Abstract
Objective
To test the added value of Calcium and vitamin D (CaD) for fracture prevention among women taking postmenopausal hormone therapy (HT).
Methods
A prospective, partial-factorial design, randomized controlled double blind trial amongst Women’s Health Initiative post-menopausal participants, ages 50–79, at 40 centers in the US, with 7.1 years average follow-up. 27,347 women were randomized to HT (conjugated estrogen 0.625 mg alone, or CEE 0.625 mg daily plus medroxyprogesterone acetate 2.5mg) and 36,282 women randomized to either 1000mg elemental calcium (carbonate) plus 400 IU of vitamin D3 daily each compared to placebo. A total of 16,089 women were in both arms. The predefined outcomes were adjudicated hip fractures and measured bone mineral density.
Results
Interaction between HT and CaD on hip fracture (P-interaction = 0.01) was shown. The effect of CaD was stronger among women assigned to HT (HR, 0.59; 95%CI, 0.38–0.93) than placebo (HR, 1.20; 95%CI, 0.85, 1.69). The effect of HT on hip fracture was stronger among women assigned to active CaD (HR, 0.43; 0.28–0.66) than placebo (HR, 0.87; 95%CI, 0.60–1.26). CaD supplementation enhanced the anti-fracture effect of the HT at all levels of personal calcium intake. There was no interaction of HT and CaD on change in hip or spine BMD.
Conclusions
Postmenopausal women at normal risk of hip fracture on HT, supplementation with CaD significantly reduced incident hip fracture beyond HT alone; at all levels of personal baseline total calcium intake.
Keywords: calcium, vitamin D, hormone therapy, estrogens
Introduction
Controversy surrounds use of supplemental calcium and vitamin D (CaD). The literature is full of conflicting reports and strong opinions. Major authorities have questioned the health benefits/risks of widespread supplementation.1 The U.S. Preventive Services Task Force recently published recommendations advising against routine supplementation with CaD.2
In practice most trials of osteoporosis medications have included CaD supplementation for both the active and placebo groups.3 There is little data supporting this practice. Conflicting results have been published from prior trials. One publication looking at response to bisphosphonates showed improved response in BMD and turnover markers with higher vitamin D serum levels.4 Using data from the Fracture Intervention Trial of alendronate, Antoniucci et al, showed baseline vitamin D levels did not change outcomes.5 Looking at a relatively small number of subjects in zolentonate trials an Australian group was unable to show a statistically significant effect of either dietary calcium and vitamin D or vitamin D levels on BMD or turnover makers.6 Another recent publication only showed a response in those with initially low vitamin D levels.7
The use of postmenopausal hormones is also controversial. Some groups support the use estrogen therapy for the treatment of osteoporosis;8 although it may not be considered the standard of care.
The Women’s Health Initiative (WHI) clinical trials which did not select women based on low bone density or osteoporosis, demonstrated that estrogen therapy, with or without progestin, increased bone density and reduced fracture risk similarly.9, 10 In this manuscript we address the following major research questions: 1) Does CaD supplementation increase bone health benefits in post-menopausal women on estrogen therapy? 2) Does estrogen therapy increase bone health benefits in women taking CaD supplementation?
WHI offers a unique opportunity to address these questions through secondary analyses of data from prospective, partial-factorial, randomized trials. WHI used a partial factorial design for the two hormonal trials (CEE-alone and CEE + MPA trials) and a trial of CaD supplementation.11 This permits the exploration of the effects of CaD on hip fractures in women receiving hormonal supplementation in a double blinded prospective study, and vice versa.
Women were randomized to either, both, or neither of the hormonal and CaD trials. After the Hormone Trial (HT) intervention ended, only a small percentage of participants reported using hormone therapy; < 5% of women in the active arms and < 3% of women, in the placebo arms of either WHI HT trial continued to take hormones.12 Incident hip fracture outcomes were assessed and adjudicated by investigators blinded to treatment assignments.13 Four clinical sites assessed BMD in a subsample women using DXA at baseline, years 1, 3 and 6 post HT randomization.
Material and Methods
WHI recruited postmenopausal women ages of 50 to 79 from 40 US clinical centers between 1993 and 1998. Women with a uterus had 0.625 mgs of congregated equine estrogen (CEE) combined with 2.5 mg/day of medroxyprogesterone acetate (MPA) compared to placebo and women who had had a hysterectomy had 0.625 mg/day of CEE compared to placebo. Women were simultaneously recruited to participate in a distinct overlapping randomized controlled intervention, the WHI Dietary Modification (DM) trial. Participants enrolled in either the DM and/or HT trials, were invited to join the CaD trial at their first or second annual follow-up visit (figure 1). Those who consented were randomized to either 1000 mg/day of elemental calcium as calcium carbonate plus 400 IU of vitamin D3 daily or placebo.
Figure 1.
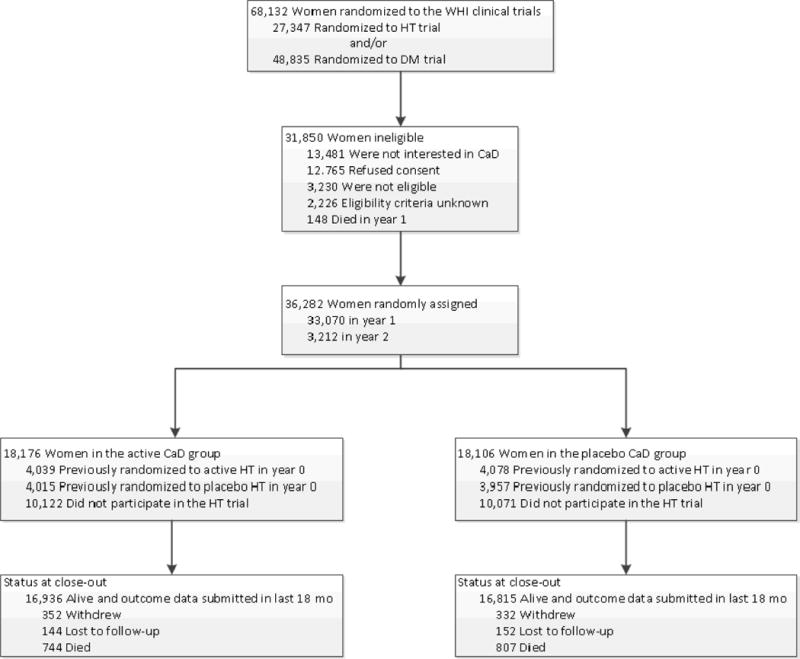
Consort diagram of WHI clinical trial.
HT denotes hormone therapy, DM dietary modification, CaD calcium with Vitamin D. All 68132 women were included in the analysis, with survival time beginning at randomization into HT, DM or both. Women were later randomized to CaD at years 1 and 2, or did not participate in the CaD trial. Hip fracture incidence and summary statistics, from CaD randomization through study close-out (March 31, 2005), are summarized in Table 2.
Detailed descriptions of eligibility criteria and recruitment methods have been published.14 DM participants are included in this analysis because some of them were randomized to CaD and contribute to the comparison of CaD with placebo.
Hip fractures were evaluated through the originally specified date of trial completion, March 31, 2005. Hip and other fractures were self-reported semi-annually. Hip fractures were verified by review of radiographic and/or operative reports by blinded physician adjudicators.
We combined the fracture outcomes of the two hormone arms. We postulated that the effect on fracture is predominantly mediated by the CEE component of the therapy, and thus was similar. The original publications reporting hip fracture rates in the two arms of the hormone trials gave very similar results, CEE + MPA hazard ratio (HR) for hip fracture compared to placebo 0.66 (95% CI 0.45–0.98) and CEE alone trial, HR = 0.61 (95% CI 0.41–0.91)15, 16. Some previous studies also suggested similar effects of CEE and CEE + MPA on bone,17 while others in a modified intension to treat protocol noted increases in spine BMD, with the addition of MPA.18 Because of these conflicting results we validated the decision to combine the groups by examining the changes in BMD among women in the active therapy arms of the two HT trials and found the effects of CEE alone and CEE + MPA to be similar. (Appendix, figure 1.)
Appendix.
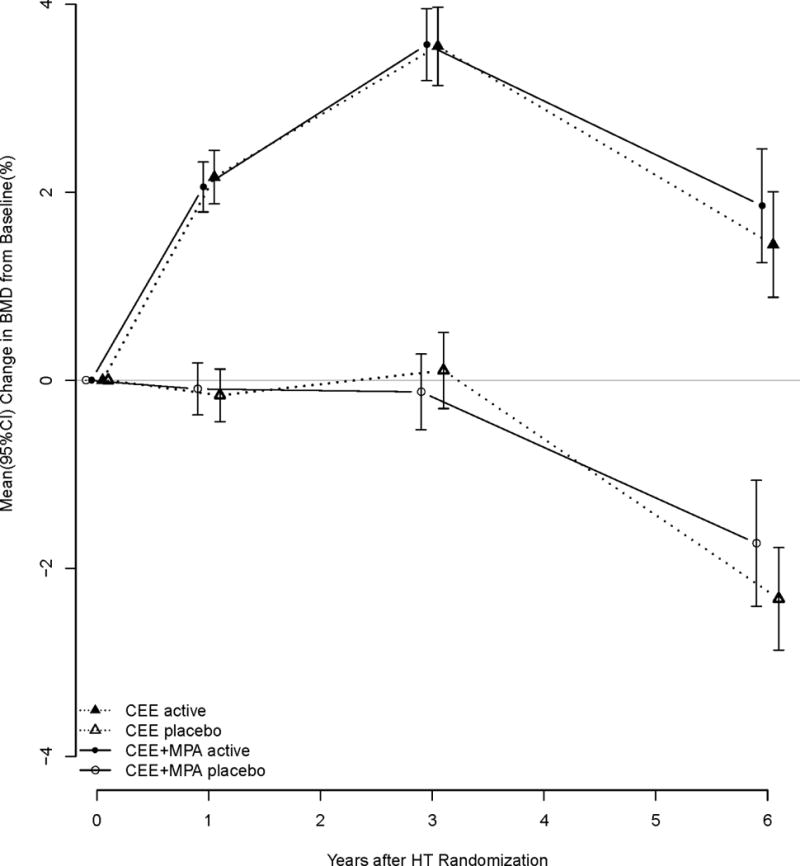
Change in hip bone mineral density by HT trial.
Comparison of the changes in BMD for women in the two hormone arms of WHI, Estrogen alone and estrogen with progestin compared to placebos.
Women in the CaD trial were permitted to take their own calcium and vitamin D supplementation. Information regarding the amounts of dietary calcium and vitamin D intake and personal supplementation were available from baseline questionnaires. Combing self-reported intake with trial intakes permitted us to investigate a larger range of total CaD intake in conjunction with HT in relation to hip fracture incidence.
A subset of women in WHI had BMD measured at 3 clinical centers (4 sites), Birmingham, Alabama; Pittsburgh, Pennsylvania; and Tucson or Phoenix, Arizona. They were chosen to provide maximal racial diversity and were not representative of the CT as a whole. DXA (Hologic QDR 2000 or 4500w) using standard protocols for positioning and analysis was used to assess BMD at the hip, spine and total body at baseline, and years 1, 3 and 6 of follow-up. The demographic profiles were similar between the entire cohort and this subset. All DXA technicians were trained and certified by the DXA manufacturer and by the WHI BMD coordination center at the University of California at San Francisco.
The research protocols where approved by the universities human subjects committees.
Statistical Methods
Primary analyses included all 68,132 randomized participants in the WHI clinical trials, using time-to event methods through study close-out (3/31/05), and were based on the intention-to-treat principle. Hazard ratios (HR) for hip fracture were estimated from a proportional hazards model that included a categorical variable for HT (active/placebo/not randomized), a similar time-dependent categorical variable for CaD, and their corresponding interaction terms. Models were stratified by prevalent condition, 5-year age group, dietary modification randomization arm and hysterectomy status, event times began at randomization into HT or DM trial.
We conducted a sensitivity analysis in which we censored HT trial participants at the time that the HT interventions ended (7/7/02 for the CEE + MPA trial and 2/29/04 for the CEE trial). While the focus was on the 2x2 factorial portion of HT (active vs. placebo) x CaD (active vs. placebo), inclusion of women that were not randomized ensures that the estimated main effects in these analysis correspond to estimates that would have been obtained if the complete trials were examined individually; designs for these individual trials have been previously published17, 18. We planned analyses apriori according to baseline subgroups of age (< 60, > = 60 years), total calcium intake (< 600, 600- < 1200, > = 1200 mg), and HT personal use at baseline. Statistical significance of the subgroup analysis was based on the p-value for interaction.
Further analysis for the effect modification of HT by CaD was investigated by creating a time-dependent quasi-subgroup of self-reported nutrient intake at baseline that was updated at CaD randomization by adding the nutrients associated with study pills. The effect of HT was then allowed to vary continuously with nutrient values as a spline, with smoothing parameter chosen objectively through generalized cross-validation and statistical significance based on the test of interaction. Adherence sensitivity analyses of the primary analysis were conducted by censoring follow-up beginning 6 months after participants became non adherent. Separate analyses were performed at adherence thresholds that corresponded to ingestion of < 50% and < 80% of CaD study pills.
Cumulative hazard functions for the 2 × 2 factorial were computed using Simon-Makuch estimates to accommodate time-dependent randomization into the CaD trial.19 At time zero all participants begin in the groups "Not randomized to CaD/active HT" or "Not randomized to CaD/placebo HT." Participants are later allowed to change groups after randomization into the CaD trial. Longitudinal change in BMD was evaluated in the 2x2 factorial among women in the BMD subsample. The within individual correlation was accounted for in a repeated measures regression model with an unstructured correlation matrix.
Baseline characteristics for women in the 2 × 2 factorial were compared by randomization group using Chi-squared and t tests. Annualized rates of clinical events were estimated by dividing the number of events by person-time. All statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina) and R software version 2.11 (R Foundation for Statistical Computing, http://www.r-project.org/). All statistical tests were 2-sided.
Results
Baseline characteristics by CaD and HT randomization arms are displayed in Table 1. With the exception of the clinically insignificant difference in mean ages across randomization groups, risk factors were similar.
Table 1.
Baseline Characteristics of the 2x2 Factorial portion of the WHI Hormone Therapy and Calcium with Vitamin D Trials (n = 16089) by Randomization Assignment.
| CaD x HT Randomization Assignment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Active CaD/ | Active CaD/ | Placebo CaD/ | Placebo CaD/ | ||||||
| Active HT | Placebo HT | Active HT | Placebo HT | ||||||
| N | % | N | % | N | % | N | % | P20 | |
| Age group at screening | 0.35 | ||||||||
| 50–59 | 1433 | 35.5 | 1412 | 35.2 | 1422 | 34.9 | 1342 | 33.9 | |
| 60–69 | 1812 | 44.9 | 1812 | 45.1 | 1833 | 44.9 | 1760 | 44.5 | |
| 70–79 | 794 | 19.7 | 791 | 19.7 | 823 | 20.2 | 855 | 21.6 | |
|
| |||||||||
| Race/ethnicity | 0.26 | ||||||||
| White | 3336 | 82.6 | 3251 | 81.0 | 3347 | 82.1 | 3251 | 82.2 | |
| Black | 331 | 8.2 | 407 | 10.1 | 363 | 8.9 | 383 | 9.7 | |
| Hispanic | 229 | 5.7 | 229 | 5.7 | 217 | 5.3 | 184 | 4.6 | |
| American Indian | 21 | 0.5 | 16 | 0.4 | 18 | 0.4 | 18 | 0.5 | |
| Asian/Pacific Islander | 70 | 1.7 | 64 | 1.6 | 80 | 2.0 | 70 | 1.8 | |
| Unknown | 52 | 1.3 | 48 | 1.2 | 53 | 1.3 | 51 | 1.3 | |
|
| |||||||||
| Smoking status | 0.34 | ||||||||
| Never | 2050 | 51.3 | 1983 | 49.9 | 2112 | 52.1 | 2050 | 52.4 | |
| Past | 1559 | 39.0 | 1571 | 39.6 | 1551 | 38.3 | 1483 | 37.9 | |
| Current | 386 | 9.7 | 416 | 10.5 | 387 | 9.6 | 377 | 9.6 | |
|
| |||||||||
| > 2 Alcohol servings/day | 175 | 4.4 | 170 | 4.3 | 157 | 3.9 | 135 | 3.4 | 0.13 |
|
| |||||||||
| Self-reported health | 0.16 | ||||||||
| Excellent | 711 | 17.7 | 706 | 17.7 | 689 | 17.0 | 630 | 16.0 | |
| Very good | 1661 | 41.4 | 1682 | 42.2 | 1749 | 43.1 | 1736 | 44.1 | |
| Good | 1344 | 33.5 | 1292 | 32.4 | 1338 | 33.0 | 1259 | 32.0 | |
| Fair/poor | 292 | 7.3 | 309 | 7.7 | 281 | 6.9 | 311 | 7.9 | |
|
| |||||||||
| Treated diabetes (pills or shots) | 219 | 5.4 | 197 | 4.9 | 209 | 5.1 | 219 | 5.5 | 0.58 |
|
| |||||||||
| Personal CEE use status | 0.34 | ||||||||
| Never used | 2651 | 65.7 | 2607 | 65.0 | 2640 | 64.8 | 2591 | 65.5 | |
| Past user | 1025 | 25.4 | 1014 | 25.3 | 1008 | 24.7 | 999 | 25.3 | |
| Current user | 361 | 8.9 | 392 | 9.8 | 429 | 10.5 | 366 | 9.3 | |
|
| |||||||||
| Duration of Personal CEE use | 0.78 | ||||||||
| Non-user | 2651 | 65.6 | 2607 | 64.9 | 2640 | 64.7 | 2591 | 65.5 | |
| < 5 yrs | 870 | 21.5 | 848 | 21.1 | 863 | 21.2 | 829 | 21.0 | |
| 5 - < 10 yrs | 264 | 6.5 | 267 | 6.7 | 271 | 6.6 | 254 | 6.4 | |
| = 10 yrs | 254 | 6.3 | 293 | 7.3 | 304 | 7.5 | 283 | 7.2 | |
|
| |||||||||
| Father - Hip Fx After 40 | 80 | 2.2 | 97 | 2.7 | 89 | 2.4 | 99 | 2.7 | 0.42 |
|
| |||||||||
| Mother - Hip Fx After 40 | 412 | 10.6 | 415 | 10.8 | 392 | 10.0 | 395 | 10.4 | 0.72 |
|
| |||||||||
| History of fracture age 55+ | 470 | 16.0 | 436 | 14.8 | 462 | 15.5 | 483 | 16.4 | 0.35 |
|
| |||||||||
| Times fell down last 12 months | 0.36 | ||||||||
| None | 2452 | 65.6 | 2513 | 66.7 | 2535 | 67.5 | 2442 | 66.0 | |
| 1 time | 785 | 21.0 | 724 | 19.2 | 746 | 19.9 | 760 | 20.5 | |
| 2 times | 322 | 8.6 | 361 | 9.6 | 304 | 8.1 | 330 | 8.9 | |
| 3 or more times | 177 | 4.7 | 168 | 4.5 | 173 | 4.6 | 167 | 4.5 | |
|
| |||||||||
| Supplemental Vitamin D Use | 1662 | 41.2 | 1602 | 39.9 | 1684 | 41.3 | 1675 | 42.3 | 0.18 |
|
| |||||||||
| Supplemental Calcium Use | 1979 | 49.0 | 1915 | 47.7 | 1993 | 48.9 | 1968 | 49.7 | 0.33 |
|
| |||||||||
| CaD x HT Randomization Assignment | |||||||||
|
|
|||||||||
| Active CaD/ | Active CaD/ | Placebo CaD/ | Placebo CaD/ | ||||||
| Active HT | Placebo HT | Active HT | Placebo HT | ||||||
|
|
|||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P | |
|
| |||||||||
| Age at screening | 62.7 | 7.1 | 62.9 | 7.1 | 62.8 | 7.1 | 63.1 | 7.1 | 0.02 |
|
| |||||||||
| Body-mass index (kg/m2), baseline | 29.3 | 6.1 | 29.3 | 6.0 | 29.1 | 6.0 | 29.3 | 6.1 | 0.26 |
|
| |||||||||
| Physical Activity (Total MET-hours/wk) | 11.2 | 13.7 | 10.8 | 13.1 | 10.7 | 12.8 | 10.7 | 12.9 | 0.24 |
|
| |||||||||
| Total personal Ca (mg) intake | 1097.4 | 654.7 | 1092.9 | 663.9 | 1096.0 | 675.9 | 1096.8 | 655.9 | 0.99 |
|
| |||||||||
| Total personal Vitamin D (IU) intake | 335.1 | 259.1 | 332.4 | 248.1 | 340.3 | 260.3 | 343.4 | 261.1 | 0.23 |
For the primary outcome of hip fracture, after an average (SD) follow-up of 7.2(1.4) years from CaD randomization, there is a significant interaction between HT and CaD (p-int = 0.01; see Table 2). The effect of HT (active vs. placebo) on hip fracture prevention was stronger among women assigned to active CaD (HR, 0.43; 95% CI 0.28–0.66) than women assigned to placebo (HR, 0.87; 95% CI, 0.60–1.26). Likewise, the effect of CaD (active vs. placebo) is stronger among women assigned to active HT (HR, 0.59; 95% CI, 0.38–0.93) than women assigned to placebo (HR, 1.20; 95% CI, 0.85, 1.69). Plots of the cumulative hazards display the synergistic effect of being on both active therapies compared to each individual therapy alone (Figure 2). While the HT interventions stopped prior to the 2005 close-out, the main effect of HT was still protective at the end of the study period (HR, 0.76; 95% CI, 0.62, 0.93).
Table 2.
Hip Fracture Incidence and Hazard Ratios* of the 2 × 2 Factorial portion of the WHI Hormone Therapy and Calcium with Vitamin D Trial.
| HT Randomization Assignment | ||||||
|---|---|---|---|---|---|---|
| Active | Placebo | |||||
| CaD Randomization Assignment | N | (Annual %) | N | (Annual %) | HR21 (95%CI) | |
| Active | 31 | (0.11) | 70 | (0.25) | 0.43 | (0.28, 0.66) |
| Placebo | 52 | (0.18) | 61 | (0.22) | 0.87 | (0.60, 1.26) |
|
| ||||||
| HR1 (95%CI) | 0.59 | (0.38, 0.93) | 1.20 | (0.85, 1.69) | P-interaction = 0.01 | |
Hazard ratios (HR) from a proportional hazards model where fit on the entire WHI Clinical Trial (n = 68132) and included a time-dependent categorical variable (not randomized/placebo/active) for CaD, an indicator variable for HT, and their corresponding interaction term. Models were stratified by prevalent condition (hip fracture after the age of 55), 5-year age group, dietary modification randomization arm and hysterectomy status.
Summary statistics for subjects that were not randomized to HT or CaD are not shown. Events are through March 31, 2005. Time to event equals zero at time of HT randomization. Hazard ratios (active HT vs. placebo HT) by CaD randomization group. HR (95%CI) for main effect of HT = 0.76 (0.62, 0.93). HR (95%CI) among those not randomized to CaD = 0.94 (0.70, 1.26). Hazard ratios (active CaD vs. placebo CaD) by HT randomization group. HR (95%CI) for main effect of CaD = 0.87 (0.72, 1.07). HR (95%CI) among those not randomized to HT = 0.83 (0.62, 1.12)
Figure 2.
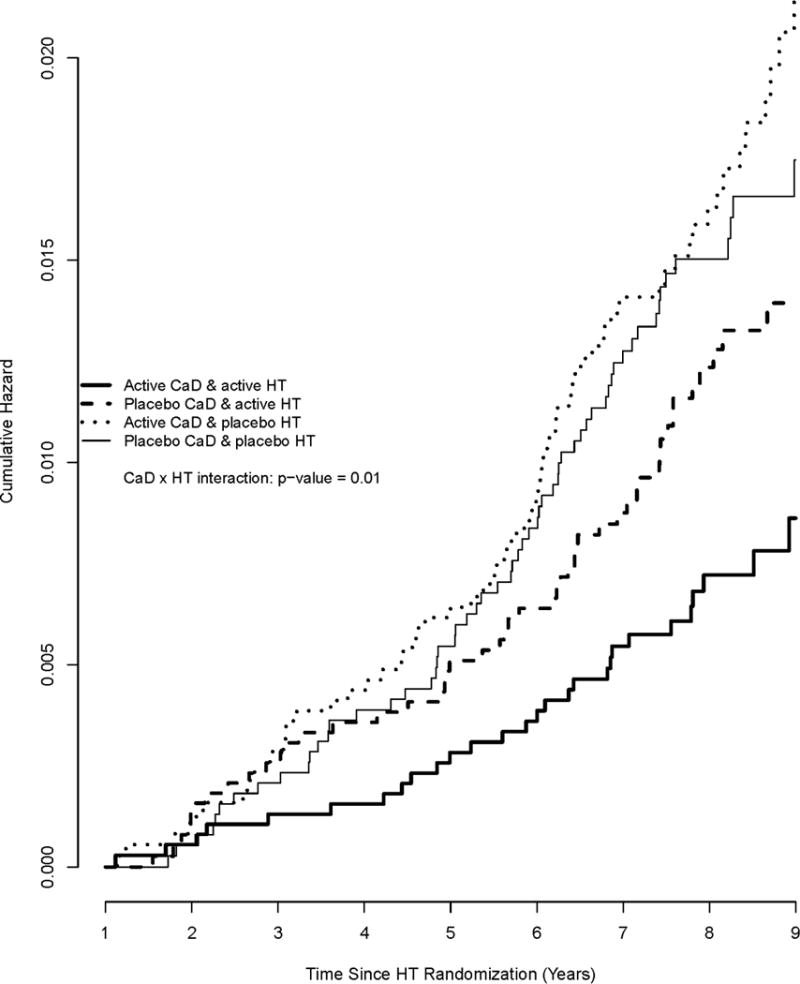
Cumulative hazards for the incidence of hip fracture by HT and CaD randomization. Cumulative hazard functions of hip fracture that allow for randomization into the CaD trial as a time-dependent variable.
In a sensitivity analysis that censored participants once the HT intervention ended, the main effect of HT became stronger (HR, 0.67; 95%CI, 0.52–0.86), and the effect of HT on hip fracture prevention was greater among women assigned to active CaD (HR, 0.46; 95% CI 0.27–0.80) than women assigned to placebo CaD (HR, 0.78; 95% CI, 0.49–1.26) after an average (SD) follow-up of 5.2(1.4) years. However, this sensitivity analysis was limited by 40% fewer hip fracture cases (129 vs. 214); the interaction between HT and CaD was not statistically significant (p-interaction = 0.15; see appendix, Table 1).
The interaction between HT and CaD translates into 11 hip fractures per 10,000 person years (pys) for women assigned to both active HT and active CaD compared to 18 per 10,000 pys for women assigned to active HT only, 25 per 10,000 pys for women on active CaD only, and 22 cases per 10,000 pys in those in both placebo arms. (Hip fractures per year in the placebo and CaD arms are not statistically different.)
The subgroup analysis of baseline total personal calcium intake showed a similar pattern to Table 2, the benefit of CaD (active vs. placebo) for hip fracture prevention was enhanced among women taking HT compared to women on placebo for all categories of baseline calcium intake. The benefit of HT for hip fracture prevention was enhanced among women randomized to CaD regardless of subgroup of personal calcium intake, indicating that the synergistic interaction of HT × CaD was consistent across all levels of personal calcium intake (p-trend = 0.86; data not shown). This observation was further supported when baseline levels of nutrient intakes and study pills were considered together. The beneficial effect of HT was evident at about 1200mgs of baseline personal Ca use and continued to increase thereafter (p-int = 0.006; Figure 3a). Similarly, benefit of HT continued to increase at levels higher than 400 IUs of vitamin D (p-int = 0.02; Figure 3b).
Figure 3AB.
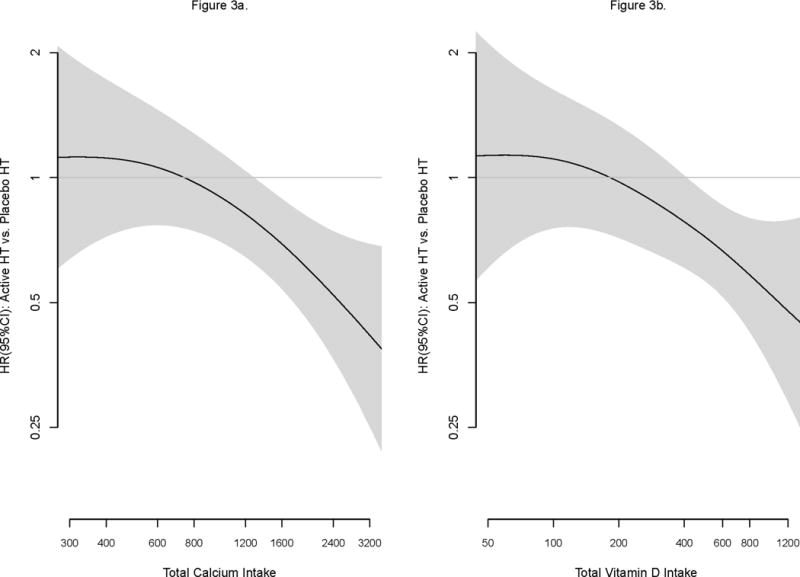
Effect of HT modified by calcium and vitamin D on hip fractures.
Non-parametric spline fits of the HR (95% confidence interval in shaded region) of the effect of HT (active vs. placebo) by level of total calcium (left panel) and total vitamin D (right panel). The smoothness of each fit was chosen objectively by generalized cross-validation.
There were too few cases to reliably test whether or not the synergistic effects of HT × CaD were consistent across subgroups of age and self-report of prior menopausal hormone use at baseline (yes/no); there were only 20 hip fracture cases among women < 60. There were only 13 hip fracture cases among women reporting personal use of menopausal hormone therapy at baseline. At the 0.05 level of significance, less than one interaction P-value could be statistically significant based on chance alone.
The adherence analyses of those who took over 50 or 80% of the allocated CaD therapy or placebo suggested the same synergistic pattern of HT × CaD, but the synergistic pattern was not statistically significant at either threshold (p-interaction 50% = 0.17, p-interactions 80% = 0.85). We suspect that the smaller sample size in the adherence analyses may have limited our power to detect statistically significant associations.
We found no evidence for potential interactions of CaD and post-menopausal HT in relation to total fractures (p-interactions = 0.97) or clinical vertebral fractures (p-interactions = 0.79, data not shown).
The intermediate end point of bone mineral density (BMD) was investigated in 1058 (261 active HT/active CaD, 275 active HT/placebo, 272 placebo/active CaD, 250 placebo/placebo) women who were randomized in the CaD and HT trials. Percent change in BMD of the total hip is presented in Figure 4. There was no evidence of a synergistic effect between estrogen therapy and CaD on hip BMD (p-interactions = 0.79) or spine BMD (data not shown). Note the graphs are not adjusted for the aging of the cohort which could be expected to result in a decreasing BMD in the range of 0.05% per year in post menopausal women.20
Figure 4.
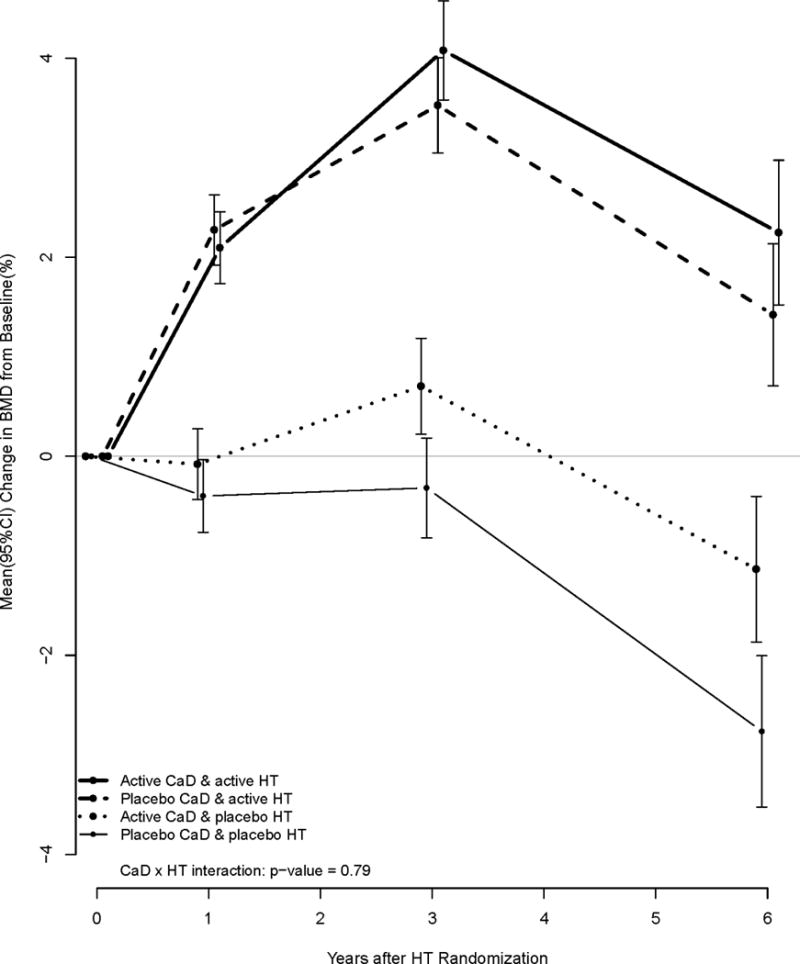
Change in hip bone mineral density by HT and CaD randomization.
BMD measures that occurred after 07Jul02 and 29Feb04 for the CEE + MPA and CEE trials were set to missing, respectively.
Discussion
The previously published WHI paper on calcium plus vitamin D supplementation and the risk of fractures found no overall significant benefit of CaD in the intention to treat analysis. Our further investigation of the WHI data shows that, although by itself CaD may not be of statistically proven benefit for fracture prevention, they have a significant effect when used with HRT. The current study provides evidence that supplemental CaD augments the protective effects of HT against hip fracture, and vice versa.
We were able to show an interaction of CaD and HT only for hip fractures. We postulate that this is a function of the imprecision of the diagnosis of the non-hip fractures. The end point of hip fracture is very precise because it is based on hospitalization. The other fracture endpoints are all based on self report. It is well documented that the diagnosis of vertebral fractures is inaccurate.21 We believe this imprecision in outcomes translated into too much “noise” to permit the accurate evaluation of the interaction of CaD and HT. One could also speculate that there is a differential effect of CaD on certain bone sites but we have no way of evaluating this.
While these data do not allow us to define a specific optimal threshold for CaD intake, both subgroup analyses regarding calcium and vitamin D intake suggest higher benefits of HT on hip fracture risk reduction associated with increased intake of CaD. In the subgroup analysis of baseline calcium, the benefit of HT was larger for women randomized to CaD compared to those given placebo for all subgroups of baseline calcium intake. Looking at total calcium intake the benefit of HT had a strong positive association with total calcium intakes greater than 1200mg. A similar positive association was observed with vitamin D intake. We found no evidence for an upper limit to the hip fracture risk reduction associated with calcium or vitamin D among women taking HT.
Women in WHI were not specifically recruited based on BMD or diagnosis of osteoporosis. Many of those tested in the BMD subgroup had BMD in the normal range. Nonetheless, the supplementation with CaD decreased the risk of hip fracture in postmenopausal women assigned to HT. In the entire cohort, the supplementation with CaD in WHI was not sufficient in an intention to treat analysis, to show a statistically significant decrease in hip fractures when it was not combined with post-menopausal hormone therapy. A sensitivity analysis of adherent women did show an effect of CaD supplementation in older women and those most adherent to their study pills.
Although, only a subset of women in WHI were evaluated by DXA visual comparison of the profile plots of mean change in hip BMD suggest no interaction, since the profiles are decidedly additive. The actions of the HT and CaD on hip fracture prevention may be mediated through mechanisms other than increased BMD. These potential mechanisms could include improved balance, muscle strength, bone architecture, bone strength, among others.
The study also has limitations. Because of the combined nature of the CaD intervention we are unable to separate out the effects of the specific components. Our ability to estimate required CaD dosages is limited by the self report of information regarding calcium and vitamin D intake instead of directly observed intake. The women in the WHI probably have a higher dietary CaD intake than the average postmenopausal women which should have been expected to bias the results toward the null. We also lacked information regarding sun light exposure. Blood Vitamin D levels were not measured for most of the study participants; therefore, personal intakes of vitamin D may not reflect actual circulating values.
Strengths of this study include large sample size, over 15,000 women; extensive information regarding dietary intakes, randomized controlled trial study design. Compared to prior studies, the partial factorial design of the current study gives it more strength to evaluate the effects of CaD on hip fracture risk in women on CEE.
Conclusion
These results suggest that women taking post menopausal estrogens should also take supplemental calcium and vitamin D. Because of the study design we are unable to suggest a specific level of supplementation; benefit appears to increase with increasing total calcium and vitamin D intake. Dosage choice should be made so as to minimize side effects. It may be possible to extrapolate to women on other forms of osteoporosis therapy suggesting that they also take CaD supplementation but we have no evidence for this and further research is obviously needed.
Acknowledgments
This research was partially funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Lewis reports: grant with Novo Nordisk. Dr. Jackson reports: grant with Pfizer. All other authors have no financial interests to disclose, and no conflicts of interest
References
- 1.ACatharine Ross, Manson JoAnn E, Abrams Steven A, Aloia John F, Brannon Patsy M, Clinton Steven K, Durazo-Arvizu Ramon A, Gallagher J Christopher, Gallo Richard L, Jones Glenville, Kovacs Christopher S, Mayne Susan T, Rosen Clifford J, Shapses Sue A. 2010 The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. Journal of Clinical Endocrinology & Metabolism. 97(4):1146–52. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer VA, on behalf of the U.S. Preventive Services Task Force Vitamin D and Calcium Supplementation to Prevent Fractures in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013 Feb 26; doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 4.Peris P, Martínez-Ferrer A, Monegal A, Martínez de Osaba MJ, Muxi A, Guañabens N. 825 hydroxyvitamin D serum levels influence adequate response to bisphosphonate treatment in postmenopausal osteoporosis. Bone. 2012;51(1):54–56. doi: 10.1016/j.bone.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Antoniucci DM, Vittinghoff E, Palermo L, Black DM, Sellmeyer DE. Vitamin D insufficiency does not affect response of bone mineral density to alendronate. Osteoporos Int. 2009;20(7):1259–66. doi: 10.1007/s00198-008-0799-4. [DOI] [PubMed] [Google Scholar]
- 6.Bourke S, Bolland MJ, Grey A, Horne AM, Wattie DJ, Wong S, Gamble GD, Reid IR. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2012;24(1):349–54. doi: 10.1007/s00198-012-2117-4. 2013 Jan. [DOI] [PubMed] [Google Scholar]
- 7.Olmos JM, Hernández JL, Llorca J, Nan D, Valero C, González-Macías J. Effects of 25-Hydroxyvitamin D3 Therapy on Bone Turnover Markers and PTH Levels in Postmenopausal Osteoporotic Women Treated with Alendronate. Clin Endocrinol Metab. 2012;97(12):4491–7. doi: 10.1210/jc.2012-2999. 2012 Dec. [DOI] [PubMed] [Google Scholar]
- 8.POSITION STATEMENT. The Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(No. 3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, Naughton MJ, Satterfield S, Bassford T. Women’s Health Initiative Investigators. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: Results from the Women’s Health Initiative randomized trial. J Bone Miner Res. 2006;21(6):817–28. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 10.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 11.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML. WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–45. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. alidity of self-report for fractures among a multiethnic cohort of postmenopausal women: Results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004V;11(3):264–74. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 14.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.The Writing Group for the PEPI. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276(17):1389–96. [PubMed] [Google Scholar]
- 18.Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early post menopausal women. JAMA. 2009;287(20):2668–76. doi: 10.1001/jama.287.20.2668. [DOI] [PubMed] [Google Scholar]
- 19.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non-responder bias. Statistics in Medicine. 1984;3:35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 20.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc Feb. 2005;53(2):183–9. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 21.Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE. Fracture Intervention Trial Research Group. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005;20(7):1216–22. doi: 10.1359/JBMR.050314. [DOI] [PubMed] [Google Scholar]


