Abstract
Photoperiodism is a biological phenomenon, common among organisms living outside of the tropics, by which environmental day length is used to ascertain time of year to engage in seasonally-appropriate adaptations. White-footed mice (Peromyscus leucopus) are small photoperiodic rodents which display a suite of adaptive winter responses to short day lengths mediated by the extended duration of nightly melatonin secretion. Exposure to short days alters hippocampal dendritic morphology, impairs spatial learning and memory, and impairs hippocampal long term potentiation (LTP). To determine the role of melatonin in these photoperiod-induced alterations of behavioral, neuroanatomical, and neurophysiological processes in this species, we implanted male mice subcutaneously with melatonin or empty Silastic capsules and exposed them to long or short day lengths. After ten weeks, mice were assessed for hippocampal LTP, tested for spatial learning and memory in the Barnes maze, and morphometric analysis of neurons in the hippocampus using Golgi staining. Extending the duration of melatonin exposure, by short day exposure or via melatonin implants, impaired both Schaffer collateral LTP in the CA1 region of the hippocampus and spatial learning and memory, and altered neuronal morphology in all hippocampal regions. The current results demonstrate that chronic melatonin implants reproduce the effects of short days on the hippocampus and implicate melatonin signaling as a critical factor in day-length induced changes in the structure and function of the hippocampus in a photoperiodic rodent.
Keywords: photoperiod, melatonin, hippocampus, LTP, Barnes maze, neuronal morphology
1. Introduction
Monitoring of environmental day length in mammals living outside of the tropics is critical to coordinate seasonally-appropriate adaptations in physiology and behavior to promote survival and reproductive success. In mammals, nocturnal melatonin (MEL) synthesis and secretion from the pineal gland is the putative endocrine signal of day length (Yellon et al., 1982, Carter and Goldman, 1983, Bittman and Karsch, 1984, Reiter et al., 2010). However, it is the duration of elevated MEL exposure, not peak concentrations, that conveys environmental photic information (Bittman and Karsch, 1984, Bartness et al., 1993, Reiter, 1993). Thus, short and extended durations of MEL exposure are physiologically indicative of long and short days respectively. Extending the daily duration of MEL exposure via exogenous supplementation is interpreted biologically as a short day, and can drive seasonally-appropriate adaptive responses (Tamarkin et al., 1977, Johnston and Zucker, 1980, Carter and Goldman, 1983, Dark et al., 1983, Bartness et al., 1993).
The most striking and extensively studied of these day length (photoperiodic) driven adaptive responses are seasonal rhythms of reproduction (Malpaux et al., 2001). Small mammals such as rodents generally reproduce in long days. As autumnal day lengths shorten, and the duration of nocturnal melatonin synthesis lengthens, the reproductive axis is inhibited, presumably to shunt energy away from reproduction to maximize survival across the harsh short days of winter (Walton et al., 2011b). However, melatonin can directly and indirectly affect photoperiod-driven seasonal rhythms in non-reproductive physiology, immune function, and behavior, which occur via both gonadal steroid-dependent and -independent mechanisms (Walton et al., 2011b).
Among photoperiodic rodents, white-footed mice (Peromyscus leucopus) are particularly well-suited to investigate photoperiod-induced changes in brain function and behavior. Compared to counterparts held in long summer-like day lengths, white-footed mice held in short winter-like days lengths have marked changes in cognitive ability and in the function and connectivity of underlying brain regions associated with these abilities, including olfactory memory and neurogenesis (Walton et al., 2012b), fear memory and the amygdala (Walton et al., 2012a), and spatial memory and the hippocampus (Pyter et al., 2005, Workman et al., 2009). Indeed, we have recently reported that short-day exposure impairs long term potentiation (LTP), the putative mechanism for how memories are formed and stored in the brain (Bliss and Collingridge, 1993), in the Schaffer collateral pathway of the hippocampus, and this impairment of LTP is associated with impaired spatial learning and memory (Walton et al., 2011a).
Although melatonin has been implicated in driving photoperiodic reproductive responses in white-footed mice (Johnston and Zucker, 1980, Petterborg and Reiter, 1980, Dowell and Lynch, 1987, Carlson et al., 1989), the role of melatonin in photoperiodic hippocampal plasticity remains largely unknown. To determine the role of melatonin in photoperiodic brain plasticity, adult male white-footed mice were given subcutaneous implants of Silastic capsules filled with melatonin or left empty, then exposed to long or short day lengths for ten weeks to evoke the full short day phenotype in the blank implant group. Chronic Silastic melatonin implants were used in the current study because they are equally effective as timed injections in reproducing the effects of short day lengths, without the unnecessary chronic repeated stress of months of daily injections (Johnston and Zucker, 1980). After ten weeks in their respective day length and melatonin treatments, one cohort of mice was assessed for Schaffer collateral LTP, and a separate cohort of mice was assessed for spatial learning and memory in the Barnes maze. At the conclusion of spatial testing, brains were Golgi impregnated and the morphology of neurons in the CA1, CA3, and dentate was assessed using computerized morphometric analyses.
2. Experimental procedures
2.1 Animals
A total of sixty-eight male white-footed mice (P. leucopus) were used in this study. All mice were bred in our colony maintained at The Ohio State University, which was derived from wild-caught stock obtained through the Peromyscus Genetic Stock Center at the University of South Carolina. Mice were group housed with same-sex littermates from weaning until reaching adulthood (60-90 days of age). Mice were then pseudo-randomly assigned to the four experimental groups and singly housed thereafter. The pseudo-random assignment ensured that pups from each breeding pair were distributed among experimental groups to avoid litter-specific effects. Throughout the study, mice were housed in cages (32 cm × 18 cm × 14 cm), maintained at constant temperature and humidity (21±4°C, 50±5%), and given ad libitum access to food (Harlan Teklad 8640, Indianapolis, IN, USA) and filtered tap water. All husbandry was provided by Ohio State University Laboratory Animal Resources staff. All animal procedures were approved by the Ohio State University Institutional Animal Care and Use Committee, and were in compliance with guidelines established by the National Institutes of Health and the United States Department of Agriculture (Institute of Laboratory Animal Resources, U.S., 1996).
2.2 Melatonin implants and photoperiod treatment
Under dim lighting, implants were prepared by packing Silastic tubing (1.47 mm ID × 1.96 mm OD × 15mm length; Dow Corning) 10mm with melatonin powder (MEL; Sigma #, St Louis, MO, USA) or left empty, and sealed with Silastic glue (Turek et al., 1976). Prior to use, implants were rinsed twice with 70% EtOH, and soaked in sterile saline. Mice were randomly assigned to treatment group and implanted subcutaneously with either empty (BL) or melatonin (MEL) packed capsules, which remained in place for the duration of the study. At the conclusion of the study, melatonin delivery was verified by visually inspecting the implants and via radioimmunoassay of plasma collected in the middle of the light phase. After surgery, mice were pseudo-randomly assigned to either remain in long day lighting (LD; 16 h light: 8 h dark) or transferred to short day lighting (SD; 8 h light: 16 h dark) forming four experimental groups: LD-BL, LD-MEL, SD-BL, SD-MEL. For both photoperiods, lights were extinguished at 15:00 EST. Mice remained in their respective photoperiods for 10 weeks to induce the suite of adaptive responses to day length (Pyter et al., 2005, Walton et al., 2011a).
2.3 Long-term potentiation (LTP)
After 10 weeks in photoperiod, mice (n = 8 from each group) were tested for hippocampal LTP in the Schaffer collateral pathway as described previously (Walton et al., 2011a). Briefly, during the light phase, mice were rapidly decapitated, and 400 μm thick hippocampal slices were prepared. Slices were held at 28 °C in carboxygenated (95% O2-5% CO2) aCSF containing (in mM) 124 NaCl, 4 MgSO4, 4 KCl, 1.0 Na2HPO4, 4 CaCl2, 26 NaHCO3, and 10 D-glucose. A bipolar stimulating electrode (67 μm, NiCr) was placed in the Schaffer collateral pathway in the striatum radiatum. Following 90 min incubation, a slice was placed in a recording chamber affixed to a microscope (model E600FN, Nikon) with a custom stage (Syskiyou Instruments, Grants Pass, OR). The chamber was continuously perfused with carboxygenated ACSF at a rate of 2 ml/min. Field excitatory post-synaptic potentials (fEPSPs) were recorded using patch clamp amplifier (model 2400, AM Systems) with a glass pipette (1–2 MΩ) filled with aCSF. After obtaining a stimulus-response curve, the intensity of a test stimulus (0.033 Hz, 0.1 ms pulse duration) was selected to yield 30-40% of the maximal response. After 30 min of steady baseline recording, LTP was induced using two trains of tetanus stimuli (100 Hz for 1s, 5 min apart) and fEPSPs were recorded during baseline stimulation and lasted for 60 min post-tetanus.
2.4 Spatial learning and memory
After ten weeks in their respective photoperiods, thirty-six mice (n = 9 from each group) were tested for spatial learning and memory ability in a Barnes maze as previously described (Walton et al., 2011a). The Barnes maze (122 cm diameter, white in color), had eighteen 9.5 cm diameter escape holes distributed evenly around the perimeter (ENV-563-R, MedAssociates, St. Albans, VT, USA), and was completely surrounded with a 60 cm high white polycarbonate barrier which had distinct visual cues attached to it at the 4 compass points (black geometric shapes, 20-25 cm in height). The target escape hole contained a black escape box (38.7 × 12.1 × 14.2 cm) whereas the blind escape holes were blocked by black panels visually similar to the escape box. Mice were trained on the maze to find the location of the escape box across five days, which consisted of three 120 s trials per day, with an inter-trial interval of 5-7 min. At the end of each trial, mice were allowed to remain in the escape box for 45-60 s, then returned to their home cage. For training trials, latency to escape, number of errors (attempt to escape in a blind escape hole), and path length were recorded using a video tracking system (HVS Image, Buckingham, UK). After 5 days of training, on the sixth day of testing, mice were given a single 90 s probe trial where they could not escape from the maze (escape box replaced with a blind escape box). For the probe trial, the number of errors and time spent in the vicinity (quadrant of the maze centered at the escape hole) of the former escape hole were recorded. To avoid transfer of olfactory cues, the maze was wiped with 70% EtOH between trials, and the surface was rotated 90° each day, without changing the orientation of the escape box with the extramaze spatial cues.
2.5 Hippocampal neuronal morphology
Twenty-four hours after the completion of behavioral testing, in the middle of the light phase, under deep isoflurane anesthesia, mice were killed and the brains were rapidly removed and processed for Golgi-Cox staining according to the manufacturer's instructions using a commercially available kit as previously described (Pyter et al., 2005, Workman et al., 2009, Walton et al., 2012a). After impregnation, 100 μm coronal brain sections were thaw mounted on gelatin-coated slides, processed, and coverslipped. The neuronal morphology of neurons within the hippocampus (from bregma −1.58 mm to −2.18 mm, corresponding to Plates 44-49 in (Paxinos and Franklin, 2004) was quantified by an experimenter blind to treatment groups, using a Zeiss microscope (Axio Imager A2) equipped with commercially available software (Neurolucida, MBF Bioscience, Williston, VT, USA). For a dendritic complexity analysis of pyramidal neurons in the CA1 and CA3 and for granule cells within the dentate gyrus (DG), neurons were selected if they met the following criteria: 1) were completely and evenly stained, 2) did not have truncated dendrites due to sectioning, and 3) did not overlap with other stained cells. Neurons and their processes (4-6 per region per brain) were traced at 200x and their digital reconstructions were analyzed for dendritic complexity using Sholl analysis. To determine dendritic spine density, spines from each of the neurons selected above were counted on four 20 μm unbranched dendrite segments that were at least 80 μm away from the cell body at 1000x. Any protrusion from the dendrite shaft was counted as a spine, independent of morphology (Pyter et al., 2005, Workman et al., 2011, Walton et al., 2012a).
2.6 Radioimmunoassay
To verify delivery of melatonin from the Silastic implants, 24 h after the completion of behavioral testing, in the middle of the light phase, terminal blood samples were collected through the retro-orbital sinus. Protected from light and held at 4 °C throughout, blood was centrifuged at 6000 RCF for 30 min; plasma was drawn off and frozen at −80 °C until assay. Plasma was assayed for melatonin concentration using a commercially available I125 RIA kit (#01-RK-MEL2; Alpco Immunoassays, Salem, NH, USA) according to manufacturer's instructions. Intra-assay coefficient of variation was <10%.
2.7 Statistics
Plasma melatonin concentrations were analyzed using a Student's t test. Reproductive tissue masses, Barnes maze probe, neuronal cell body, dendrite length, and spine density data were analyzed by ANOVA. Significant differences were followed up by LSD post-hoc tests. Barnes maze acquisition, LTP, and dendritic Sholl analysis data were analyzed using repeated measures ANOVA. All analyses were performed using SPSS software (v.19, IBM, Armonk, NY, USA). For all analyses α was set at 0.05 and mean differences were considered statistically significant if p ≤ 0.05.
3. Results
3.1 Melatonin assay
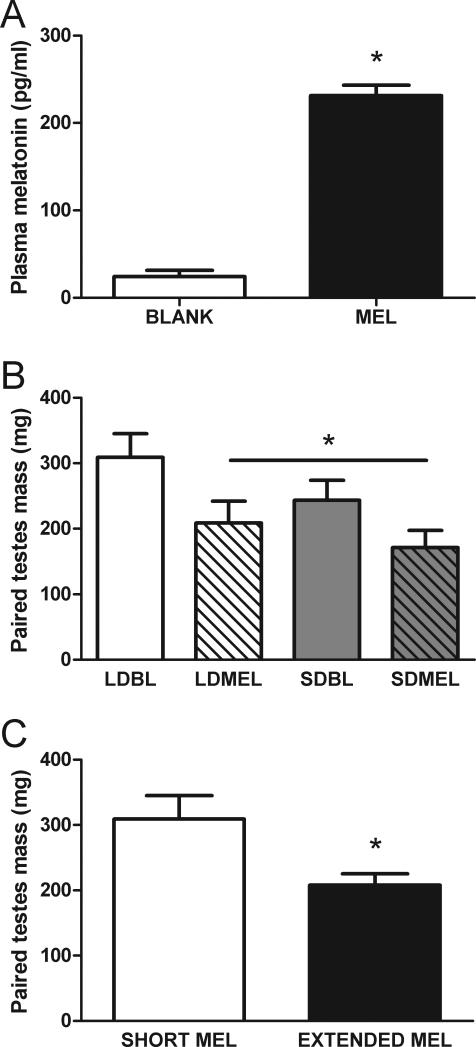
Compared to mice receiving empty capsules, 12 weeks after implantation, in the middle of the light phase, mice with melatonin capsules had elevated plasma melatonin concentrations (Fig 1A; t11 = −14.378, p < 0.05).
Figure 1.
Melatonin delivery from implants and reproductive responses to extended melatonin exposure. A) Independent of photoperiod, 12 weeks after implant surgery, plasma melatonin concentrations in the middle of the light phase were elevated in mice with melatonin implants. B) Extending the duration of daily melatonin exposure by short day lengths (SDBL) or by melatonin implants (LDMEL, SDMEL) reduces paired testes mass. C) Extended duration of daily melatonin exposure reduces paired testes mass (data collapsed by melatonin exposure duration from B). *p < 0.05.
3.2 Reproductive responses to melatonin and SD
Compared to mice in LD with empty implants, extending the daily duration of melatonin exposure via exposure to either short day lengths or melatonin implants, reduced paired testes mass in all other groups (F(3,32) = 3.460, p < 0.05 Fig 1B. t34 = 2.758, p < 0.05 Fig 1C).
3.3 Spatial learning and memory
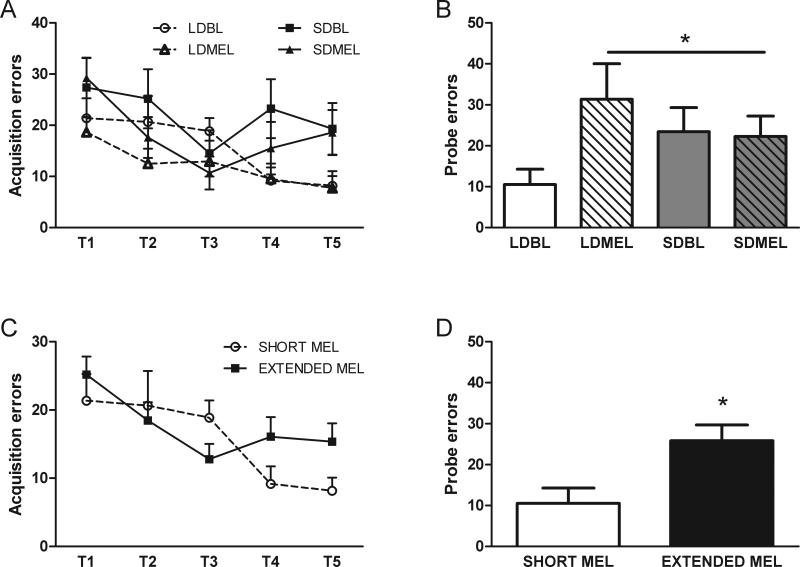
There were no effects of photoperiod (F(1,24) = 3.209, p > 0.05), melatonin implants (F(1,24) = 1.797, p > 0.05), or interaction of day length and melatonin treatment (F(1,24) = 0.085, p > 0.05) on acquisition errors across days in the Barnes maze (Fig 2A). However, compared to LDBL mice, extending melatonin exposure via implants or SD exposure impaired spatial memory (increased errors) during the probe trial (p < 0.05 all comparisons, Fig 2B). When extended melatonin exposure groups are collapsed, as above, in comparison to the short duration of melatonin exposure (LDBL), there were no differences due to extended melatonin duration in acquisition (F(1,26) = 0.029, p > 0.05, Fig 2C) and spatial memory, as measured by the number of errors, was impaired by extended melatonin exposure in the probe trial (t32 = −2.091, p < 0.05, Fig 2D).
Figure 2.
Spatial learning and memory in the Barnes maze. Photoperiod and melatonin treatment did not affect errors (incorrect escape choice) during acquisition trials (A,C). Both SD exposure and melatonin implants impair spatial memory during the probe trial (B,D). Data in C and D are collapsed by melatonin exposure duration from A and B respectively. *p < 0.05.
3.4 Hippocampal long-term potentiation
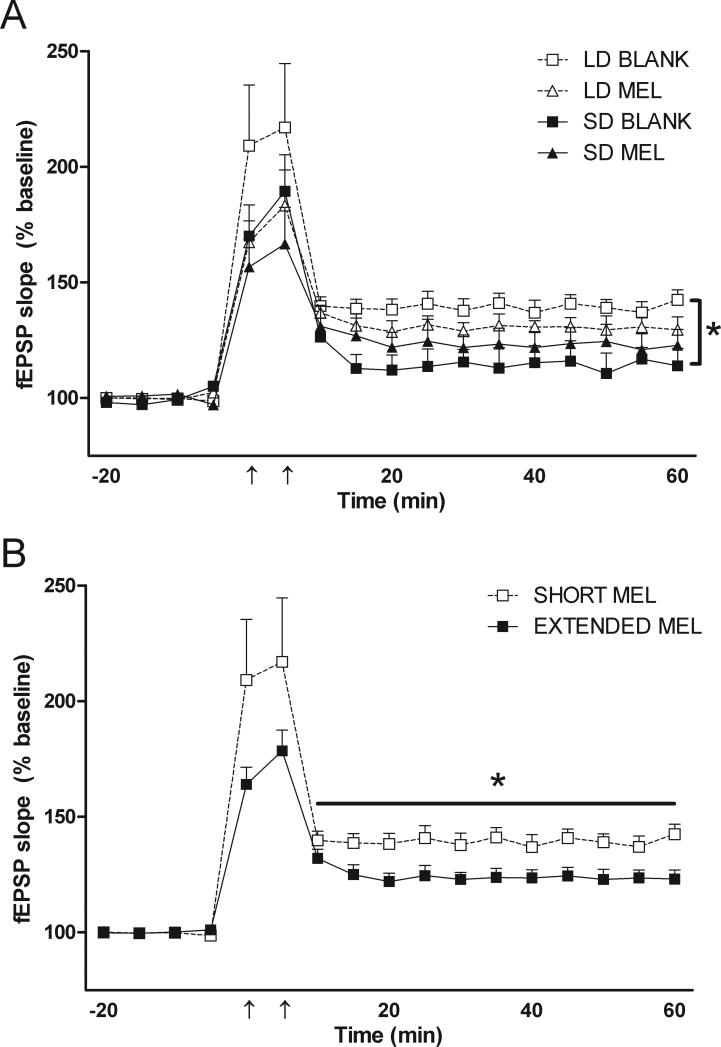
In all 4 groups of mice, LTP was induced with two trains of tetanus stimuli (100Hz, 1sec, and 5 min apart). Consistent with our previous report (Walton et al., 2011a), exposure to short day lengths impaired Schaffer collateral LTP in the CA1 region of the hippocampus in mice with blank implants (F(1,17) = 7.351, p < 0.05, Fig 3A). When analyzed with respect to extended melatonin exposure, compared to mice with a short daily duration of melatonin exposure (LDBL), mice with extended melatonin duration (LDMEL, SDBL, SDMEL) had impaired hippocampal LTP (F(1,19) = 4.676, p < 0.05, Fig 3B).
Figure 3.
Schaffer collateral long-term potentiation. Compared to long day lengths (LDBL), exposure to short day lengths (SDBL) impairs hippocampal LTP (A). When compared to mice exposed to a short duration of melatonin (i.e. LDBL), extending melatonin exposure duration with melatonin implants or exposure to short day lengths (i.e. LDMEL, SDMEL, and SDBL) impairs CA1 LTP (B). Data in B are collapsed by melatonin exposure duration from A. *p < 0.05 repeated measures ANOVA. Horizontal bar in B indicates time points included in ANOVA for A and B.
3.5 Morphometry of hippocampal neurons
3.5.1 Cell soma
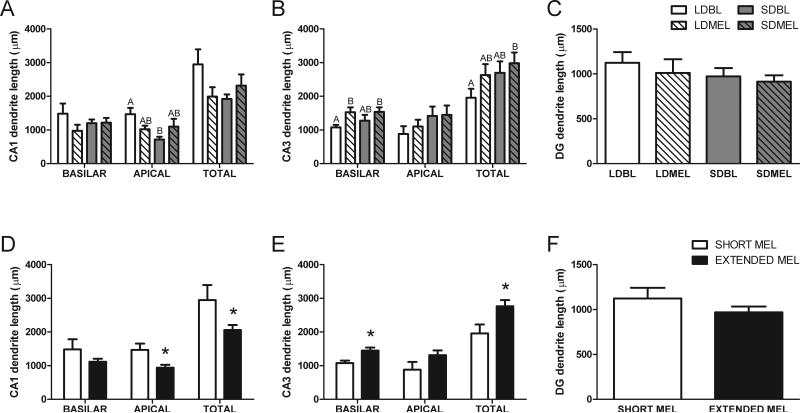
Neither photoperiod nor melatonin affected cell body size in CA1 and CA3 neurons (p > 0.05, data not shown). In the dentate gyrus, extending the duration of melatonin exposure reduces cell soma size (short duration 221.5 ± 17.6 μm2, extended duration 163.5 ± 8.9 μm2; p < 0.05).
3.5.2 Dendritic analysis
Extending the duration of melatonin exposure reduced the overall amount of dendritic length in CA1 pyramidal neurons (t22 = −2.520, p < 0.05); this effect was significant in the apical dendrites (t22 = −2.896, p < 0.05, Fig 4D). Conversely, extending melatonin exposure increased total dendritic length in CA3 neurons (t24 = 2.355, p < 0.05), with the greatest effect in the basilar dendritic field (t24 = 2.452, p < 0.05, Fig 4E). Melatonin did not affect total amounts of dendritic length of the granule cells within the dentate gyrus (t19 = 1.165, p > 0.05, Fig 4F).
Figure 4.
Dendritic length of hippocampal neurons. Extending melatonin exposure duration reduces total and apical dendritic length of pyramidal neurons in CA1 (D). Extending melatonin increases total dendritic length of the CA3 neurons, mainly in the basilar dendrites (E). Shared letters indicate no significant differences LSD post-hoc test. Data in D, E, and F are collapsed by melatonin exposure duration from A, B, and C respectively. A, B, and C share key depicted in C. D, E, and F share key depicted in F. *p < 0.05 Student's t test
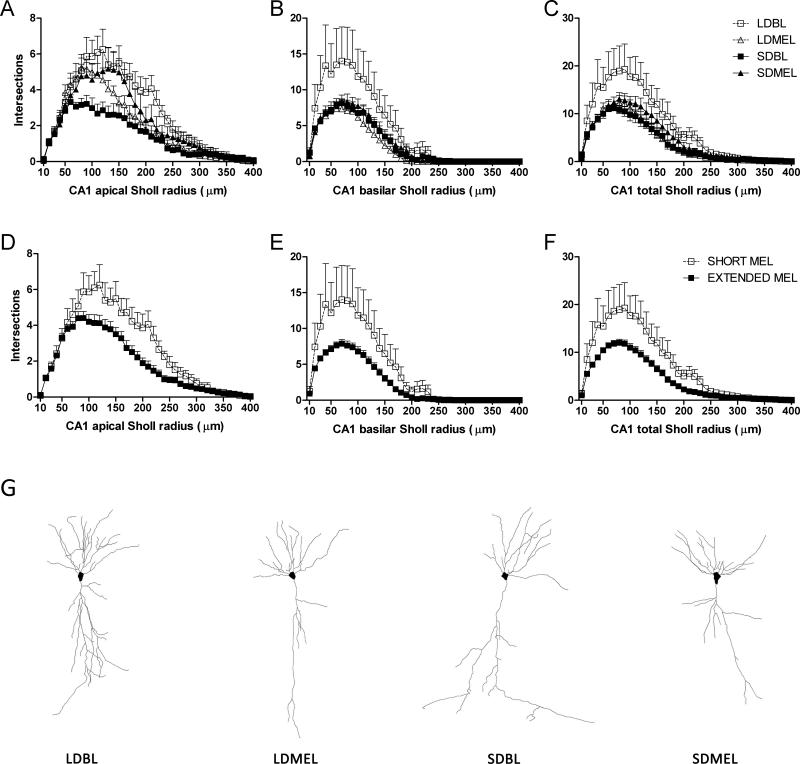
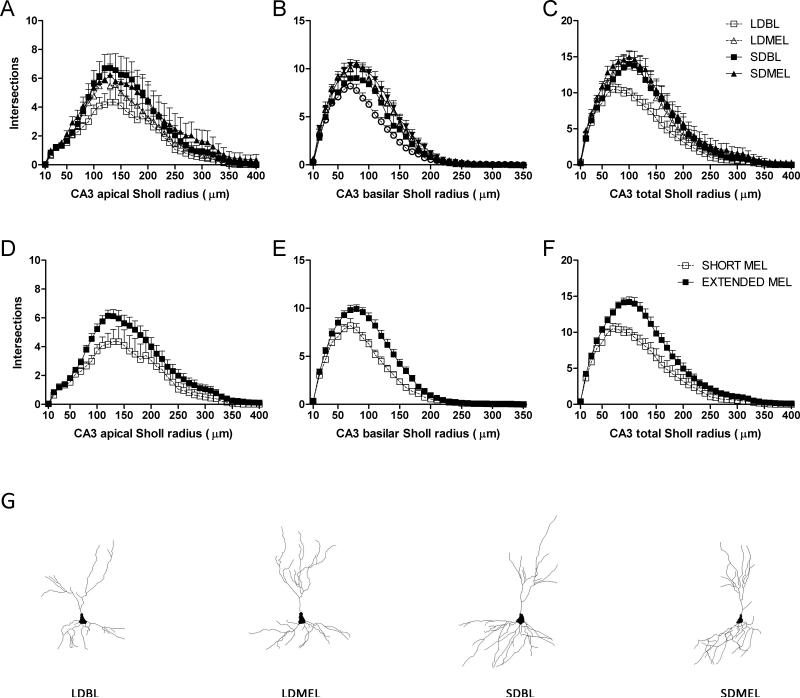
3.5.3 Dendritic complexity
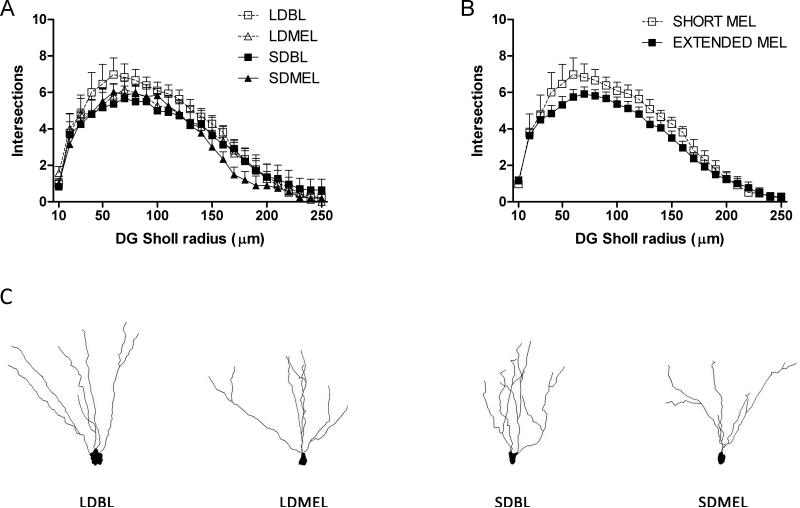
Extending the duration of melatonin exposure reduced total dendritic complexity, as measured by the number of intersections with outwardly concentric circles centered on the call soma (Sholl analysis), of the CA1 neurons (F(1,22) = −6.198, p < 0.05 Fig 5C,F). Reduced complexity was found in both apical (F(1,22) = −5.990, p < 0.05 Fig 5A,D) and basilar (F(1,22) = −4.416, p < 0.05 Fig 5B,E) dendritic fields. As with total dendritic length, extended melatonin duration increased overall dendritic complexity in CA3 neurons (F(1,24) = 6.287, p < 0.05 Fig 6C,F), and increased complexity was found in both apical (F(1,24) = 4.302, p < 0.05 Fig 6A,D) and basilar (F(1,22) = 7.061, p < 0.05 Fig 6B,E) CA3 dendritic fields. No differences were observed in DG granule neurons (F(1,19) = 1.065, p > 0.05 Fig 7).
Figure 5.
CA1 dendritic complexity. Extended duration of melatonin exposure reduces complexity in CA1 apical (A,D), basilar (B,E), and total (C,F) dendritic fields. Representative Neurolucida tracings of CA1 pyramidal neurons from each experimental group (G). Data in D, E, and F are collapsed by melatonin exposure duration from A, B, and C respectively. A, B, and C share key depicted in C. D, E, and F share key depicted in F.
Figure 6.
CA3 dendritic complexity. Extended duration of melatonin exposure increases complexity in CA3 apical (A,D), basilar (B,E), and total (C,F) dendritic fields. Representative Neurolucida tracings of CA3 pyramidal neurons from each experimental group (G). Data in D, E, and F are collapsed by melatonin exposure duration from A, B, and C respectively. A, B, and C share key depicted in C. D, E, and F share key depicted in F.
Figure 7.
Dentate dendritic complexity. Extended duration of melatonin exposure did not affect dendritic complexity in dentate granule cells. Representative Neurolucida tracings of DG granule cells from each experimental group (G). Data in B are collapsed by melatonin exposure duration from A.
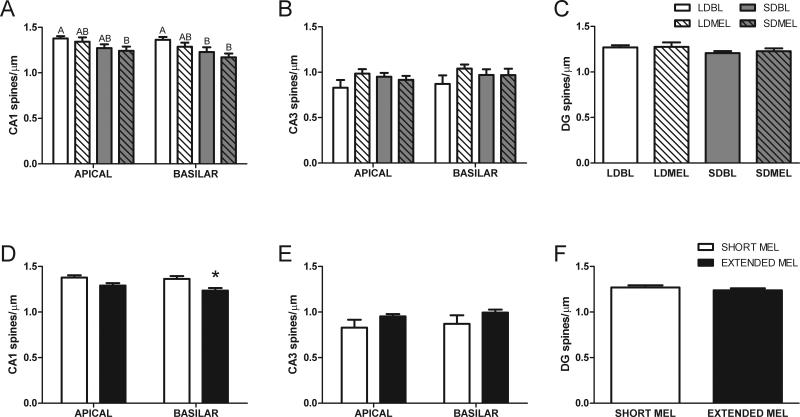
3.5.4 Spine density
Extended melatonin exposure reduced spine density in the basilar tips of CA1 dendrites (t21 = −2.265, p < 0.05, Fig 8D), without affecting spine density in the CA1 apical dendrites (t21 = 1.685, p = 0.11, Fig 8D), CA3 neurons (apical: t20 = −2.265, p = 0.07; basilar: t21 = 1.568, p = 0.13, Fig 8E), or DG neurons (t21 = −0.690, p > 0.05, Fig 8F).
Figure 8.
Neuronal spine density in hippocampal neurons. Extended duration melatonin exposure reduces spine density in the basilar dendrites of CA1 without affecting neuronal spine density in other areas of the hippocampus. Data in D, E, and F are collapsed by melatonin exposure duration from A, B, and C respectively. Shared letters indicate no significant differences LSD post-hoc test. A, B, and C share key depicted in C. D, E, and F share key depicted in F. *p < 0.05 Student's t test.
4. Discussion
Extending the daily duration of melatonin exposure by exposure to short days impairs hippocampal LTP, spatial learning and memory, and alters neuronal morphology of hippocampal neurons (current study, Pyter et al., 2005, Workman et al., 2009, Walton et al., 2011a). The current findings demonstrate that extending the duration of melatonin exposure via exogenous melatonin delivery through chronic subcutaneous implants reproduces the effects of short day exposure, independent of environmental day length, on hippocampal function and morphology in white-footed mice, providing further evidence for the role of melatonin in seasonal brain plasticity in photoperiodic rodents.
Melatonin has direct effects on spatial memory and neuronal physiological plasticity in the hippocampus. In both rats and mice, acute application of melatonin to the hippocampus impairs both LTP in the CA1 region (El-Sherif et al., 2002, Feng et al., 2002, Wang et al., 2005, Talaei et al., 2010) and hippocampal-mediated learning and memory (Collins and Davies, 1997, Feng et al., 2002, Cao et al., 2009). Melatonin also alters hippocampal function in an indirect manner. When tested during the light phase when endogenous melatonin levels are at nadir (Figure 1A), SDBL mice have impaired performance in spatial tasks (Figure 2) and impaired hippocampal physiological plasticity in the form of LTP (Figure 3, Walton et al., 2011a). Independent of environmental day length, extending the daily duration of melatonin exposure with chronic implants, which is interpreted biologically as a short day, reproduces the effects of SD exposure on both spatial ability (Figure 2) and LTP (Figure 3). Although melatonin potentially directly affected hippocampal function during behavioral testing in mice with melatonin implants, that LDMEL and SDMEL mice do not differ from SDBL mice (Figure 2) argues against this possibility and rather suggests that these effects are mediated via indirect mechanisms. Additionally, that LTP experiments were performed in vitro in the absence of both endogenous and exogenous melatonin, and that extended melatonin duration groups (LDMEL, SDBL, SDMEL) did not differ significantly from each other (Figure 3), further supports indirect mechanisms underlying the effects of melatonin on hippocampal plasticity described herein.
Extended melatonin exposure (short days or chronic melatonin implants) may indirectly affect hippocampal LTP and learning and memory by altering GABA signaling. Mice lacking a functional GABAA receptor in the hippocampus display enhanced spatial learning and memory and LTP (Collinson et al., 2002), and blockade of GABAA receptors increases the slope of fEPSPs in the CA1 region (Schummers and Browning, 2001). Melatonin stimulates glutamic acid decarboxylase, thus increasing GABA accumulation in the brain by altering turnover rate (Rosenstein and Cardinali, 1986, Golombek et al., 1996). Both GABA turnover rate and postsynaptic GABAA receptor activity display circadian rhythms in the brains of Syrian hamsters (Mesocricetus auratus) held in long days, with peak levels in both occurring in phase with pineal melatonin rhythms (dark phase) (Kanterewicz et al., 1993, Kanterewicz et al., 1995). However, upon exposure to short day lengths GABA turnover rate is increased and the circadian phase relationship is lost (Kanterewicz et al., 1993). Furthermore, melatonin can enhance GABA binding in the forebrain 26 hours after an injection (Niles et al., 1987). Thus, it is possible that the effects of melatonin reported here on LTP and spatial memory are indirectly mediated through altered GABA signaling. However, the role of altered GABA signaling in the photoperiodic impairment of LTP and spatial learning and memory has not been described.
Independent of potential changes in GABA signaling, the indirect effects of extended melatonin duration on LTP and learning and memory described above may simply be mediated by altered neuronal morphology in the hippocampus (Figures 4-8, Pyter et al., 2005). Short day lengths, and thus extended melatonin exposure, reduce hippocampal volume in multiple rodent species (reviewed in Yaskin, 2011), including white-footed mice (Pyter et al., 2005). Some of the reduction in hippocampal volume may be accounted for by reduced dendritic complexity and dendritic length in CA1 (Figures 4 & 5), which may also account for the impaired LTP and spatial memory. Regarding brain size, the CA1 dendritic reductions combined with the smaller soma in DG granule cells in extended melatonin exposure groups may also contribute to reduced volume via tighter packing of the hippocampal neurons in SD exposed brains. Although this hypothesis is appealing, it remains to be determined empirically whether this is the case by careful stereological quantification of hippocampal neurons in both extended and short duration melatonin exposed brains, which is beyond the scope of the current study. Arguing against neuronal morphologicial changes contributing to smaller hippocampi in SD by tighter neuronal packing is the increase in dendritic length and complexity in CA3 (Figures 4 & 6). The alteration of CA3 neuronal morphology may not arise as direct result of melatonin exposure, but it could be driven indirectly by gonadal regression induced by extended melatonin exposure (Figure 1), as reduced testosterone concentrations enhance mossy fiber connectivity in the CA3 (Skuckas et al., 2013). There are two main types of CA3 pyramidal neurons identified by differences in apical dendrite length and branch morphology: long-shaft and short-shaft (Fitch et al., 1989), and in at least one photoperiodic rodent species, Siberian ground squirrels (Citellus undulates), CA3 pyramidal neurons change rapidly between long- and short-shaft types dependent on torpid state (Popov et al., 1992). Thus, the current findings provide evidence that extended melatonin exposure in white-footed mice may either 1) shift phenotype of the CA3 pyramidal neurons from long- to short-shaft, or 2) make short-shaft CA3 neurons more susceptible to impregnation via the Golgi-Cox method.
Photoperiodic rodents, such as white-footed mice, are seasonally reproductive, and extended duration of melatonin exposure inhibits the hypothalamic-pituitary-gonadal axis and drives regression of the reproductive system (Figure 1C, reviewed in Walton et al., 2011b). It is possible that the indirect effects of melatonin on spatial memory and hippocampal LTP described herein may be modulated through altered gonadal sex steroid signaling. Gonadal sex steroids (estrogen and testosterone) can affect spatial memory (Galea et al., 1994, Galea et al., 1996), hippocampal morphology (Cooke and Woolley, 2005), and hippocampal LTP (Gupta et al., 2001, Leranth et al., 2003). Consistent with the current findings, testosterone rescued photoperiodic impairment of spatial navigation ability in gonadectomized male white-footed mice, but only in short days (Pyter et al., 2006). However, in that study it was also reported that day length (and thus melatonin duration) did not affect hippocampal androgen or estrogen receptor expression, and that gonadal steroids had no effect on spatial performance in mice held in long days, so the effects of testosterone on hippocampal mediated behaviors were indirect effects. Similarly, the impaired LTP of mice with extended melatonin exposure is not likely a direct effect of reduced testosterone levels as androgens generally impair LTP in the hippocampus (Harley et al., 2000, Hebbard et al., 2003, Skuckas et al., 2013).
The suite of hippocampal-mediated behavioral, physiological, and morphological changes driven by melatonin signaling described in the current study may also have ecological significance. Male white-footed mice maintain and defend breeding territories during the long days of spring and summer (King, 1968), and enhanced hippocampal function and spatial memory are necessary to support these behaviors. However, during the short days of winter when energy conservation is critical, the attenuation of hippocampal function and associated hippocampal-mediated behaviors is likely adaptive response to conserve energy to survive the harsh energy-restricted short days of winter (Jacobs, 1996, Nelson et al., 2010, Walton et al., 2011b). These photoperiod-mediated hippocampal changes do not occur in isolation, as short day male white-footed mice have enhanced olfaction and olfactory neurogenesis, potentially to enhance winter foraging efficiency (Walton et al., 2012b). Short day mice also have enhanced amygdala function and fear memory, potentially to promote survival during winter when mice must increase foraging for food in an environment devoid of understory for cover from predation (Bratton, 1976, Walton et al., 2012a).
In conclusion, the current results demonstrate that the naturally occurring short day impairment in the structure and function of the hippocampus can be reproduced by chronic melatonin implants independent of environmental day length, and thus implicate melatonin signaling as a critical factor in day-length induced changes in the structure and function of the hippocampus in a photoperiodic rodent. Although the exact mechanisms underlying melatonin's effects on the hippocampus are largely undescribed, hippocampal responses to extended melatonin exposure are likely the result of multiple direct and indirect neuroendocrine responses to changing day length which underlie the adaptive photoperiodic responses to short day lengths.
Short days impair CA1 LTP, spatial learning & memory, and alter neuronal morphology
Chronic melatonin implants reproduce effects of short days on hippocampal function
Melatonin signaling drives photoperiodic plasticity in the hippocampus
Acknowledgements
We thank James Spieldenner, Roxanne Demarest, Erika Sulecki, Kara Ruder, Tracy Bedrosian, and Laura Fonken for technical assistance. This work was supported by NIH R01MH057535.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
JCW, ZC, JT, and RJN designed the experiments. JCW and ZC performed the experiments and analyzed the data. JCW, ZC, JT, and RJN interpreted the data and wrote the manuscript.
References
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? Journal of Pineal Research. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Karsch FJ. Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biology of Reproduction. 1984;30:585–593. doi: 10.1095/biolreprod30.3.585. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bratton SP. Resource Division in an Understory Herb Community - Responses to Temporal and Microtopographic Gradients. American Naturalist. 1976;110:679–693. [Google Scholar]
- Cao XJ, Wang M, Chen WH, Zhu DM, She JQ, Ruan DY. Effects of chronic administration of melatonin on spatial learning ability and long-term potentiation in lead-exposed and control rats. Biomed Environ Sci. 2009;22:70–75. doi: 10.1016/S0895-3988(09)60025-8. [DOI] [PubMed] [Google Scholar]
- Carlson LL, Zimmermann A, Lynch GR. Geographic differences for delay of sexual maturation in Peromyscus leucopus: effects of photoperiod, pinealectomy, and melatonin. Biol Reprod. 1989;41:1004–1013. doi: 10.1095/biolreprod41.6.1004. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Collins DR, Davies SN. Melatonin blocks the induction of long-term potentiation in an N-methyl-D-aspartate independent manner. Brain Res. 1997;767:162–165. doi: 10.1016/s0006-8993(97)00733-6. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Dark J, Zucker I, Wade GN. Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. American Journal of Physiology. 1983;245:R334–338. doi: 10.1152/ajpregu.1983.245.3.R334. [DOI] [PubMed] [Google Scholar]
- Dowell SF, Lynch GR. Duration of the melatonin pulse in the hypothalamus controls testicular function in pinealectomized mice (Peromyscus leucopus). Biol Reprod. 1987;36:1095–1101. doi: 10.1095/biolreprod36.5.1095. [DOI] [PubMed] [Google Scholar]
- El-Sherif Y, Hogan MV, Tesoriero J, Wieraszko A. Factors regulating the influence of melatonin on hippocampal evoked potentials: comparative studies on different strains of mice. Brain Res. 2002;945:191–201. doi: 10.1016/s0006-8993(02)02752-x. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhang LX, Chao DM. Role of melatonin in spatial learning and memory in rats and its mechanism. Sheng Li Xue Bao. 2002;54:65–70. [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Res. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. Journal of Experimental Biology. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Pevet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev. 1996;20:403–412. doi: 10.1016/0149-7634(95)00052-6. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hebbard PC, King RR, Malsbury CW, Harley CW. Two organizational effects of pubertal testosterone in male rats: transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp Neurol. 2003;182:470–475. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Jacobs LF. The economy of winter: phenotypic plasticity in behavior and brain structure. Biological Bulletin. 1996;191:92–100. doi: 10.2307/1543068. [DOI] [PubMed] [Google Scholar]
- Johnston PG, Zucker I. Antigonadal effects of melatonin in white-footed mice (Peromyscus leucopus). Biol Reprod. 1980;23:1069–1074. doi: 10.1095/biolreprod23.5.1069. [DOI] [PubMed] [Google Scholar]
- Kanterewicz BI, Golombek DA, Rosenstein RE, Cardinali DP. Diurnal changes of GABA turnover rate in brain and pineal gland of Syrian hamsters. Brain Res Bull. 1993;31:661–666. doi: 10.1016/0361-9230(93)90138-2. [DOI] [PubMed] [Google Scholar]
- Kanterewicz BI, Rosenstein RE, Golombek DA, Yannielli PC, Cardinali DP. Daily variations in GABA receptor function in Syrian hamster cerebral cortex. Neurosci Lett. 1995;200:211–213. doi: 10.1016/0304-3940(95)12112-h. [DOI] [PubMed] [Google Scholar]
- King JA. Biology of Peromyscus (Rodentia) American Society of Mammalogist; Stillwater, Okla: 1968. [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. Journal of Biological Rhythms. 2001;16:336–347. doi: 10.1177/074873001129002051. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Denlinger DL, Somers DE. Photoperiodism : The Biological Calendar. Oxford University Press; Oxford ; New York: 2010. [Google Scholar]
- Niles LP, Pickering DS, Arciszewski MA. Effects of chronic melatonin administration on GABA and diazepam binding in rat brain. J Neural Transm. 1987;70:117–124. doi: 10.1007/BF01252513. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- Petterborg LJ, Reiter RJ. Effect of photoperiod and melatonin on testicular development in the white-footed mouse, Peromyscus leucopus. J Reprod Fertil. 1980;60:209–212. doi: 10.1530/jrf.0.0600209. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48:45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus). Journal of Neuroscience. 2005;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Trainor BC, Nelson RJ. Testosterone and photoperiod interact to affect spatial learning and memory in adult male white-footed mice (Peromyscus leucopus). Eur J Neurosci. 2006;23:3056–3062. doi: 10.1111/j.1460-9568.2006.04821.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Progress in Brain Research. 2010;181:127–151. doi: 10.1016/S0079-6123(08)81008-4. [DOI] [PubMed] [Google Scholar]
- Rosenstein RE, Cardinali DP. Melatonin increases in vivo GABA accumulation in rat hypothalamus, cerebellum, cerebral cortex and pineal gland. Brain Res. 1986;398:403–406. doi: 10.1016/0006-8993(86)91505-2. [DOI] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for GABA(A) and NMDA receptors in ethanol inhibition of long-term potentiation. Brain Res Mol Brain Res. 2001;94:9–14. doi: 10.1016/s0169-328x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Skuckas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G, Schroeder CE, MacLusky NJ, Scharfman HE. Testosterone Depletion in Adult Male Rats Increases Mossy Fiber Transmission, LTP, and Sprouting in Area CA3 of Hippocampus. Journal of Neuroscience. 2013;33:2338–2355. doi: 10.1523/JNEUROSCI.3857-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaei SA, Sheibani V, Salami M. Light deprivation improves melatonin related suppression of hippocampal plasticity. Hippocampus. 2010;20:447–455. doi: 10.1002/hipo.20650. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Hollister CW, Lefebvre NG, Goldman BD. Melatonin induction of gonadal quiescence in pinealectomized Syrian hamsters. Science. 1977;198:953–955. doi: 10.1126/science.563102. [DOI] [PubMed] [Google Scholar]
- Turek FW, Desjardins C, Menaker M. Melatonin-induced inhibition of testicular function in adult golden hamsters. Proc Soc Exp Biol Med. 1976;151:502–506. doi: 10.3181/00379727-151-39245. [DOI] [PubMed] [Google Scholar]
- Walton JC, Chen Z, Weil ZM, Pyter LM, Travers JB, Nelson RJ. Photoperiod-mediated impairment of long-term potentiation and learning and memory in male white-footed mice. Neuroscience. 2011a;175:127–132. doi: 10.1016/j.neuroscience.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JC, Haim A, Spieldenner JM, Nelson RJ. Photoperiod alters fear responses and basolateral amygdala neuronal spine density in white-footed mice (Peromyscus leucopus). Behav Brain Res. 2012a;233:345–350. doi: 10.1016/j.bbr.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JC, Pyter LM, Weil ZM, Nelson RJ. Photoperiod mediated changes in olfactory bulb neurogenesis and olfactory behavior in male white-footed mice (Peromyscus leucopus). PLoS One. 2012b;7:e42743. doi: 10.1371/journal.pone.0042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol. 2011b;32:303–319. doi: 10.1016/j.yfrne.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–2237. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Bowers SL, Nelson RJ. Enrichment and photoperiod interact to affect spatial learning and hippocampal dendritic morphology in white-footed mice (Peromyscus leucopus). European Journal of Neuroscience. 2009;29:161–170. doi: 10.1111/j.1460-9568.2008.06570.x. [DOI] [PubMed] [Google Scholar]
- Workman JL, Manny N, Walton JC, Nelson RJ. Short day lengths alter stress and depressive-like responses, and hippocampal morphology in Siberian hamsters. Horm Behav. 2011;60:520–528. doi: 10.1016/j.yhbeh.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Yaskin VA. Seasonal changes in hippocampus size and spatial behaviour in mammals and birds. Zh Obshch Biol. 2011;72:27–39. [PubMed] [Google Scholar]
- Yellon SM, Tamarkin L, Pratt BL, Goldman BD. Pineal melatonin in the Djungarian hamster: photoperiodic regulation of a circadian rhythm. Endocrinology. 1982;111:488–492. doi: 10.1210/endo-111-2-488. [DOI] [PubMed] [Google Scholar]