Abstract
Multiple biological processes are related to cognitive impairment in older adults, but their combined impact on cognition in midlife is not known. Using an array of measurements across key regulatory physiological systems and a state-of-the-art cognition battery that is sensitive to early changes, on a large, national sample of middle-aged and older adults, we examined the associations of individual biological systems and a combined, multi-system index, allostatic load, with cognitive performance. Allostatic load was strongly inversely associated with performance in both episodic memory and executive function. Of seven biological systems, only the cardiovascular system was associated inversely with both; inflammation was associated inversely with episodic memory only, and glucose metabolism with executive function only. The associations of allostatic load with cognition were not different by age, suggesting that the implications of high allostatic load on cognitive functioning are not restricted to older adults. Findings suggest that a multi-system score, like allostatic load, may assist in the identification of adults at increased risk for cognitive impairment at en early age.
Keywords: adult cognition, allostatic load, inflammation, dysregulated physiology
1. Introduction
Cognitive impairment presents an immense burden on older adults, their families, and society. Clinically manifest diseases such as diabetes, stroke, and cardiovascular disease, which are causally related to neuronal loss and interruption of neural circuits (Whitmer et al., 2005) account for only a fraction of the population variance in performance on cognition tests: Diabetes and stroke together explain less than 1% of population test score variance (Zelinksi et al., 1998; Zelinksi and Gilewski, 2003) and the four leading medical conditions together explain 1.6% or less (Verhaeghen et al., 2003). In addition, chronic health conditions do not appear to significantly influence the rate of cognitive decline with aging, and many older adults experience cognitive impairment in the absence of diagnosed medical conditions (Chodosh et al., 2010; Deary et al., 2009). Therefore, with the rapid aging of the world’s population, there is an urgent need to delineate the sub-clinical biological processes that influence the risk of cognitive impairment in older ages.
Risk factors for cardiovascular disease, such as high blood pressure (Dahle et al., 2009), high levels of low density lipoprotein (LDL) cholesterol, insulin resistance (Neumann et al., 2008; S Roriz-Filho et al., 2009), increased visceral adiposity, metabolic syndrome (Cavalieri et al., 2010), and chronic inflammation (Laurin et al., 2009; Marsland et al., 2008; Ownby, 2010) are also recognized risk factors for cognitive decline, and appear to contribute to such decline even in the absence of overt cardiovascular disease (Duron and Hanon, 2008). These risk factors are causally related to sub-clinical atherosclerosis, which can lead to ischemic damage in the brain without causing symptomatic strokes (Lazarus et al., 2005; Vermeer et al., 2003). In addition, high levels of circulating insulin, such as are seen in insulin resistance (pre-diabetes), may have more direct impact on the development of Alzheimer’s type dementia (Neumann et al., 2008; Qiu and Folstein, 2006; S Roriz-Filho et al., 2009).
In addition to clinical cardiovascular risk factors, sub-clinical changes in neuroendocrine regulatory systems have also been suggested as more proximal biological changes on the pathway to cognitive decline. Cortisol, the primary hormonal agent of the hypothalamic-pituitary-adrenal (HPA) system, for instance, is known to promote neuronal death in experimental settings and contribute to hippocampal atrophy in normal human aging (Lupien et al., 1998; Porter and Landfield, 1998); not surprisingly, high circulating levels of cortisol are associated with poor performance on tests of cognition (Fiocco et al., 2006; Karlamangla et al., 2005a). Catecholamines, such as epinephrine and norepinephrine, the primary agents of the sympathetic nervous system, are also associated inversely with adult cognition (Karlamangla et al., 2005b). The parasympathetic system, indexed by heart rate variability, on the other hand, is associated positively with cognition (Hansen et al., 2004; Kim et al., 2006; Zulli et al., 2005).
Multi-system indices, such as allostatic load, created to capture the combined effect of biology from multiple systems, have been found to predict cognitive decline in previously high-functioning older adults (Juster et al., 2010; Karlamangla et al., 2002; Seeman et al., 1997), but little is known about the role of allostatic load in cognitive changes in younger ages, although cognitive aging begins fairly early in life, particularly in domains such as speed of processing (Grodstein, 2011; Salthouse, 1996, 2009; Singh-Manoux et al., 2011).
Accordingly, our objective was to determine the cross-sectional associations of a cumulative, multi-system index, allostatic load, with cognitive performance in a large-scale, national sample of young, middle-aged, and older adults with a wide range of education levels, using a state-of-the-art cognitive battery, that was designed to be sensitive to early changes in young and middle ages. The specific questions addressed were: 1) Are some biological systems more strongly related to cognitive functioning than others? 2) Is there a bigger ‘signal’ in a multi-system index, like allostatic load, than in individual systems? 3) Is the association of allostatic load with cognitive functioning stronger in older adults than in young and middle aged adults?
2. Methods
Data came from the second wave of the Midlife in the United States Study (MIDUS), which included telephone assessment of cognitive functioning and blood and urine assays for biomarkers on sub-samples. The MIDUS study, initiated in 1995, was designed to determine how social, psychological, and behavioral factors inter-relate to influence mental and physical health. The first wave collected sociodemographic and psychosocial data on 7,108 Americans, ages 25 to 74 years, from a sample of English-speaking, non-institutionalized adults residing in the contiguous 48 states, whose household included at least one telephone (recruited by random digit dialing), with oversampling of 5 metropolitan areas, twin pairs, and siblings (Brim et al., 2004). Of the original 7,108 MIDUS participants, 4,963 (70%) were successfully re-contacted and completed the MIDUS II 30-minute phone interview and two self-assessment questionnaires 9-10 years later. As in other longitudinal studies, retention was higher among those who were White, married, and had higher levels of education (Radler and Ryff, 2010). To increase the representation of African Americans from urban, low socioeconomic strata in the sample, 592 African American residents were recruited from Milwaukee, WI to participate in MIDUS II.
In addition to the phone interview and self-administered questionnaires, MIDUS II also conducted telephone-based assessment of cognitive function and detailed blood and urine based measurement of biomarkers. Of the 4,963 participants who completed the MIDUS II survey, 4,512 participated in the MIDUS II Cognition Project and completed the telephone assessment of cognitive functioning. Of the 3,191 MIDUS II participants who were deemed medically safe to travel, 1,255 agreed to participate in the MIDUS II biomarker project, which required a 2-day commitment, including travel to one of three general clinical research centers (GCRC): University of California at Los Angeles, Georgetown University, and University of Wisconsin. Reasons given for nonparticipation were travel, family obligations, and being too busy. Data were collected during a 24-hour stay at a GCRC between July 2004 and May 2009. The protocol included a medical history and physical examination, medication review (including examination of pill bottles by staff), a 12-hour overnight urine collection and a fasting blood draw (Love et al., 2010). Blood and urine samples were frozen and shipped to a central laboratory for assays.
Of the 1,152 MIDUS II respondents who participated in both the Cognition and Biomarker projects, 11 participants did not get an allostatic load score because they were missing data for more than one physiological system score (described below), and an additional 65 participants lacked complete covariate data, leaving us with 1,076 participants to constitute our study sample. The resulting study sample was very similar to the complete MIDUS Cognition and Biomarker Project samples with respect to major demographic and health characteristics (Table 1).
Table 1. Descriptive Statistics: median (inter-quartile range) for continuous variables and percentage for categorical variables.
| Study Sample (N=1,076) |
BioMarker Sample (N=1,255) |
Cognition Sample (N=4,512) |
|
|---|---|---|---|
| Age (years) | 57.0 (49.0, 66.0) | 57.0 (48.0, 65.0) | 55.0 (46.0, 65.0) |
| Sex: Female | 57.0 | 56.8 | 55.1 |
| Race White | 82.2 | 77.2 | 84.9 |
| African American | 12.8 | 17.7 | 10.2 |
| Multi-racial | 3.44 | 3.51 | 3.02 |
| Education: High school or less | 25.3 | 27.7 | 33.2 |
| Some college, but did not graduate | 29.4 | 29.9 | 30.0 |
| Parent Education: High school or less | 58.4 | 58.7 | 62.4 |
| Some college, but did not graduate | 18.0 | 18.0 | 15.6 |
| Primary language: English | 97.0 | 97.1 | 96.6 |
| Neurological condition | 10.4 | 11.4 | 10.6 |
| Systolic blood pressure (mm Hg) | 130 (119, 143) | 130 (119, 143) | - |
| Resting pulse pressure (mm Hg) | 54.0 (46, 64.0) | 54.0 (45.0, 64.0) | - |
| Resting heart rate (beats per minute) | 69.0 (64.0, 76.0) | 70.0 (64.0, 79.0) | - |
| Blood glycosylated hemoglobin (%) | 5.82 (5.60, 6.20) | 5.86 (5.60, 6.24) | - |
| Fasting blood glucose (mg/dL) | 96.0 (90.0, 104.0) | 96.0 (90.0, 105) | - |
| Homeostasis model assessed insulin resistance | 2.37 (1.41, 4.22) | 2.40 (1.43, 4.35) | - |
| Body mass index (kg/m2) | 28.5 (25.1, 32.6) | 28.6 (25.2, 33.0) | - |
| Waist to hip circumference ratio | 0.89 (0.82, 0.96) | 0.89 (0.82, 0.97) | - |
| Low density lipoprotein cholesterol (mg/dL) | 102 (81, 129) | 101 (80, 127) | - |
| High density lipoprotein cholesterol (mg/dL) | 52.0 (42.0, 65.4) | 52.8 (42.5, 66.0) | - |
| Serum triglycerides (mg/dL) | 106 (77.0, 155) | 106 (77.0, 155) | - |
| Serum C-reactive protein (mg/L) | 1.37 (0.68, 3.45) | 1.44 (0.69, 3.64) | - |
| Serum interleuken 6 (ng/L) | 2.07 (1.34, 3.39) | 2.15 (1.36, 3.47) | - |
| E-selectin (ng/mL) | 38.2 (27.8, 50.2) | 39.0 (28.1, 51.9) | - |
| Intracellular adhesion molecule-1 (mg/L) | 274 (222, 334) | 273 (219, 335) | - |
| Fibrinogen (mg/dL) | 338 (286, 396) | 341 (290, 399) | - |
| Urine cortisol (mg /gram of creatinine) | 12.0 (7.20, 20.0) | 12.0 (6.70, 19.0) | - |
| Serum dehydroepiandrosterone sulfate (ug/dL) | 86.5 (52, 141) | 86.0 (51, 141) | - |
| Urine epinephrine (mg/gram of creatinine) | 1.69 (1.16, 2.50) | 1.67 (1.13, 2.47) | - |
| Urine norepinephrine (mg/gram of creatinine) | 25.0 (18.2, 32.8) | 24.8 (18.1, 33.0) | - |
| Heart rate resting variability | - | ||
| Low frequency power (msec2) | 244 (114, 505) | 246 (115, 515) | - |
| High frequency power (msec2) | 134 (57.9, 297) | 140 (59, 305) | - |
| R-R interval standard deviation (msec) | 32.4 (23.8, 43.6) | 32.5 (23.7, 44.6) | - |
| Rootmeansquare successive differences (msec) | 18.0 (12.1, 27.1) | 18.4 (12.1, 27.6) | - |
| System-level dysregulation scores | - | ||
| Cardiovascular | 0.33 (0, 0.67) | 0.33 (0. 0.67) | - |
| Glucose metabolism | 0 (0, 0.33) | 0 (0, 0.67) | - |
| Lipid metabolism | 0.20 (0, 0.40) | 0.20 (0, 0.40) | - |
| Inflammation | 0.20 (0, 0.40) | 0.20 (0, 0.40) | - |
| Hypothalamic-pituitary-adrenal axis | 0 (0, 0.50) | 0 (0, 0.50) | - |
| Sympathetic nervous system | 0 (0, 0.50) | 0 (0, 0.50) | - |
| Parasympathetic nervous system | 0 (0, 0.50) | 0 (0, 0.50) | - |
| Allostatic load score | 1.85 (1.03, 2.73) | 1.90 (1.03, 2.77) | - |
| SGST Mixed mode reaction time (seconds) | 1.02 (0.92, 1.17) | - | 1.03 (0.92, 1.18) |
| Episodic memory score | 0.01 (−0.61, 0.64) | - | −0.02 (−0.64, 0.64) |
| Executive function score | 0.18 (−0.44, 0.77) | - | 0.00 (−0.68, 0.70) |
Abbreviation: SGST = Stop and Go Switch Test
2.1. Measurements: Performance on Tests of Cognition
After a brief hearing check, cognition was assessed using the Brief Test of Adult Cognition by Telephone (BTACT) and the Stop and Go Switch Task (SGST), a telephone test of task switching and inhibitory control processes, designed to be especially sensitive to early changes in cognitive functioning (Lachman and Tun, 2008; Tun and Lachman, 2006, 2008). The BTACT includes six accuracy measures of key domains of cognitive aging. These are immediate and delayed 15-word-list free recall measures of episodic verbal memory, digits backward span measure of working memory, a verbal fluency (number of words produced in 60 seconds from the category of animals) measure of executive function and semantic memory, a five-number series pattern completion measure of inductive reasoning, and a backward counting (from 100 in 30 seconds) measure of speed of processing. The SGST provides both accuracy and reaction time measures; we focus on reaction times. Participants were told to respond as quickly as possible to the spoken words “Red” and “Green” either in the normal response mode (i.e., respond “Go” to the stimulus “Green” and “Stop” to the stimulus “Red”) or the reverse response mode (i.e., respond “Stop” to the stimulus “Green” and “Go” to the stimulus “Red”). They first completed single-mode baseline blocks of 20 trials in each of the normal and reverse response modes separately. These were followed by the mixed-mode block that required alternating between the normal and reverse response modes each time a cue to switch was given; this task-switching test assessed executive functions of switching and inhibitory control, and consisted of 14 practice/warm-up trials with mode switching followed by 32 scored trials where reaction times were measured. The switch cue (“normal” or “reverse”) was heard at random intervals of 2-6 trials, in order to minimize predictability and maximize sensitivity to age effects (Kray and Lindenberger, 2000; Van Asselen and Ridderinkhof, 2000). Stimulus and switch timing were controlled by computer, with one-second intervals between a response and the next stimulus, and between mode-switch cue and the following stimulus. Using sound editing software, response times were calculated from onset of stimulus to onset of response, averaged over the 32 scored trials (both normal and reverse mode trials in the mixed mode block), and multiplied by −1, so higher scores would correspond to faster reaction times. In order to ensure that participants were performing the task as directed, response times from 110 participants who did not meet 75% or better accuracy criteria, or had extreme values (> 4 seconds) were deemed missing (Tun and Lachman, 2008).
Two summary measures, an episodic memory measure and an executive function measure, were created based on exploratory and confirmatory factor analysis of BTACT item scores and SGST mixed-mode response times (Lachman et al., 2010). The episodic memory measure is comprised of scores on immediate and delayed word recall; the remaining four BTACT items (backward counting, digit span backward, number series, and category fluency) and the SGST mixed-mode latency measure comprise the executive function score. Each summary score was computed as the mean of standardized z-scores of component items, which was then also standardized to mean zero and standard deviation (SD) one. Individuals with missing component scores got a summary score only if they had scores for at least half the components (at least one of two components for the episodic memory score, and at least three of five components for the executive function score). Individuals with scores based on incomplete data were flagged, and these flags were included as covariates in regression models. Thus, while episodic memory score was available for 1,072 participants in the sample (and 33 were flagged for missing one of the two components: either immediate or delayed recall), executive function score was available for all 1,076 participants (and 99 were based on missing data for up to two of the five components).
2.2. Measurements: Biomarkers
Functioning of major physiological systems thought to be related to adult cognition was assessed via a comprehensive range of biological and anthropometric measurements, between two and five measures per system; cardiovascular functioning: resting systolic blood pressure, pulse pressure, and heart rate; glucose metabolism: blood levels of glycosylated hemoglobin, fasting glucose, and homeostasis model assessment of insulin resistance; lipid metabolism: body mass index, waist-to-hip circumference ratio, and serum levels of low and high density lipoprotein (LDL and HDL, respectively) cholesterol and triglycerides; chronic inflammation: serum levels of C-reactive protein, interleukin-6, E-selectin, intracelleular adhesion molecule-1, and fibrinogen; hypothalamic pituitary adrenal axis functioning: overnight urinary excretion of cortisol and serum levels of dehydroepiandrosterone sulfate (DHEA-S); sympathetic nervous system functioning: overnight urinary epinephrine and norepinephrine; and parasympathetic nervous system functioning: resting heart rate variability (HRV) parameters: low-frequency spectral power, high-frequency spectral power, the standard deviation of R-R (heartbeat to heartbeat) intervals, and the root mean square of successive differences. Details of laboratory assays and HRV measurement protocols have been published (Crowley et al., 2011; Love et al., 2010). The choice of biomarkers assessed for each system was based on biological plausibility and prior empirical evidence of responsiveness to life stresses as well as links to long-term health outcomes (Gruenewald et al., 2012; Karlamangla et al., 2002; McEwen 2000; Seeman et al., 2010), and limited by considerations of cost and participant burden. With respect to inflammation, for example, C-reactive protein, interleukin-6, E-selectin, intracelleular adhesion molecule-1, and fibrinogen, have each been linked to psychosocial stressors (Brunner et al., 1996; Friedman et al., 2005; Friedman et al., 2009; Packard et al., 2012; Taylor et al., 2006;) and to downstream health outcomes (Harris et al., 1999; Hwang et al., 1997; Peters et al., 2013).
2.1.1. Allostatic Load
Multi-system dysregulation, or allostatic load, has been proposed as the accumulated biological signature of recurring exposure to stressors, and the biological pathway from life stresses to ill health (McEwen and Stellar 1993). We computed allostatic load as the sum of seven system-level dysregulation scores. Dysregulation scores for each system (range, 0-1) were calculated as the proportion of that system’s biomarkers in the highest-risk quartile of its distribution. Despite differences between systems in numbers of biomarkers measured, each system was thus scored on the same 0-1 scale. It should be noted that the highest-risk quartile is the bottom quartile for HDL cholesterol, DHEA-S, and the four resting HRV measures, which are each associated inversely with adverse outcomes; it is the top quartile for all other biomarkers, which are generally associated positively with adverse health outcomes. Quartile cut points used for the scoring were from biomarker distributions in the MIDUS II Biomarker Sample with participants from the Milwaukee sample excluded (so as to more closely resemble distributions from a national sample).
Resulting cut points are very close to disease/treatment thresholds for clinical risk factors such as blood pressure, glucose, lipids, and body mass index (Table 2). Of note, participants on anti-hypertensive medications were scored as being in the high-risk quartile of systolic blood pressure, those on diabetes medications as in the high-risk quartile of fasting glucose and of glycosylated hemoglobin, those on heart rate reducing medications (e.g., beta blockers and atrio-ventricular nodal blockers) as in the high-risk quartile of resting heart rate, those on statins, cholesterol absorption inhibitors, niacin, and/or bile acid sequestrants as in the high-risk quartile of LDL cholesterol, and those on fibrates as in the high-risk quartile of serum triglycerides, even if the measured value of the biomarker was not in the high-risk zone. Use of medications typically prescribed to lower a clinical risk factor is an indication of native dysregulation of that biomarker and of exposure to high-risk levels of the risk factor prior to (and during titration of) therapy. Since effects of dysregulated biology on cognition (and most chronic health outcomes) are cumulative over time, historical exposure to high-risk levels is also of interest.
Table 2. Cut points for System-level and Allostatic Load Scoring.
| Biomarkers by System | Cut Points |
|---|---|
| Cardiovascular | |
| Systolic blood pressure (mm Hg)a | ≥143 |
| Resting pulse pressure (mm Hg) | ≥65 |
| Resting heart rate (beats per minute)a | ≥77 |
| Glucose metabolism | |
| Blood glycosylated hemoglobin (%)a | ≥6.1 |
| Fasting blood glucose (mg/dL)a | ≥105 |
| Homeostasis model assessed insulin resistance | ≥4.04 |
| Lipid metabolism | |
| Body mass index (kg/m2) | ≥32.3 |
| Waist to hip circumference ratio | ≥0.97 |
| Low density lipoprotein cholesterol (mg/dL)a | ≥128 |
| High density lipoprotein cholesterol (mg/dL) | ≤41.4 |
| Serum triglycerides (mg/dL)a | ≥160 |
| Inflammation | |
| Serum C-reactive protein (mg/L) | ≥3.18 |
| Serum interleuken 6 (ng/L) | ≥3.18 |
| E-selectin (ng/mL) | ≥50.6 |
| Intracellular adhesion molecule-1 (mg/L) | ≥330 |
| Fibrinogen (mg/dL) | ≥390 |
| Hypothalamic-pituitary-adrenal axis | |
| Urine Cortisol (mg /g of creatinine) | ≥21.0 |
| Serum dehydroepiandrosterone sulfate (ug/dL) | ≤51.0 |
| Sympathetic nervous system | |
| Urine epinephrine (mg/g of creatinine) | ≥2.54 |
| Urine norepinephrine (mg/g of creatinine) | ≥33.3 |
| Parasympathetic (heart rate variability) | |
| Low frequency power (msec2) | ≤114 |
| High frequency power (msec2) | ≤54.2 |
| R-R interval standard deviation (msec) | ≤23.5 |
| Root mean square successive differences (msec) | ≤11.8 |
Scored as high-risk if taking medications that are generally prescribed to lower these risk factors, even if the measured biomarker is below the cut point.
System scores were only computed if participants had data on half or more of the system’s biomarkers. Fewer than 20 participants got system scores based on incomplete biomarker data. The multi-system allostatic load score, range 0-7, was computed only for participants who had scores for at least six of the seven systems, with the missing system score imputed (for 105 participants) as described below. For 83 patients who were missing the parasympathetic score but had data on all other systems, we imputed the allostatic load score from the participants’ scores on the other six systems, age, gender, and race, using a regression equation derived from those with complete biomarker data. For the 22 participants who were each missing exactly one of the other 6 system scores, the missing system score was imputed as zero (since the sample median for five of the seven system scores, and the sample mode for all system scores was zero). An allostatic load imputation flag was created to indicate people with allostatic load score based on six system scores, and was included as a covariate in regression models.
2.3. Measurements: Demographic and Socioeconomic
Age, gender, highest achieved education level, chronic health conditions, primary language spoken at home when growing up, and highest educational level attained by father (or other male head of household) and mother (or other female head of household) were obtained from self reports. The higher of mother’s and father’s education levels was recorded as parent education level. Race/ethnicity was self-identified as white, Black/African-American, other, or multiracial. If a participant reported a different primary race at the MIDUS I and II assessments, then the participant was classified as multiracial. Since the number of participants in the Other and multi-racial groups was small (n=55), for the purposes of this analysis, we combined them with the African-American group, and denoted the bigger group Non-white.
2.4. Statistical Analyses
We first examined LOESS smoothed plots of the two summary cognition scores (episodic memory and executive function) as a function of allostatic load. Since these revealed a monotonic relationship, we next examined the cognition scores as a linear function of the continuous allostatic load score and used multiple linear regression to adjust for age (continuous, linear plus quadratic), gender, own education (continuous plus 3-level categorical: high school or less, less than 4 years of college, vs. 4 or more years of college), parent education (continuous plus 3-level categorical as above), race (White vs. Non-white), primary language (English vs. not English), neurological conditions such as stroke or Parkinson’s disease (yes/no), and three interaction terms for own education (continuous) with age (continuous), race, and gender. The choice of covariates was designed to minimize residual confounding by stable, individual-level characteristics known to have large associations with cognitive functioning. In particular, we included multiple terms for parent education, age, and own education to capture non-linear associations with age, step effects of education credentials, and differential influences of education by cohort, race, and gender. To account for clustering within family members (siblings and twins), we used STATA’s cluster option with robust, empirical estimation of standard errors (StatCorp, 2007).
We also examined the individual system-level dysregulation scores as predictors of the two summary cognition scores, adjusted for the same covariates, in separate linear regression models. To test the appropriateness of the equi-weighted scoring of allostatic load, we compared the proportion of variance in cognition scores explained (R-squared) by allostatic load (plus covariates) to the proportion explained by a model which had all seven system-level scores (plus same covariates). Since the latter allows for different contributions to the prediction by different systems, this comparison serves as an empirical test of the appropriateness of the equi-weighted approach to allostatic load scoring.
Finally, we tested for modification of the allostatic load effect by gender and age, by separately adding interactions with gender and dichotomized age (65 years or older vs. younger than 65) to the allostatic load model. All analyses were conducted using STATA version 10.1 (StataCorp LP: College Station, TX).
3. Results
The study sample (N=1,076) was similar to the complete MIDUS Cognition and Biomarker Project samples (N=4,512 and 1,255 respectively) with respect to major demographic and health characteristics (Table 1). Median age in the study sample was 57 years, 57% were female, and 82% were Caucasian. Median allostatic load score was 1.9 and inter-quartile range (IQR) was (1.0, 2.7); median cognition scores were 0.01 (IQR −0.61, +0.64) for episodic memory and 0.18 (IQR: −0.44, +0.77) for executive function. All three scores had symmetric, near-normal distributions; skew ranged from −0.01 to 0.45 (normal if 0) and kurtosis from 2.5 to 3.1 (normal if 3). The seven system scores that contribute to allostatic load had means between 0.23 and 0.38, and standard deviations (SD) between 0.26 and 0.36. The seven system scores were not highly correlated with each other; pair-wise correlation coefficients ranged from 0.04 to 0.40; median value 0.16.
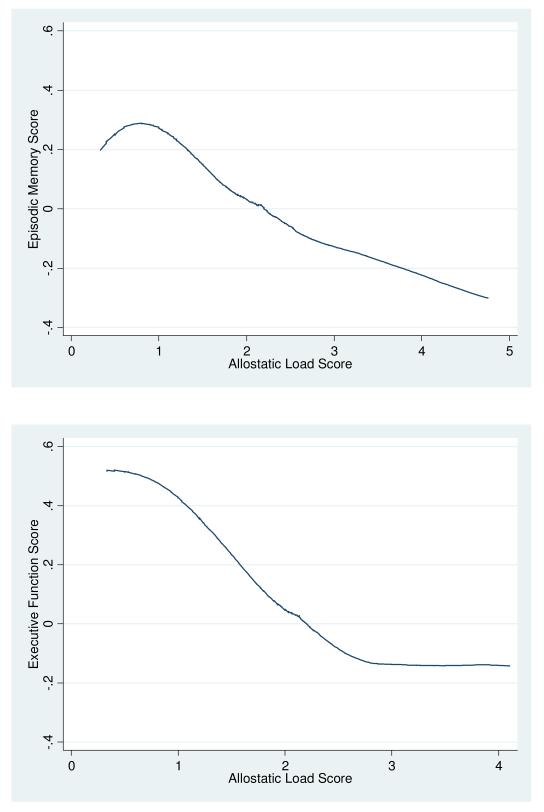
In LOESS-smoothed (bandwidth 0.8) plots of cognition as a function of allostatic load, both summary cognition scores had monotonically decreasing relationships with increasing allostatic load score over its entire range, without any obvious threshold or non-linear pattern (Figure 1).
Figure 1.
LOESS smoothed (bandwidth 0.8) plots of cognition scores versus allostatic load score
Panel A: Episodic Memory
Panel B: Executive Function
In linear regression analysis with robust estimation of standard errors accounting for within-family clustering, the continuous allostatic load score was strongly and inversely related to both cognition scores before adjusting for covariates: Each unit increment in the allostatic load score was associated with 0.170 decrement in episodic memory score (95% confidence interval (CI): −0.215, −0.126; p<0.001) and 0.239 decrement in executive function score (95% CI: −0.286, −0.192; p<0.001). The proportion of cognition score variance explained by the allostatic load score (in the sample that needed no imputation for allostatic load score or the cognition score) was 4.9% for episodic memory and 7.3% for executive function. In the same sample, in parallel models that included all seven system scores together, the proportion of variance explained by the seven system scores was only moderately higher: 5.9% for episodic memory and 8.7% for executive function. In parallel models that examined one system score at a time, the cardiovascular system score had the strongest associations with both cognition scores, and explained 3.1% of the variance in episodic memory and 5.1% of the variance in executive function.
After adjusting for age, sex, race, own education level (including education interactions with age, sex, and race), parent education, primary language, prevalent neurological conditions, and imputation flags for allostatic load and the cognition score, the associations reduced in magnitude but remained strong: Each unit increment in the allostatic load score was associated with 0.065 decrement in episodic memory score (p=0.008) and 0.055 decrement in executive function score (p=0.02) – See Table 3. This translates to 0.074 decrement in episodic memory (95% confidence interval (CI): −0.129, −0.019) and 0.063 decrement in executive function (95% CI: −0.144, −0.014) per SD increment in allostatic load. Adjusted for these covariates, an individual with allostatic load score at the 75th percentile (of 2.7) would score 0.11 lower on episodic memory and 0.09 lower on executive function than an individual with allostatic load score at the 25th percentile (of 1.0). A unit increment in allostatic load score had the same adjusted association with episodic memory as being 3.5 years older or having one less year of education; a unit increment in allostatic load had the same effect on executive function as being 2.5 years older. The average age and education associations with cognition scores (from models without non-linear age or education terms, and without age or education interaction terms) were 0.019 decrement in episodic memory score (95% confidence interval, −0.023, −0.014) and 0.022 decrement in executive function score (95% confidence interval, −0.027, −0.018) per additional year of age, and 0.068 increment in episodic memory score (95% confidence interval, 0.046, 0.090) and 0.100 increment in executive function score (95% confidence interval, 0.079, 0.122) per additional year of education.
Table 3. Adjusted associationsa of biology with cognition scores.
| Episodic Memory Score (N=1,072) |
Executive Function Score (N=1,076) |
|
|---|---|---|
| Allostatic load score (range 0-7, SD 1.14) | − 0.065** (−.113, −.017) | − 0.055* (−.100, −.010) |
|
| ||
| System-level scores (range 0-1) | ||
|
| ||
| Cardiovascular system score (SD 0.34) | − 0.25** (−.41, −.10) | − 0.17* (−.32, −.02) |
| Glucose metabolism score (SD 0.35) | − 0.13‡ (−.28, +.02) | − 0.20** (−.34, −.06) |
| Lipid metabolism score (SD 0.26) | − 0.11 (−.30, +.08) | − 0.19‡ (−.39, +.00) |
| Inflammation score (SD 0.26) | − 0.20* (−.39, −.01) | − 0.07 (−.24, +.11) |
| HPA axis score (SD 0.31) | + 0.05 (−.12, +.21) | + 0.02 (−.13, +.17) |
| Sympathetic system score (SD 0.35) | − 0.05 (−.20, +.10) | + 0.01 (−.12, +.14) |
| Parasympathetic system score (SD 0.36) | − 0.07 (−.21, +.07) | − 0.07 (−.21, +.06) |
Abbreviation: SD = Standard deviation; HPA = Hypothalamic-pituitary-adrenal
Results from separate models for allostatic load and each system score as primary predictor, adjusted for age (continuous, linear plus quadratic), sex, race (White vs. non-White), education (high school or less vs. some college vs. 4-year college graduate or more), parental education (continuous and high school or less vs. some college vs. 4-year college graduate or more), primary language (English vs. not), and neurological conditions (yes/no), and interactions for education with race, gender and age. Associations presented as point estimates (95% confidence limits). Confidence limits based on robust estimates of standard error that account for clustering within families.
p < 0.1;
p < 0.05;
p< 0.01
Adjusted for all covariates, only three of the seven system-level dysregulation scores had statistically significant inverse relationships with cognition, but only one system score (namely, the cardiovascular system score) had a statistically significant inverse relationship with both episodic memory and executive function (Table 3). Cardiovascular dysregulation had the strongest association with episodic memory: Each SD increment in the cardiovascular system score was associated with 0.086 decrement in episodic memory score (p=0.001). In addition, inflammation was also associated with lower scores on episodic memory (p=0.04). Glucose dysregulation had the strongest association with executive function: Each SD increment in the glucose metabolism score was associated with 0.067 decrement in executive function score (p=0.06).
In interaction testing, neither gender nor old age (being 65 years or older) modified the associations of allostatic load with episodic memory (p values 0.2 for gender and 0.3 for age) and executive function (p values 0.5 for gender and 0.4 for age).
3.1. Sensitivity Analyses
Parallel analyses with an alternately scored allostatic load which ignored medication use and relied only on measured values of the 24 biomarkers yielded very similar results: Adjusted effect size per unit increment in allostatic load score was −0.063 (p=0.049) for episodic memory and −0.056 (p=0.053) for executive function.
In analyses restricted to the sample that had complete data for allostatic load (i.e., no missing system-level score) as well as complete data for cognition summary score (i.e., no missing component scores; N=938 for episodic memory and 886 for executive function), the adjusted associations of allostatic load with cognition summary scores remained as strong: −0.079 (p=0.003) for episodic memory and −0.060 (p=0.014) for executive function.
4. Discussion
As hypothesized, the multi-system allostatic load score had strong inverse associations with both episodic memory and executive function in this national cross section of adults, aged 34 years and older. In unadjusted analyses using LOESS plots, both episodic memory and executive function score gradually declined by around half a standard deviation as allostatic load increased over the sample from 0.5 to 3.5. Since no single system contributed more than one point to the allostatic load score, this decline over the entire observed range of allostatic load is consistent with the hypothesis that cognitive performance is lower when more systems are dysregulated.
As to the individual systems themselves, only the cardiovascular system score was associated strongly with both episodic memory and executive function. In addition, inflammation was associated inversely with episodic memory but not with executive function, and the glucose metabolism score was associated inversely with executive function but not with episodic memory. Hypertension and inflammation have both been linked to changes in the hippocampus (Marsland et al., 2008; Sabbatini et al., 2002), and hypertension and glucose dysregulation have also been linked to white matter lesions (van Dijk et al., 2004; Yau et al., 2010), pointing to probable mechanisms by which peripheral biology may influence episodic memory and executive function.
This study highlights the need to combine information from multiple systems when assessing an individual’s sub-clinical physiological status relevant to cognitive health. Median allostatic load score in this national sample was 1.9, but median system dysregulation scores were no greater than 0.33, which implies that the majority of the population has dysregulation in multiple systems. The differential associations of individual systems with cognitive function observed here (in that some systems were more strongly correlated with cognition scores than others) might suggest that some systems should be weighted more heavily than others in the creation of a multi-system score. However, the model with seven system scores explained only a modestly greater proportion of the variance in cognition scores than the model with the single allostatic load score, suggesting that the equi-weighted approach to multi-system scoring adopted here performs reasonably well in predicting cognitive function in a cross section of the population.
The associations of allostatic load with episodic memory and executive function were not different by age, suggesting that the implications of high allostatic load on cognitive functioning are not restricted to older adults, where most previous studies have been conducted. This finding is consistent with a recent study that found that a biomarker risk score based on traditional clinical risk factors for cardiovascular disease is inversely associated with cognitive functioning in adults aged 20-59 years (Kobrosly et al., 2012).
Study limitations relating to the cross-sectional design should be noted. Cross-sectional differences in cognitive functioning in this sample may be dominated by stable, between-person differences in levels of peak functioning achieved (secondary to genetics, native intelligence, childhood circumstances, and education level, for example) and less indicative of differences in cognitive decline from previously achieved peaks (Deary et al., 2010). We adjusted for differences in education, primary language spoken, parent’s education, and demographic characteristics; but will not have completely controlled for all genetic and environmental factors that contribute to differences in peak cognitive abilities. However, there is good evidence that cognitive declines begin early in life (Salthouse, 1996; Singh-Manoux et al., 2011); between-person differences in cognitive decline rates would also contribute to cognitive performance gradients seen in this study. Also, social stressors over the life course (including childhood) can affect the level of peak cognitive functioning via allostatic load pathways (Luecken, 2006; Lupien et al., 2009; Tun et al., 2013). For instance, childhood socioeconomic conditions appear to influence childhood cognitive ability via effects on allostatic load (Lupien et al., 2001), and allostatic load in childhood is negatively associated with cognitive functioning in young adults (Evans and Schamberg, 2009). Even if allostatic load were related only to peak levels of cognitive function and not to rates of cognitive aging, it would still mean that allostatic load would predict incidence of dementia, since people with high allostatic load and thus, lower peak cognitive abilities, would reach the dementia threshold at younger ages (Karlamangla et al., 2009; Meng and D’Arcy 2012; Schmand et al., 1997; Stern 2009). A related limitation of the cross-sectional design is the inability to infer a causal role for allostatic load in poor cognitive functioning. We cannot rule out the possibility that lower cognitive abilities cause the physiological dysfunction that was seen associated with it. At least one prior study has shown that childhood intelligence predicts inflammation in adulthood (Luciano et al., 2009). Another possible explanation for the findings is a common cause, such as genes (including but not limited to ApoE genotype) and childhood circumstances, that leads to both high allostatic load and poor performance on cognition testing (Deary et al., 2009); genotype date was not available in the study, and controls for own and parental educational attainment may only partly alleviate this concern.
We submit, however, that these limitations are outweighed by several notable strengths, including sample size and diversity, sensitivity of the cognition tests to early changes, comprehensive assessment of biomarkers across multiple regulatory systems, incorporation of medication use in the assessment of native dysregulation, and empirical testing of the equi-weighted operationalization of allostatic load. The study is based on a large national data set that includes a more diverse sample than many previous studies with regard to age and education levels. The study also includes a broad test battery that covers key aspects of cognition that are associated with cognitive aging and are sensitive to changes across the adult lifespan (Lachman and Tun, 2008; Tun and Lachman, 2008). The inclusion of fasting blood assays allowed measurement of LDL cholesterol, triglycerides, and insulin resistance, and the collection of overnight urines in a standardized GCRC setting allowed for neuro-endocrine hormone measurements from the sympathetic and HPA systems. In addition, the measurement of heart rate variability allowed assessment of the functioning of the parasympathetic system for the first time in a large national sample.
In conclusion, the multi-system allostatic load score is strongly and inversely associated with cognitive functioning in middle-aged and older adults; the greater the ‘reach’ of dysregulation across physiological systems, the lower the individual’s performance on cognition testing. This study showed that this association is equally strong in those younger and older than 65 years of age, and that a single multi-system score predicts cognitive performance across two major domains, episodic memory and executive function. A multi-system score, like allostatic load, may have the potential to shed light on the biological underpinnings of poor cognition, to assist in the identification of adults at increased risk for early onset of cognitive impairment and dementia (Lindeboom and Weinstein, 2004; Storandt, 2008), and to inform development and testing of preventive interventions designed to delay its onset in our rapidly aging population.
Acknowledgements
This work was partially funded by the National Institutes of Health under the following grants: 2P0 1AG020166-07A2, 1R01 AG032271-01A1), M01-RR000865, and 2P30-AG028748-06.
This work was funded by the National Institutes of Health, Bethesda, MD, USA.
The Institutional Review Boards at the University of Wisconsin at Madison, Georgetown University, and University of California at Los Angeles approved the study protocols for the Midlife in the United States Study (MIDUS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of disclosure
There are no actual or potential conflicts of interest.
References
- Brim OG, Ryff CD, Kessler RC. How Healthy are We?: A National Study of Well-being at Midlife. University of Chicago Press; Chicago: 2004. [Google Scholar]
- Brunner E, Davey-Smith G, Marmot M, Cammer R, Bekinka M, O’Brien J. Childhood social circumstances and psychosocial and behavioral factors as determinants of plasma fibrinogen. Lancet. 1996;347:1008–1013. doi: 10.1016/s0140-6736(96)90147-6. [DOI] [PubMed] [Google Scholar]
- Cavalieri M, Ropele S, Petrovic K, Pluta-Fuerst A, Homayoon N, Enzinger C, Grazer A, Katschnig P, Schwingenschuh P, Berghold A, Schmidt R. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care. 2010;33:2489–2495. doi: 10.2337/dc10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley OV, McKinley PS, Burg MM, Schwartz JE, Ryff CD, Weinstein M, Seeman TE, Sloan RP. The interactive effect of change in perceived stress and trait anxiety on vagal recovery from cognitive challenge. Intl J Psychophysiol. 2011;82:225–232. doi: 10.1016/j.ijpsycho.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognitive decline. Psych Aging. 2009;24(1):154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB, Starr JM. Age-associated cognitive decline. Br Med Bull. 2009;92:135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging? Psychol Aging. 2010;25(1):219–28. doi: 10.1037/a0017750. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Mgt. 2008;4(2):363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. PNAS. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: Relation to cognitive functioning. Stress. 2006;9(3):143–152. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005;102(51):18757–62. doi: 10.1073/pnas.0509281102. Epub 2005 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, Ryff CD. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS Study. Brain, Behavior and Immunity. 2009;23(5):684–692. doi: 10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F. How early can cognitive decline be detected? BMJ. 2011;344:d7652. doi: 10.1136/bmj.d7652. doi: 10.1136/bmj.d7652. [DOI] [PubMed] [Google Scholar]
- Gruenewald T, Karlamangla AS, Hu P, et al. History of socioeconomic disadvantage and multi-system physiological health in later life. Soc Sci Med. 2012;74(1):75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Sollers JJ, 3rd, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur J Appl Physiol. 2004;93:263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hwang S-J, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases. The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on heatlh and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Wight RG, Seeman TE, Chodosh J. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. Amer J Epidemiol. 2009;170(3):331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiol Aging. 2005a;26(Suppl 1):S80–S84. doi: 10.1016/j.neurobiolaging.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Greendale GA, Seeman TE. Increase in urinary epinephrine excretion is positively associated with subsequent cognitive decline in elderly men: MacArthur Studies of Successful Aging. Psychoneuroendocrinology. 2005b;30(5):453–460. doi: 10.1016/j.psyneuen.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline: MacArthur Studies of Successful Aging. J Clin Epidemiol. 2002;55(7):696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disable women in the community: Women’s Health and Aging Study I. J Amer Geriatr Soc. 2006;54(11):1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Seplaki CL, Jones CM, van Wijngaarden E. Physiologic dysfunction scores and cognitive function test performance in US adults. Psychosom Med. 2012;74(1):81–8. doi: 10.1097/PSY.0b013e3182385b1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychol Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 2010;18(1):4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Tun PA. Cognitive testing in large-scale surveys. Assessment by telephone. In: Hofer SM, Alwin DF, editors. Handbook of Cognitive Aging: Interdisciplinary Perspectives. Sage Publications; Thousand Oaks: 2008. pp. 506–523. [Google Scholar]
- Laurin D, David Curb J, Masaki KH, White LR, Launer LJ. Midlife C-reactive protein and risk of cognitive decline: A 31-year follow up. Neurobiol Aging. 2009;30:1724–1727. doi: 10.1016/j.neurobiolaging.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R, Prettyman R, Cherryman G. White matter lesions on magnetic resonance imaging and their relationship with vascular risk factors in memory clinic attenders. Int J Geriatr Psychiatry. 2005;20(3):274–9. doi: 10.1002/gps.1283. [DOI] [PubMed] [Google Scholar]
- Lindeboom J, Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, and vascular cognitive impairment. Eur J Pharmacol. 2004;490:83–86. doi: 10.1016/j.ejphar.2004.02.046. [DOI] [PubMed] [Google Scholar]
- Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Marioni RE, Gow AJ, Starr JM, Deary IJ. Psychosom Med. 2009;71:404–409. doi: 10.1097/PSY.0b013e3181a24fb9. [DOI] [PubMed] [Google Scholar]
- Luecken LJ. Early family adversity and cognitive performance in aging: A lifespan developmental model. J Soc Clin Psychol. 2006;25(1):33–52. [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleuken-6 covaries inversely with hippocampal grey matter volume in middle aged adults. Biol Psychiatry. 2008;64(6):484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative Analyses. PLoS ONE. 2012;7(6):e38268. doi: 10.1371/journal.pone.0038268. doi:10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann KF, Rojo L, Navarrete LP, Farías G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: Molecular links and clinical implications. Curr Alz Res. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Bezlyak V, McLean JS, et al. Early life socioeconomic adversity is associated in adult life with chronic inflammation, carotid atherosclerosis, poorer lung function, and decreased cognitive performance: A cross-sectional, population-based study. BMC Public Health. 2011;11:42. doi: 10.1186/1471-2458-11-42. doi:10.1186/1471-2458-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SAE, Woodward M, Rumely A, et al. Direct comparisons of three alternative plasma fibrinogen assays with the von Clauss assay in prediction of cardiovascular disease and all-causes mortality: The Scottish Heart Health Extended Cohort. Brit J Haematology. 2013;162(3):392–399. doi: 10.1111/bjh.12389. [DOI] [PubMed] [Google Scholar]
- Porter NM, Landfield PW. Stress hormones and brain aging: Adding injury to insult? Nat Neurosci. 1998;1(1):3–4. doi: 10.1038/196. [DOI] [PubMed] [Google Scholar]
- Qiu W, Folstein MF. Insulin, insulin-degrading enzyme, and amyloid-β peptide in Alzheimer’s disease: Review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff C. Who Participates? Accounting for Longitudinal Retention in the MIDUS National Study of Health and Well-Being. J Aging Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S Roriz-Filho J, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriguti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochimica et Biophysica Acta. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Sabbatini M, Catalani A, Consoli C, Marletta N, Tomassoni D, Avola R. The hippocampus in spontaneously hypertensive rats: An animal model of vascular dementia. Mech Ageing Dev. 2002;123(5):547–559. doi: 10.1016/s0047-6374(01)00362-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychol Med. 1997;27(6):1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation - Allostatic load and its health consequences. Arch Intern Med. 1997;157:2259–2268. Erratum in: Arch Intern Med; 159(11):1176. [PubMed] [Google Scholar]
- Seeman TE, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. N.Y. Acad. Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of the onset of cognitive decline: Results from Whitehall II prospective cohort study. BMJ. 2011;344:d7622. doi: 10.1136/bmj.d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . Stata 10 Base Reference Manual. Stata Press; College Station: 2007. [Google Scholar]
- Storandt M. Cognitive deficits in the early stages of Alzheimer’s Disease. Curr Dir Psychol Sci. 2008;17:198–202. [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing. 2006;35(6):629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national sample of adults: education, sex, and task complexity matter. Dev Psychol. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, Miller-Martinez D, Lachman ME, Seeman T. Social strain and executive function across the lifespan: The dark (and light) sides of social engagement. Aging, Neuropsychology & Cognition. 2013;20(3):320–328. doi: 10.1080/13825585.2012.707173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asselen M, Ridderinkhof KR. Shift costs of predictable and unexpected set shifting in young and older adults. Psychologica Belgica. 2000;40:259–273. PMCID: PMC3508192. [Google Scholar]
- Van Dijk EJ, Breteler MMB, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: Cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychol. 2003;22:559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, dan Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Yau PL, Javier DC, Ryan CM, Tsui WH, Ardekani BA, Ten S, Convit A. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53(11):2298–306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinksi EM, Gilewski MJ. Effects of demographic and health variables on Rasch scaled cognitive scores. J Aging Health. 2003;15:435–464. doi: 10.1177/0898264303253499. [DOI] [PubMed] [Google Scholar]
- Zelinksi EM, Crimmins A, Reynolds S, Seeman T. Do medical conditions affect cognition in older adults? Health Psychol. 1998;17:504–512. doi: 10.1037//0278-6133.17.6.504. [DOI] [PubMed] [Google Scholar]
- Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, De Vecchi M, Romanelli G, Grassi V, Padovani A. QT dispersion and heart rate variability abnormalities in Alzheimer’s disease and in mild cognitive impairment. J Amer Geriatr Soc. 2005;53(12):2135–2139. doi: 10.1111/j.1532-5415.2005.00508.x. [DOI] [PubMed] [Google Scholar]