84 |
| Both sexes | ||||||||
| Diabetes No | 1202 | 272 (22.6) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 248 | 76 (30.6) | 1.39 (1.08–1.79) | .01 | 1.42 (1.06–1.91) | .02 | 1.58 (1.16–2.15) | .004 |
| aMCI§ | ||||||||
| Men | ||||||||
| Diabetes No | 583 | 88 (15.1) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 139 | 33 (23.7) | 1.91 (1.29–2.84) | .001 | 1.87 (1.19–2.93) | .007 | 2.23 (1.40–3.55) | .0007 |
| Women | ||||||||
| Diabetes No | 619 | 90 (14.5) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 109 | 20 (18.3) | 1.23 (0.75–2.01) | .42 | 1.35 (0.76–2.41) | .31 | 1.19 (0.63–2.23) | .60 |
| Both sexes | ||||||||
| Diabetes No | 1202 | 178 (14.8) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 248 | 53 (21.4) | 1.58 (1.17–2.15) | .003 | 1.58 (1.12–2.25) | .01 | 1.73 (1.20–2.50) | .003 |
| naMCI§ | ||||||||
| Men | ||||||||
| Diabetes No | 583 | 41 (7.0) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 139 | 13 (9.4) | 1.24 (0.66–2.34) | .50 | 1.38 (0.67–2.88) | .38 | 1.68 (0.78–3.60) | .18 |
| Women | ||||||||
| Diabetes No | 619 | 30 (4.8) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 109 | 9 (8.3) | 1.68 (0.78–3.62) | .19 | 1.17 (0.46–2.95) | .74 | 1.10 (0.41–2.98) | .85 |
| Both sexes | ||||||||
| Diabetes No | 1202 | 71 (5.9) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Yes | 248 | 22 (8.9) | 1.37 (0.84–2.24) | .20 | 1.28 (0.72–2.25) | .40 | 1.44 (0.79–2.61) | .23 |
Model 1 is adjusted for sex and years of education (≤12 vs >12).
Model 2 includes Model 1 variables with additional adjustment for APOE ε4 genotype, hypertension, obesity, depression, statin use, moderate exercise, coronary artery disease, dyslipidemia, and stroke.
Model 3 includes Model 2 variables with exclusion of subjects with a history of stroke (n = 108).
Clinical subtype of MCI could not be determined for 24 subjects.
The association of diabetes with MCI varied by number of domains and by sex (Table 3). The associations were stronger for multiple-domain aMCI (MDaMCI) than for single domain aMCI (SDaMCI) in men and women combined, and in men. In men, diabetes was strongly associated with MDaMCI and the risk for multiple-domain naMCI (MDnaMCI) was nonsignificantly elevated 2-fold. In women, diabetes was strongly associated with single-domain naMCI (SDnaMCI), and the risk of SDaMCI was nonsignificantly elevated.
Table 3. Association of type 2 diabetes with MCI subtype, number of domains, and sex.
| MCI Outcome | Number | Events, n (%) | HR (95% CI)* | P |
|---|---|---|---|---|
| SDaMCI | ||||
| Men | 722 | 77 | ||
| Diabetes no | 583 | 58 (9.9) | 1.00 (reference) | |
| Yes | 139 | 19 (13.7) | 1.63 (0.97–2.75) | .07 |
| Women | 728 | 77 | ||
| Diabetes no | 619 | 62 (10.0) | 1.00 (reference) | |
| Yes | 109 | 15 (13.8) | 1.45 (0.81–2.58) | .21 |
| Both sexes | 1450 | 154 | ||
| Diabetes no | 1202 | 120 (10.0) | 1.00 (reference) | |
| Yes | 248 | 34 (13.7) | 1.53 (1.04–2.25) | .03 |
| MDaMCI | ||||
| Men | 722 | 44 | ||
| Diabetes no | 583 | 30 (5.1) | 1.00 (reference) | |
| Yes | 139 | 14 (10.1) | 2.42 (1.31–4.48) | .005 |
| Women | 728 | 33 | ||
| Diabetes no | 619 | 28 (4.5) | 1.00 (reference) | |
| Yes | 109 | 5 (4.6) | 0.84 (0.32–2.18) | .71 |
| Both | 1450 | 77 | ||
| No diabetes | 1202 | 58 (4.8) | 1.00 (reference) | |
| Diabetes | 248 | 19 (7.7) | 1.68 (1.01–2.77) | .04 |
| SDnaMCI | ||||
| Men | 722 | 41 | ||
| Diabetes no | 583 | 33 (5.7) | 1.00 (reference) | |
| Yes | 139 | 8 (5.8) | 0.99 (0.45–2.18) | .98 |
| Women | 728 | 32 | ||
| Diabetes no | 619 | 23 (3.7) | 1.00 (reference) | |
| Yes | 109 | 9 (8.3) | 2.32 (1.04–5.20) | .04 |
| Both sexes | 1450 | 73 | ||
| Diabetes no | 1202 | 56 (4.7) | 1.00 (reference) | |
| Yes | 248 | 17 (6.9) | 1.41 (0.81–2.46) | .23 |
| MDnaMCI | ||||
| Men | 722 | 13 | ||
| Diabetes no | 583 | 8 (1.4) | 1.00 (reference) | |
| Yes | 139 | 5 (3.6) | 2.11 (0.70–6.33) | .18 |
| Women | 728 | 7 | ||
| Diabetes no | 619 | 7 (1.1) | ||
| Yes | 109 | 0 (0) | – | – |
| Both sexes | 1450 | 20 | ||
| Diabetes no | 1202 | 15 (1.2) | 1.00 (reference) | |
| Yes | 248 | 5 (2.0) | 1.26 (0.46–3.48) | .65 |
HR (95% CI), adjusted for age, sex, and education. Estimates were 1.58 (1.01-2.47) for MDMCI and 1.49 (1.09-2.05) for SDMCI.
Table 4 shows associations of diabetes-related measures with MCI. Early age at diagnosis of diabetes, longer duration of diabetes, and worse glycemic control were associated with increased risk. The frequency of subjects with diabetes for longer than 15 years was higher in men than in women (27% vs. 21% of women; P =.03); men had a trend toward earlier onset of diabetes before age 70 years (56% of men vs. 46% of women P =.10). There was a dose response association with type of treatment for diabetes (P for trend = .004 for Model 1). Diabetic retinopathy and peripheral neuropathy (Model 3) were associated with an increased risk. In subtype analyses, similar associations were observed for aMCI, and insulin use was associated with risk of naMCI (2.74 [1.31–5.73]; other data are not presented).
Table 4. Clinical characteristics of type 2 diabetes and risk of MCI.
| Full sample | Restricted sample | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Number of subjects n | Number of MCI events n (%) | Model 1* | Model 2† | Model 3‡ | ||||
|
|
|
|
||||||
| Clinical characteristic | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| No diabetes | 1202 | 272 (22.6) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Diabetes onset, years | ||||||||
| >65 | 175 | 52 (29.7) | 1.23 (0.92–1.66) | .17 | 1.24 (0.88–1.74) | .22 | 1.24 (0.86–1.78) | .24 |
| ≤65 | 73 | 24 (32.9) | 1.94 (1.27–2.97) | .002 | 2.10 (1.31–3.36) | .002 | 3.21 (1.98–5.19) | ,.0001 |
| Disease duration, years | ||||||||
| ≤7.94 (median) | 124 | 36 (29.0) | 1.32 (0.93–1.86) | .12 | 1.43 (0.97–2.09) | .07 | 1.45 (0.97–2.16) | .07 |
| >7.94 (median) | 124 | 40 (32.3) | 1.47 (1.05–2.05) | .03 | 1.42 (0.96–2.10) | .08 | 1.74 (1.16–2.62) | .008 |
| Glycemic control | ||||||||
| HbA1c < 7% | 183 | 52 (28.4) | 1.25 (0.93–1.68) | .14 | 1.27 (0.90–1.78) | .17 | 1.36 (0.95–1.93) | .09 |
| HbA1c ≤ 7% | 47 | 18 (38.3) | 1.76 (1.08–2.87) | .02 | 1.76 (1.03–3.00) | .04 | 2.40 (1.38–4.20) | .002 |
| Diabetes treatment | ||||||||
| No treatment | 73 | 16 (21.9) | 0.93 (0.56–1.55) | .79 | 0.91 (0.50–1.67) | .77 | 0.83 (0.43–1.61) | .58 |
| Oral antidiabetic | 116 | 39 (33.6) | 1.44 (1.03–2.02) | .03 | 1.44 (0.98–2.11) | .06 | 1.71 (1.16–2.54) | .007 |
| Insulin | 59 | 21 (35.6) | 2.01 (1.28–3.15) | .002 | 2.17 (1.32–3.55) | .002 | 2.51 (1.49–4.21) | .0005 |
| Retinopathy | 37 | 14 (37.8) | 1.77 (1.02–3.05) | .04 | 1.92 (1.05–3.49) | .03 | 2.37 (1.28–4.39) | .006 |
| Neuropathy | 78 | 29 (37.2) | 1.44 (0.98–2.12) | .07 | 1.48 (0.96–2.28) | .08 | 1.61 (1.02–2.55) | .04 |
| Nephropathy§ | 18 | 5 (27.8) | 1.09(0.45–2.66) | .85 | 0.92 (0.34–2.49) | .88 | 1.57 (0.58–4.23) | .37 |
Model 1 is adjusted for sex and years of education (≤12 vs >.12). The reference group for all analyses is subjects without type 2 diabetes.
Model 2 includes Model 1 variables with additional adjustment for APOE ε4 genotype, hypertension, obesity, depression, statin use, moderate exercise, coronary artery disease, dyslipidemia, and stroke.
Model 3 includes Model 2 variables with exclusion of subjects with a history of stroke (n = 108).
Diabetic nephropathy does not include subjects with nephropathy that could be attributed to hypertension.
The HR (95% CI) for any MCI was stronger when partial participants were censored (1.52 [1.17–1.97]). When subjects with new onset of diabetes were characterized as exposed (n = 47), the risk of MCI remained elevated (HR, 1.35 [1.06-1.72]). There was no suggestion of bias due to differential mortality or loss to follow-up by diabetes and or by sex. Among all losses to follow-up including deaths (n = 335), the frequency of diabetes was 10% in men vs. 11% in women (P = .51).
4. Discussion
In this elderly population-based cohort, type 2 diabetes was associated with an increased risk of MCI. Diabetes was associated with a stronger risk of aMCI and MDaMCI in men than in women, a 2-fold increased risk of MDnaMCI in men, and with a strong association of SDnaMCI in women. The stronger association with MDaMCI than with SDaMCI is consistent with greater extent of underlying pathology with multiple-domain clinical presentations. The stronger association with male sex provides mechanistic insights on the higher incidence and prevalence of MCI among men. Diabetes severity assessed as duration of disease, glycemic control, type of treatment, and presence of complications was associated with greater risk of MCI, consistent with our prevalence studies [34]. The association of diabetes with MCI has important public health implications given the increasing incidence of diabetes in the U.S. population. Our findings suggest that focused strategies to prevent diabetes may reduce the risk of late-life MCI and dementia.
The strong association of diabetes with MCI in men may be due to an earlier age at diagnosis of diabetes, longer duration, and a higher frequency of diabetes in men. Indeed, men in our study had an earlier onset and longer duration of diabetes than women, and we observed a higher frequency of diabetes in men than in women ages 70 to 79 years at enrollment (20.3% vs 13.6% in women; P =.02), but no difference at ages 80 to 89 (17.9% in men vs 16.2% in women, P = .57). We also observed a trend toward a stronger effect of diabetes at younger ages that was significant for aMCI and stronger associations with earlier age at onset and duration of diabetes. The 2-fold increased risk for MDaMCI and MDnaMCI in men with diabetes may partly explain the higher incidence and prevalence of MCI in men observed in our cohort [16,17]. Our secondary analyses did not suggest possible bias by differences in mortality or losses to follow-up in men and women overall or by diabetes.
The combined effects of diabetes on degenerative and cerebrovascular disease may accelerate onset of MCI. Several neurodegenerative mechanisms have been proposed for the association of diabetes with MCI. The hippocampus, entorhinal formation, and frontal cortex are potential target regions in the brain that are known to have insulin receptors through which insulin-related effects may affect cognitive function [35]. Diabetes may adversely affect amyloid processing and accumulation in target regions through effects of insulin resistance and hyperinsulinemia, dysregulation of brain insulin signaling, impaired central and peripheral glucose homeostasis [36], insulin degrading enzyme, and advanced glycation endproducts (AGEs) [7,37]. Insulin resistance and hyperinsulinemia increase brain intraneuronal β-amyloid deposition and hyperphosphorylation of tau [36]. Brain function depends on insulin sensitivity; consequently, dysregulation of brain insulin signaling in diabetes may lead to impaired central and peripheral glucose homeostasis and neurodegeneration. Impaired insulin homeostasis may increase brain β-amyloidosis through competition of insulin with β-amyloid for insulin-degrading enzyme binding sites, leading to decreased β-amyloid clearance [36,38]. Peripheral hyperinsulinemia may lead to decreased bioavailability of brain insulin, with several central effects that include downregulation of brain insulin uptake, increased intraneuronal β0-amyloid accumulation, decreased β-amyloid clearance in the brain, decreased insulin degrading enzyme, and increased cerebrovascular endothelial inflammation, all of which contribute to neurodegeneration [36]. Chronic hyperglycemia in type 2 diabetes increases production of AGEs; activation of the receptor for AGE (RAGE) leads to cyclical nuclear factor-κβ activation, production of reactive oxygen species, and upregulation of AGE and RAGE that lead to diabetes-associated neurovascular damage [39].
Diabetes is also associated with abnormalities in several structural magnetic resonance imaging markers that are indicative of neurodegenerative or vascular damage. These abnormalities include accelerated hippocampal atrophy, reduction in whole brain volume, and increased white matter hyperintensity volumes [1,36,40]. Autopsy studies have shown a greater burden of neuritic plaques and neurofibrillary tangles in key regions in the brains of diabetics [4]. Finally, small and large vessel cerebrovascular disease may contribute to the risk of aMCI and naMCI [4,12,38,41,42].
Our unpublished findings have also demonstrated strong associations of diabetes onset in midlife and in late life with imaging abnormalities that include reduced hippocampal and whole brain volumes suggesting neurodegenerative disease mechanisms and with increased risk of cortical and subcortical infarctions and increased white matter hyperintensity volume suggesting vascular disease mechanisms. In general, the effects were greater in subjects with onset of diabetes in midlife than in late life (unpublished data presented at the 2012 Alzheimer's Association International Conference, Vancouver, BC, Canada).
The association of diabetes with aMCI in our study suggests that diabetes may contribute to a diagnosis of MCI due to AD [43]. This is consistent with the association of diabetes with accelerated hippocampal atrophy [1,40] and with the high attributable risk of diabetes for AD [10]. We may have failed to observe a significant association of diabetes with any naMCI and may have underestimated the effect because subjects with MDaMCI who also have nonmemory impairment are characterized as aMCI and are not included in analyses for naMCI; indeed, 33% of subjects diagnosed with aMCI in our study also had impairment in nonmemory domains. The association of diabetes with SDnaMCI in women, but not with aMCI, compared with the 2-fold increased risk of diabetes with MDaMCI and MDnaMCI in men is consistent with the earlier age at onset or longer duration of diabetes in men versus women that may contribute to degenerative and cerebrovascular disease mechanisms and accelerate onset and severity of MCI in men.
Our findings are consistent with several studies on diabetes and cognitive decline or MCI. Diabetes was associated with cognitive decline in a multicenter study of community-dwelling elders in France [44], in a multinational European study [45], and in a clinical trial [46]. Other prospective studies have demonstrated associations of diabetes with increased risk of MCI or cognitive decline [7,47], and increased risk of aMCI and naMCI, with a stronger association with aMCI in one study [2]. Other investigators have reported cognitive decline in women with diabetes and a stronger association with disease of longer duration and with insulin treatment [5,7,48]. Indeed, the risk of SDnaMCI was significantly elevated in women with diabetes in the present study.
However, one study did not find an association of diabetes with incident MCI, but it found an increased risk with MCI progression to dementia [49]. Other investigators have reported a greater risk of dementia in persons with diabetes and APOE ε4 allele [4,50]. In the present study, there were too few subjects with an APOE ε4 allele and diabetes to meaningfully assess the interaction.
A potential limitation of our study is that nonparticipants at baseline were more likely to be older, men, have greater comorbidity, and a higher frequency of diabetes [26]. However, under-representation of men and subjects with diabetes at baseline suggests that the HRs in our study are underestimated. Although we adjusted for nonparticipation using propensity scores, there could still be residual bias. The Olmsted County population is predominantly of Northern European ancestry, and our findings may not apply to other ethnic groups.
Our study has several strengths. The study was specifically designed to study risk factors for MCI, risk factors were assessed at baseline, and the diagnosis of MCI was made using published criteria. The comprehensive cognitive evaluation by 3 independent evaluators who were kept unaware of previous diagnoses enhanced the validity of the MCI diagnoses. The internal validity of our findings was enhanced by the use of the medical records-linkage system of the Rochester Epidemiology Project to ascertain a history of diabetes and covariates and to adjust for potential participation bias using propensity scores. The population-based design reduced the potential for selection and volunteer bias and enhanced the generalizability of the findings. The prospective design allowed us to assess causal inferences.
Acknowledgments
The study was supported by funding from the National Institutes of Health U01 AG006786, P50 AG016574, K01 AG028573, K01 MH068351, and R01 AG034676; the Robert Wood Johnson Foundation (Harold Amos Program); and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program. The authors have no conflicts of interest to disclose.
References
- 1.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–5. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 3.Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33:355–61. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- 4.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77:1351–6. doi: 10.1212/WNL.0b013e3182315a56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–63. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 9.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 10.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 12.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 13.von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol. 1999;56:587–92. doi: 10.1001/archneur.56.5.587. [DOI] [PubMed] [Google Scholar]
- 14.Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S10–5. [PubMed] [Google Scholar]
- 15.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JRM, et al. Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–7. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology. 2012;78:342–51. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, et al. Risk profiles of subtypes of mild cognitive impairment: the Sydney Memory and Ageing Study. J Am Geriatr Soc. 2012;60:24–33. doi: 10.1111/j.1532-5415.2011.03774.x. [DOI] [PubMed] [Google Scholar]
- 19.Sosa AL, Albanese E, Stephan BC, Dewey M, Acosta D, Ferri CP, et al. Prevalence, distribution, and impact of mild cognitive impairment in Latin America, China, and India: a 10/66 population-based study. PLoS Med. 2012;9:e1001170. doi: 10.1371/journal.pmed.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol Scand. 2002;106:148–54. doi: 10.1034/j.1600-0404.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 21.Skup M, Zhu H, Wang Y, Giovanello KS, Lin JA, Shen D, et al. Sex differences in grey matter atrophy patterns among AD and aMCI patients: results from ADNI. NeuroImage. 2011;56:890–906. doi: 10.1016/j.neuroimage.2011.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Jr, Weiner MW, et al. Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiol Aging. 2010;31:1463–80. doi: 10.1016/j.neurobiolaging.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–96. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 24.Kaye JA, DeCarli C, Luxenberg JS, Rapoport SI. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed X-ray tomography. J Am Geriatr Soc. 1992;40:225–31. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- 25.Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, et al. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30:569–78. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- 26.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The Short Test of Mental Status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–8. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 29.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 30.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 31.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 32.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–6. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev. 1995;17:192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RO, Geda YE, Knopman DS, Christianson TJH, Pankratz VS, Boeve BF, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–73. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 36.Craft S. Insulin resistance syndrome and Alzheimer's disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26(suppl 1):65–9. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Munch G, Schinzel R, Loske C, Wong A, Durany N, Li JJ, et al. Alzheimer's disease–synergistic effects of glucose deficit, oxidative stress and advanced glycation endproducts. J Neural Transm. 1998;105:439–61. doi: 10.1007/s007020050069. [DOI] [PubMed] [Google Scholar]
- 38.Knopman DS, Roberts R. Vascular risk factors: imaging and neuropathologic correlates. J Alzheimers Dis. 2010;20:699–709. doi: 10.3233/JAD-2010-091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 40.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 41.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–5. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 42.Roberts RO, Kantarci K, Geda YE, Knopman DS, Przybelski SA, Weigand SD, et al. Untreated type 2 diabetes and its complications are associated with subcortical infarctions. Diabetes Care. 2011;34:184–6. doi: 10.2337/dc10-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–25. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 45.Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T, et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology. 2010;75:160–7. doi: 10.1212/WNL.0b013e3181e7ca05. [DOI] [PubMed] [Google Scholar]
- 46.van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- 47.Toro P, Schonknecht P, Schroder J. Type II diabetes in mild cognitive impairment and Alzheimer's disease: results from a prospective population-based study in Germany. J Alzheimers Dis. 2009;16:687–91. doi: 10.3233/JAD-2009-0981. [DOI] [PubMed] [Google Scholar]
- 48.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70-81 years. BMJ. 2004;328:548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu W, Caracciolo B, Wang HX, Winblad B, Backman L, Qiu C, et al. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59:2928–35. doi: 10.2337/db10-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]