Abstract
The metastatic dissemination and spread of malignant circulating tumor cells (CTCs) accounts for over 90% of cancer related deaths. CTCs detach from a primary tumor, travel through the circulatory system, then invade and proliferate in distant organs. The detection of CTCs from blood has been established for prognostic monitoring and is predictive of patient outcome. Analysis of CTCs could enable the means for early detection and screening in cancer, as well as provide diagnostic access to tumor tissues in a minimally invasive way. The fundamental challenge with analyzing CTCs is the fact that they occur at extremely low concentrations in blood, on the order of one out of a billion cells. Various technologies have been proposed to isolate CTCs for enrichment. Here we focus on antigen-independent approaches that are not limited by specific capture antibodies. Intrinsic physical properties of CTCs including cell size, deformability, and electrical properties are reviewed, and technologies developed to exploit them for enrichment from blood are summarized. Physical enrichment technologies are of particular interest as they have the potential to increase yield, and enable the analysis of rare CTC phenotypes that may not be otherwise obtained.
Keywords: Circulating tumor cells, cancer, physical properties, antigen-independent, enrichment
1. Introduction: clinical needs and biology of CTCs
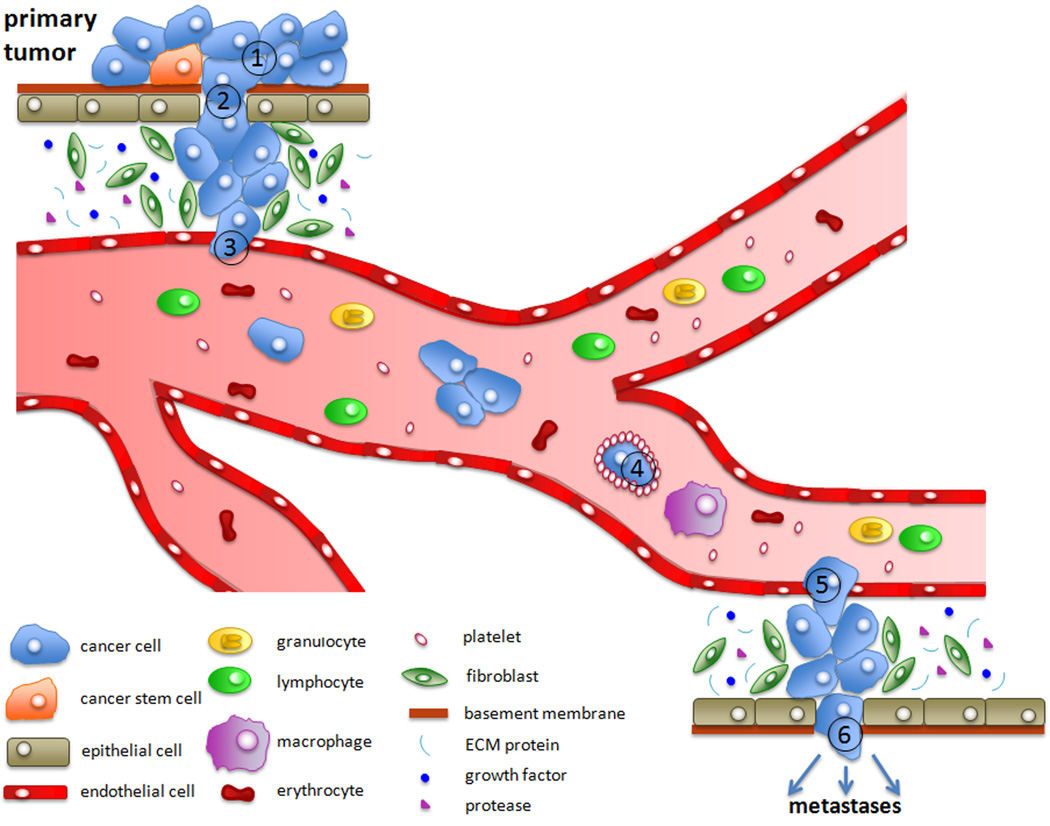
Cancer metastasis involves the spread of cancer cells from an initial site to form distant secondary tumors and is the main cause of death in cancer patients 1. It is thought that primary tumor cells undergo the process of metastasis in the following schematic steps: (1) localized invasion, whereby the tumor cells detach from the primary tumor and breach the basement membrane (which makes the tumor malignant), (2) intravasation into blood or lymphatic circulation systems, which allows for transport via circulation and interactions with blood components, (3) arrest in microvessels of various organs, (4) extravasation and migration into the distant tissue followed by colonization to form micrometastases, and (5) stimulation of angiogenesis leading to growth into macrometastases (metastatic tumors) (Fig. 1) 2. However, this process is highly inefficient, and less than 0.01% of CTCs will seed metastases 3, 4. The fact that CTCs occur at extremely low concentrations and are obscured by billions of cells in peripheral blood has hindered the understanding of their mechanism of action, as well as their clinical importance 5.
Fig. 1.
Overview of the process of metastasis: Progression from a primary epithelial cancer cell to an invasive, metastatic cell involves several steps. First, cancer cells undergo EMT to (1) reduce adhesion to neighboring cells and (2) dissolve the basement membrane through the secretion of extracellular matrix metalloproteases (MMPs). (3) Intravasation, or the entry of a cancer cell into the bloodstream, is achieved by the release of molecules, such as vascular endothelial growth factor (VEGF), that stimulate angiogenesis. In the bloodstream, cancer cells can interact with platelets (4), which protect the cancer cell from the immune system. After reaching the secondary site, cancer cells can exit the bloodstream (5) by inducing endothelial cell retraction or death. Lastly, the cancer cells undergo MET (6) and continue to proliferate at the metastatic site. 157
Conventional cancer treatments elicit only a transient response in patients with metastatic disease and as a result, these patients often relapse within 12 to 24 months of therapeutic intervention 6–8. Although quality of life may improve, the increase in survival rates has thus far been minimal. It has been long known that the presence of CTCs is indicative of shorter survival times 9–12. Detecting, isolating, and analyzing CTCs has the potential to improve diagnosis, allow prognostic monitoring, and enable targeted treatment strategies that are based on the metastatic cells most responsible for cancer mortality. CTCs may be sampled repeatedly in a minimally invasive way to monitor therapeutic efficacy and to account for constantly evolving tumor phenotypes.
There is currently only one US Food and Drug Administration (FDA) cleared technology for CTC enrichment, CellSearch® (Veridex, LLC, Raritan, NJ, USA). Enumeration of CTCs enriched with this technology has been established as a prognostic marker and predictor of patient outcome in metastatic breast 13, prostate 14, and colon cancers 15. CellSearch® is based on immunomagnetic enrichment, employing antibody-coated magnetic beads to isolate cells that express the epithelial cell adhesion molecule (EpCAM). CTC identification criteria includes (1) positive expression of monoclonal antibodies targeting cytokeratins (CK), a class of intermediate filaments present in epithelial cells; (2) negative expression of a leukocyte specific antibody targeting the leukocyte common antigen, CD45; and (3) positive expression of a nuclear stain, DAPI. In addition, a cell must have a diameter of at least four microns to be identified as a CTC 16. Nagrath and Toner et al designed a microfluidic chip consisting of an array of silicon microposts coated with EpCAM to improve CTC enrichment. This CTC-chip captured CTCs at a high purity of 50%, with a capture efficiency of 65% and a throughput of 2.5 mL/hour 17. Various other immunoafinity-based technologies have been developed to enrich CTCs using capture antibodies that target EpCAM, including a microvortex generating herringbone chip 18, a magnetic sweeper device 19, nanostructured silicon substrates 20, selectin coated microtubes 21, and a surface functionalized medical wire that is injected in vivo for continuous sampling 22. Other approaches have employed antibodies against cytokeratins 23, prostate specific antibodies 24, 25, and a cocktail of various antibodies 26, 27. In general, immunoaffinity based approaches for positive enrichment of CTCs result in high cell purity, depending on the specificity of the antibody. Negative enrichment techniques have been demonstrated to enrich CTCs using antibodies that select and remove hematopoietic cells that express CD45, but with a purity that is orders of magnitude lower 28–30.
One major limitation of immunoaffinity methods is their reduced efficiency with capturing tumor cells with low expression of EpCAM and other epithelial antigens 31. When CTCs detach from the original tumor and intravasate into blood circulation, it has been hypothesized that phenotypic changes occur. One proposed change is an epithelial to mesenchymal transition (EMT) by which epithelial tumors cells overcome the physical constraints of cell to cell adhesions and acquire the motility and invasive characteristics of mesenchymal cells. EMT is known to be essential for cell and tissue remodeling during embryogenesis 32 and wound healing 33. During EMT, the expression of epithelial proteins such as E-cadherin is downregulated 34, while mesenchymal markers like N-cadherin are upregulated 35. Mani et al discovered that EMT generates cells with many of the properties of self-renewing stem cells. A reverse process of mesenchymal to epithelial transition (MET) during extravasation and colonization has also been proposed. Currently, the EMT/MET model is gaining more and more evidentiary support in cell line studies, animal models and clinical investigations. However, it is still unclear how widely this model applies to all metastatic diseases.
The recent discovery of the presence of cancer stem cells (CSCs) is another important concept in cancer metastasis. This rare subpopulation of cells within a tumor retains the ability to initiate and drive tumor cell expansion, as well as a capacity for self-renewal and to produce differentiated progeny 36. The existence of CSCs was first proven in acute myelogenous leukemia 37, 38, and later verified in breast 39, and brain tumors 40–42. Recently, the identification of cancer stem cells has also been reported in prostate 43, ovarian 44, and pancreatic 45 cancers based on the identification of specific cell surface markers. Conventional cancer treatments have been developed and tested based on their ability to kill the majority of the tumor population. However, CSCs can escape conventional therapy and have been shown in several tumor types to be more resistant to standard chemotherapeutic agents 46.Approaches for CTC enrichment that are independent of expression of EpCAM or other cell surface antigens have been developed on the basis of intrinsic physical properties, e.g. size, deformability, density, and electrical properties. Expression of cell antigens can vary widely among different cancer types, and immunoaffinity-based enrichment is further complicated by the loss of epithelial markers during to EMT and with rare CSC phenotypes. Physical enrichment strategies therefore have the potential for greater CTC yield, and they enable the analysis and evaluation of clinical relevance of new CTC subpopulations that do not fit within currently established definitions. There have been numerous reviews on biological and clinical studies involving CTCs 5, 47–56, and technological developments for CTC enrichment and detection 52, 57–61. Readers are urged to consult those references for more general information, as we will focus specifically on CTC enrichment based on physical properties.
2. Biological basis of physical enrichment methods
The majority of metastatic solid tumors originate from epithelial cells. In addition to biochemical differences, there are distinct physical differences between these tumor cells and blood cells. The majority of physical methods for CTC enrichment exploit differences in size, deformability and electrical (conductive and dielectric) properties between CTCs and blood cells. The physical properties of tumor cells are reviewed here.
2.1. Size of tumor cells
In general, caution should be taken when interpreting cell size measurements. The method of measurement, variation among samples, and sample preparation steps can result in different measured values even within the same cell population (e.g. a tumor cell line). Most published studies measure cell size either by optical microscopy or flow cytometry. With optical microscopy the cell diameter or area is reported, whereas with flow cytometry electrical impedance sensing (by Coulter counters) measures cell volume at DC or low frequency. In flow cytometry, optical diffraction and fluorescence measure the equivalent diffraction area or cross-sectional area respectively, and both require calibration with standards (e.g. glass or plastic beads) 62, 63. There is a significant difference when cell size (diameter) is measured on a 2D surface by microscopy versus derived from cell volume measurement in suspension by flow cytometry. For example, on typical blood smears on a 2D surface, leukocytes are severely deformed into a pancake shape and normally reported as 7–20 µm diameter particles 64. However, all leukocytes in isotonic suspension are measured to be less than 10 µm in diameter 65. The relative importance of the techniques used to measure cell size depends on the CTC enrichment approach. For filtration based methods, the cell diameter on a 2D surface is important, while for flow separation the cell volume and 3D shape are likely to be more relevant. Ambient conditions can affect the results of a cell size measurement. For example, the tonicity of the measurement media will change the volume of viable cells, and fixation processes can also alter cell size. For cell lines, cell size is affected by the culture media formulation and in general by the health and status of the cells. Considering such complexity in cell size measurements, it is critical to compare tumor cell size with blood cells using the same measurement method under the same experimental conditions to facilitate size based CTC separation.
Sizes of blood cells have been reported in many studies and textbooks (Table 1). For example, erythrocytes in 2D blood smears have a diameter of 7.0–8.5 µm 64, 66. Diameter of granulocytes are measured to be 10–20 µm using microscopy of blood smear 64, 66, 8.7–9.9 µm using flow cytometry 66, and 7.3–13.2 µm using light microscopy of cell suspensions 67. Lymphocytes are smaller than granulocytes. Their diameter is measured to be 6–18 µm in blood smears 64, 66, 7.1–10.5 µm using flow cytometry 66, and 5.2–10.1 µm using light microscopy of cell suspensions 67. Monocytes are among the largest of leukocytes, with a diameter of 12–20 µm in blood smears 64, 66 and 9.7–10.5 µm measured with flow cytometry 66.
Table I.
Size (diameter or area) of tumor cells and major blood cells
| Cell types | Cell size | Measurement methods |
|---|---|---|
| Five Tumor cell lines | 396–796 µm2 | Microscopy of fixed sample on track-etched filter 68 |
| Leukocytes | 140 µm2 | |
| Tumor cells in NCI-60 panel | Electrical measurement 69 | |
| Solid tumor cell lines | 11.7–23.8 µm | |
| Leukemia cell lines | 8.9–15.3 µm | |
| Leukocytes | 6.2–9.4 µm | |
| Tumor primary culture and cell lines | 12–40 µm | Microscopy of 38 cell samples grown in spheroids 71 |
| Tumor tissue slides | Microscopy after H-E staining 72 | |
| small-cell lung carcinoma (SCLC) | 7.2–10 µm | |
| large-cell lung carcinoma (LCLC) | 15 µm | |
| CTCs from ten prostate cancer patients | 89 µm2 | Imaging after erythrocyte lysis73 |
| LNCaP cell line | 142.9 µm2 | |
| CTCs in metastatic breast cancer patient samples |
77.59 µm2 (CVB) 62.28 µm2µ(PVB) |
Imaging after CellSearch® enrichment 77 |
| Erythrocytes | 7.0–8.5 µm | Blood smear 64, 66 |
| Granulocytes | 10–20 µm | Blood smear 64, 66 |
| 8.7–9.9 µm | Flow cytometry 66 | |
| 7.3–13.2 µm | Microscopy of cell suspension67 | |
| Lymphocytes | 6–18 µm | Blood smear 64, 66 |
| 7.1–10.5 µm | Flow cytometry 66 | |
| 5.2–10.1 µm | Microscopy of cell suspension67 | |
| Monocytes | 12–20 µm | Blood smear 64, 66 |
| 9.7–10.5 µm | Flow cytometry 66 | |
It is generally agreed that cell lines originated from solid tumors are larger than blood cells. Using optical microscopy, the average diameter of 15 cancer cell lines commonly used in our lab are between 15–25 µm, while that of the majority of leukocytes are under 12 µm (unpublished results). After partial fixation and filtration through track-etched filters, the cross-sectional area of five tumor cell lines (MCF-7, Hep3B, HepG2, LNCaP, and Hela) is measured to be 396–796 µm2 using microscopy, which is significantly larger than that of leukocytes (average of 140 µm2) measured under the same conditions 68. In a recent study, the diameters of all cell lines in the NCI-60 panel are calculated from dielectrophoretic field-flow fractionation (DEP-FFF) and compared with those of various subtypes of blood cells 69. Cell size extracted from the DEP data clearly shows the size difference of leukocytes (6.2–9.4 µm), leukemia cells (8.9–15.3 µm) and solid tumor cell lines (11.7–23.8 µm).
In examinations of routinely fixed and stained pathological tissue samples, viable epithelial cells (e.g. solid tumor cells) are always larger than virtually all normal blood cells, including leukocytes 1, 70. Measurement from 38 primary tumor tissue biopsy samples and cell lines grown in semisolid agar medium results in tumor cell sizes in the range of 12–40 µm 71. Measurement of 206 biopsy slides of small-cell lung carcinoma (SCLC) and large-cell lung carcinoma (LCLC) showed that these cells are on average 7.2–10 µm and 15 µm in diameter, respectively 72.
CTCs from clinical samples can be smaller than tumor cell lines. A study of imaging CTCs after erythrocytic lysis measured the cross-sectional area of CTCs from ten stage IV prostate cancer patients to be 89 µm2, while that of the LNCaP cell line was measured to be 142.9 µm2 under the same measurement conditions 73. However, the clinical relevance of CTCs of varying sizes is unclear. P. Kuhn's group studied cytomorphology of CTCs from patient samples of various cancer types. Erythrocytes are lysed first and, then nucleated cells are plated on slides and CTCs are detected by immunocytochemistry. A laser scanning imaging system is applied for high definition imaging of CTCs 74. In non-small lung cancer, smaller CTCs (4.3 mL−1 versus 15 mL−1 of normal CTCs) were not related to clinical features, survival and treatment variables 75. In a study of CTC detection on slides after erythrocytic lysis, CTC aggregates were found in 43% of 86 blood samples of breast, non-small cell lung, pancreatic and prostate metastatic patients 76. Area per cell for both single CTCs and CTCs in aggregates was twice that of leukocytes in the same field of view, although the length of CTCs in aggregates was smaller than that of single CTCs. It is interesting to note that in a CTC study using CellSearch® the average single imaged CTC area from central venous blood (CVB) was larger than that measured from peripheral venous blood (PVB) of the same patient (77.59 µm2 versus 62.28µm2) 77.
Smaller CTC positive particles (defined as CK+CD45−DAPI+) were reported after immunological CTC enrichment 18. CellSearch® excludes CTC positive particles smaller than 4 µm and regards them as cell fragments 48. It has been reported that a high percentage of CTCs are in different stages of apoptosis and apoptotic cells are expected to have a smaller size, thus it is possible that the smaller CTCs in clinical samples can be attributed to cell death. Mild apoptotic changes (e.g. cytoplasmic blebbing) are observed and acceptable for the CTC definition, while cells with apoptotic indicators in the nucleus are excluded from CTC counts with the assumption that these cells will not have clinical significance 70. Another potential cause of CTC size variation is that CTCs may shift from an active state to a dormant state (cell cycle G0 like), which may increase their metastatic potential and thus indicate a poorer prognosis for patients.
2.2. Deformability of tumor cells
Various biomechanical tools have been developed to measure mechanical properties of living cells 78. Local properties can be probed with atomic force microscopy (AFM) 79 and magnetic twisting cytometry (MTC) 80, while optical tweezers/laser traps 81, a microplate stretcher 82, micropipette aspiration 83 and micropillar arrays 84 can study mechanical loading to whole cells. The advantages and drawbacks of these techniques have been reviewed in detail 85–87. In general, the measurement of cell deformability is affected by the method of choice as mentioned above, cell handing (e.g. culture media, ice/room temperature, dry/wet, fixed/unfixed), and the heterogeneity of cell mechanical properties (e.g. different regions of cells, cell cycle, cell differentiation and aging). Although measurement of absolute Young's modulus might not be reliable, comparing different cell populations under the same conditions will be able to provide qualitative conclusions.
For physical enrichment of CTCs from blood, it is important to compare the elastic properties of tumor cells and blood cells (especially leukocytes). In an AFM study, the apparent stiffness of neutrophils is measured to be 156 Pa 88, which is small compared with many other types normal human cells as determined from other studies 89. However, in another AFM study, the elastic moduli of the cell body part of neutrophils is measured to be 1.548 kPa, while the leading and trailing edges are significantly lower (686 Pa and 494 Pa) 90.
Many studies have shown that tumor cells that have greater metastatic potential are more deformable. For example, M. Lekka, et al applied AFM to measure elastic properties on single cells. The Young's modulus of cancer or transfected cell lines (Hu456, T24, BC3726) was found to be one order of magnitude lower than those of non-malignant cell lines (Hu609 and HCV29) 91. In studies of breast cancer cell lines using AFM, benign MCF-10A cells were found to be 1.4–1.8 times more rigid than the cancerous but non-metastatic breast cancer cell line MCF7 (~0.5–1.1 kPa versus 0.3–0.5 kPa) 92. Similar results were obtained with an optical cell stretcher 93, 94. In another study, MCF7 cells were found to be more rigid than the metastatic breast cancer cell line MDA-MB-436 95. Similar measurements have been applied to clinical samples. S. E. Cross and colleagues used AFM to measure stiffness of live metastatic cancer cells taken from pleural fluids of patients with lung, breast and pancreatic cancer. These metastatic cancer cells are found to be 70% softer than the benign cells within the same pleural fluid samples (Young’s modulus 0.53±0.10 kPa versus 1.97±0.07 kPa) 96. Nevertheless, careful consideration should be applied when interpreting the Young's modulus of living cells measured by AFM 97. Most AFM studies fit the results into the Hertz model, which assumes the measured material to be infinitely thick, purely elastic, isotropic and homogeneous. Indentation depth, sample thickness and contact point relevant to the center of the cell can all affect the measured results. For example, reducing ramp size from 2 µm to 500 nm and ramp velocity from 2 µm/s to 500 nm/s increases the measured Young's modulus by ~5 times for the same cell lines 95. Microfluidic optical stretching is a method to measure single cell deformability at a higher throughput. Measured with a microfluidic optical stretcher, primary oral squamous cell carcinomas cells are 3.5 times more compliant than normal keratinocytes from healthy donors 98. Similar results were obtained with normal and cancerous oral epithelial cell lines. Recently D. Di Carlo’s group used hydrodynamic shearing forces to measure deformability of whole cells in microfluidic channels at a throughput of ~ 2,000 single cells per second. They found that malignant cells obtained from pleural effusions of 11 carcinoma and mesothelioma patients were at least 18% more deformable than unactivated mononuclear leukocytes and granulocytes, 5% more deformable than activated granulocytes, and similar or more deformable than activated mononuclear leukocytes 99. Microfiltration with track-etched pore filters has been used to study the deformability of tumor cells qualitatively. In a study of B16 melanoma cells and its variants, the time required to pass 50% of the tumor cells was used to correlate to cell deformability and again it was found that metastatic cells are more deformable 100. The elastic rigidity reduction during malignant transformation may be induced by a reduction of F-actin concentration 101 and a generally more fluid-like state of the cytoskeleton 102.
2.3. Electrical properties of tumor cells
It has long been known that most mammalian cells have a net negative surface charge under physiological conditions 103, 104. Moreover, cells contain a variety of polarizable particles/molecules, including proteins, peptides, and nucleic acids. Thus, cells can have complicated dielectric properties that are frequency dependent. At low frequencies, interfacial polarization dominates, which depends mainly on the conductive properties of polarizable particles and their surrounding medium. In general, the plasma membrane acts as an electrical insulator below 25 kHz and viable cells behave like insulating objects. At high frequencies (10 MHz up to GHz range), dielectric properties (permittivity) become more important and cellular features, such as nucleus-cytoplasm volume ratio and endoplasmic reticulum play important roles 105. While at intermediate frequencies, both conductive and dielectric properties can be significant. Cell size and shape, membrane integrity and morphology, and cytoskeletal structure can be explored by electrical measurements. Various techniques have been used to measure electrical properties of cells, including impedance spectroscopy, electrorotation (ROT), and dielectrophoresis (DEP). Impedance spectroscopy uses electrodes to scan the electrical impedance over a certain frequency range 106–110. It can be applied to either a cell population or single cells in a flow cytometry format. ROT can be used to measure single cell dielectric properties 111, 112. In ROT, normally four electrodes are arranged in a criss-cross configuration and the single cell is located at the center of the four electrodes. The ROT excitation signals are applied to the four electrodes in a 90 degree phase shifted fashion. The cell rotates and the rotation speed is recorded. This speed depends on the difference between the electric polarity of the cell and its surrounding medium under the frequency of the ROT excitation signal. DEP requires applying a non-uniform electromagnetic field to the cell, with the cell responding to the DEP force by either moving towards (positive DEP, pDEP) or away from (negative DEP, nDEP) the strong electromagnetic field 112–115. The DEP cross-over frequency from pDEP to nDEP is a characteristic value for cell dielectric properties given the same surrounding medium.
A. Han and colleagues measured whole cell impedance spectroscopy of various breast cancer cell lines (MCF-10A, MCF-7, MDA-MB-231, and MDA-MB-435) using a microfabricated device 116. After fitting the data into a parallel RC circuit model, the equivalent membrane capacitance and resistance were calculated for each cell line at 100 kHz. The results show that both magnitude and phase of the whole cell impedance of different cell lines are distinct.
ROT spectra (electrorotation speed versus frequency) between 1 kHz to 10 MHz were measured for metastatic breast (SkBr3) and lung (A549) cancer cell lines along with different human leukocyte subtypes, including T lymphocytes, B lymphoctyes, granulocytes and monocytes in a low-conductivity medium 117. After fitting into a single-shell dielectric model, the dielectric properties of cells, including unit membrane capacitance and cytoplasm conductivity, were extracted. The tumor cells were shown to have higher unit membrane capacitance and lower cytoplasm conductivity compared with leukocytes.
P. R. C. Gascoyne's group applied dielectrophoretic field-flow fractionation (DEP-FFF, dFFF) to study dielectric properties of cancer cells and apply it for CTC enrichment. A summary of the total cell electrical capacitance of 7 subtypes of blood cells and 9 cancer cell lines shows the capacitances of cancer cells are significantly larger than those of blood cells 118. All the data points show that the total cell capacitance scales with the cube of cell diameter, which is consistent with the general conclusion that cancer cells are larger than blood cells. In a recent study, they expanded on their previous work and compared over 50 solid cancer cell lines in the NCI-60 panel with various blood cells and leukemia cells 119. The dielectric and density properties of cells were measured by DEP-FFF. The DEP crossover frequencies of solid tumor cells were extracted from the measurements and found to be distinctly lower than for blood cells and leukemia cells. The cut-off frequency is 65 kHz. Under 1 MHz, the DEP crossover frequency of a spherical mammalian cell is inversely proportional to cell diameter and the capacitance per unit area of the cell plasma membrane. Both calculated cell diameter and the capacitance per unit area differ between cancer cells and blood cells 69.
3. Physical enrichment technologies
The rarity of CTCs in whole blood, on the order of one CTC per billions of blood cells or per millions of nucleated blood cells, poses a major challenge for enrichment. Heterogeneity among CTCs and the complexity of clinical blood samples present additional challenges. In general, to successfully evaluate technologies for CTC enrichment, multiple performance parameters (i.e. capture efficiency/recovery, enrichment against leukocytes, cell viability, processing speed, blood sample capacity, sample pre-processing requirements, cost of consumables and equipment, and repeatability and reliability) must be considered. The optimal enrichment solution may require a compromise among performance parameters, and it is likely to depend on the intended downstream application.
Various approaches have been used to exploit the differences of physical properties between tumor cells and blood cells to enrich and separate CTCs from blood samples. These technologies are summarized in Fig. 2 and discussed in detail below.
Fig. 2.
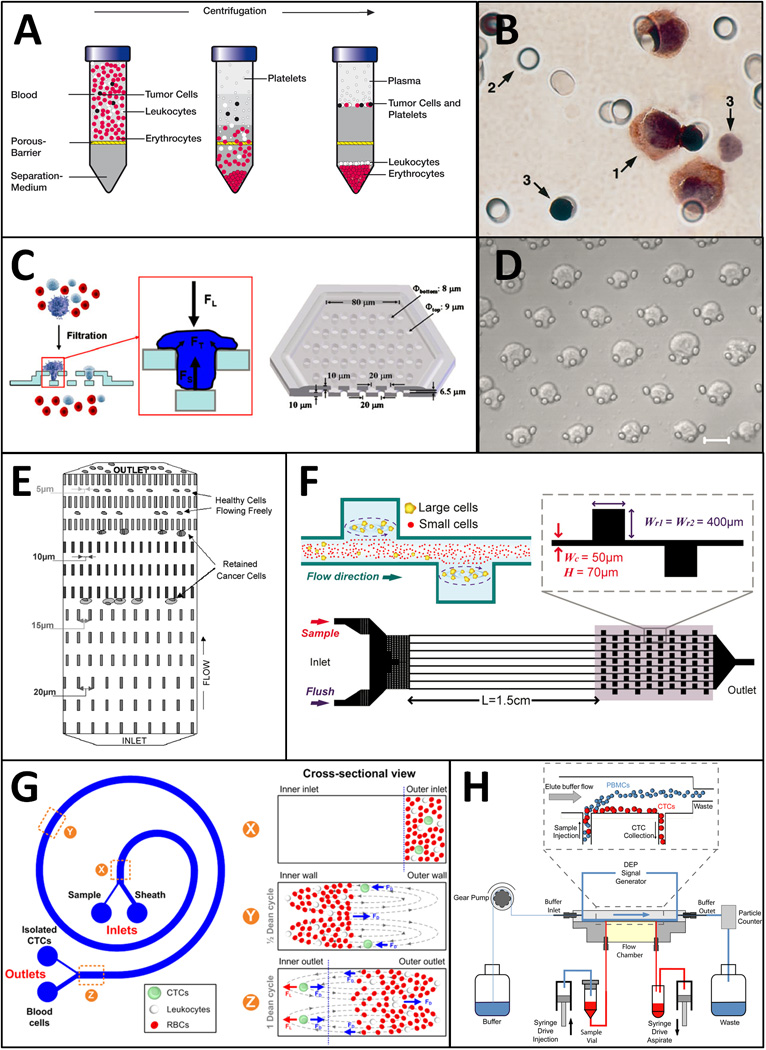
Technologies for CTC enrichment based on physical properties. A: Density gradient centrifugation with OncoQuick® (reproduced by courtesy of Grenier Bio-One GmbH, Germany). B: Track-etch filter for ISET. Arrows indicate 1: tumor cell; 2: filter pores; 3: leukocytes. (reproduced from ref. 68 with permission from Elsevier). C: 3D–microfilter device for viable CTC enrichment (reproduced from ref. 137 with permission from Springer). D: Crescent-shaped traps in a microfluidic device. Scale bar is 20 µm. (reproduced from ref. 141 with permission from Elsevier). E: Array of microfluidic traps with varying geometrical restrictions. (reproduced from ref. 91 with permission from Springer). F: Laminar vortices generated on a microfluidic device for size based CTC enrichment. (reproduced from ref. 144 with permission from AIP). G: Spiral microchannel for CTC isolation using centrifugal forces. (reproduced from ref. 148 with permission from NPG). H: DEP separation of CTCs using Apostream™ (reproduced from ref. 62 with permission from AIP).
3.1. Centrifugation
Centrifugation is a widely used technique for fractionating blood into its constituent components. Density-based gradient centrifugation segregates CTCs in the mononucleocyte fraction of blood away from the more dense cells present in the erythrocytic and granulocytic fractions. In 1959, S. H. Seal developed a floatation method using blended silicone oils as a solution for CTC enrichment that was quantitative, inexpensive and simple to perform. Using this technique, he successfully detected CTCs in 45% of patient samples obtained from a variety of cancers with cytological staining after a secondary size based filtration step 120. A synthetic polysaccharide was later incorporated into Ficoll-Paque® (Pharmacia-Fine Chemicals, Uppsala, Sweden) solution, which became the gold standard for gradient centrifugation separation of mononuclear cells from blood and bone marrow aspirates. Ficoll-Paque® was used to enrich CTCs that could be detected with a resolution of 1 cell/mL of blood in model systems using reverse transcription-PCR assays (RT-PCR) 98. This technique was used to detect CTCs in 41% of patients undergoing surgical resection in colorectal cancer 98. OncoQuick® (Greiner BioOne, Frickenhausen, Germany) is a novel technology incorporating a porous barrier that allows erythrocytes and some leukocytes to pass through while retaining CTCs in conjunction with density based centrifugation to achieve more effective enrichment (Fig. 2A). Rosenberg and Siewert et al report an enrichment ratio of 632-fold against leukocytes with OncoQuick® compared to 3.8-fold with Ficoll based centrifugation 121. The OncoQuick® method was used to enrich CTCs in blood samples obtained from 30% of 37 gastrointestinal cancer patients 121 and 40% of 63 advanced breast cancer patients 122 with RT-PCR based CTC detection. In a comparative study, CTCs were found in 23% of 61 patients using immunocytochemical detection on cytospins prepared after OncoQuick® enrichment, as opposed to 54% with the CellSearch® instrument 123.
As an inexpensive and dependable technique, centrifugation is widely employed for CTC enrichment. However, the elimination of contaminant leukocytes is limited, with the even most advanced centrifugation techniques achieving a purity of less than 1%. It is therefore commonly used as an initial step in combination with further enrichment techniques.
3.2. Microfiltration
Microfiltration technology has demonstrated the greatest potential for achieving high-throughput continuous processing of large volumes of blood. Since track-etched polymer filters were first invented in the 1960s 124, 125, they have been widely used in biological research and clinical practice for cell enrichment. In 1964, S. H. Seal once again made a pioneering contribution to the field when he applied these filters to attempt to capitalize on his stated observations that CTCs isolated with his silicone flotation method were generally larger and more rigid than blood cells 126. Decades later, track-etched polycarbonate filters with 8 µm diameter pores were effectively used for CTC enrichment and cytological detection from fixed blood samples using the isolation by size of epithelial tumor cells technique (ISET) developed by Vona and Paterlini-Brechot et al (Fig. 2B) 68. Track-etched filters have been successfully used to enrich and analyze CTCs in liver cancer 127, melanoma 76, lung cancer 128, 129, prostate cancer 130 and various cancers 131. In a comparative study, ISET detected CTCs in 95% of 60 metastatic patients with breast, prostate and lung cancer compared to 70% with CellSearch®, though CellSearch® did occasionally detect CTCs in greater quantities 102. Zheng and Tai et al used deterministic photolithography to develop an improved pore shaped microfilter that was fabricated out of a single 10 µm thick layer of parylene, reporting a capture efficiency of ~90% 132. This device was used in a blind comparison study to identify CTCs in 89% of 57 cancer patients as opposed to 46% with the CellSearch® 133. Similar porous membranes have been fabricated from electroformed nickel 134 and silicon substrates 135. While successful for CTC enumeration, these microfiltration techniques apply concentrated stresses that affect the viability of enriched CTCs 136, 137. To address this issue and enable viable CTC enrichment, a three-dimensional microfilter was designed out of two layers of parylene to incorporate support structures that mitigate cell damage (Fig. 2C) 137. Track-etched filters were also adapted for viable cell capture using the ScreenCell® (ScreenCell, Paris, France) system, which reported 74–91% recovery and 85% viability 138. Xu and Goldkorn et al. used a parylene filter designed by Tai's group 158 with slot shaped pores to detect telomerase activity from viable enriched CTCs filtered from Ficoll-Paque® isolated buffy coats of metastatic prostate cancer patients 139.
The speed and simplicity of microfiltration allows for rapid CTC enrichment from large volumes of clinical blood in minutes with minimal processing. While high capture efficiencies on the order of 90% and greater can be reliably achieved, the final purity is typically on the order of 10% or less, which may require further processing for some downstream applications.
3.3. Microfluidics
Microfluidics allows unparalleled control and ability to manipulate fluids in miniscule volumes. The past decade has seen many novel technologies proposed for biological cell sorting and analysis on microchips. Mohamed and Caggana et al used arrays with pillars of varying geometries to fractionate cells in blood and capture tumor cells (Fig. 2E) 91, 140. They demonstrated the capture of neuroblastoma and other cancer cell lines from 1:10 diluted blood samples with a processing rate of 1 mL per hour. Tan and Lim et al incorporated crescent-shaped trap arrays with a fixed 5 µm gap width within microfluidic chambers to enrich CTCs from whole blood without pre-processing, reporting a capture rate of greater than 80% and a purity of over 80% 141. This device was used to successfully detect CTCs in 1–3 mL blood samples obtained from metastatic lung cancer patients 142. Tumor cell separation based on size and deformability can also be achieved through cross-flow filtration within a serpentine microfluidic channel 136.
Inertial flow fractionation enables microfluidic enrichment of tumor cells by exploiting hydrodynamic forces to select for cells of different sizes. Microfluidic channels incorporating contraction and expansion reservoirs were developed for pinch alignment of tumor cells by Bhagat and Han et al 143 and tumor cell trapping in micro-scale vortices by Hur and Di Carlo et al (Fig. 2F) 144. These devices allow a significantly higher throughput compared to previous microfluidic approaches, but with potential reductions to cell recovery rate and enrichment against leukocytes. A similar approach was used to classify cells based on deformability 145. Sun and Jiang et al developed a double spiral microfluidic channel to hydrodynamically separate tumor cells using drag forces, reporting a recovery rate of 88.5% from diluted blood 146, 147. Lim and colleagues incorporated a spiral microfluidic channel to successfully enrich CTCs and microclusters in 20 blood samples of metastatic lung cancer patients (Fig. 2G) 148.
Carefully applied microfluidic approaches are capable of achieving excellent purity of greater than 80% and high capture rates with little disturbance to CTCs. However, this comes at the expense of throughput, requiring either reduced sample volumes or several hours to process a full tube of blood. Recently developed hydrodynamic flow sorting approaches can achieve a throughput of up to 3 mL per hour for microfluidic systems, but with a reduction in sample purity to below 10%.
3.4. Dielectrophoresis
The phenomenon of dielectrophoresis has inspired novel approaches for the separation of cells based on their electrical properties. Becker and Gascoyne et al fabricated interdigitated gold electrodes and used them to separate leukemia 149 and breast cancer cell lines 63 from healthy blood cells. Tumor cells were attracted towards the electric field generated by the electrodes by pDEP, while other cells were flushed away. The electric field was then turned off and the cells were released for collection with a recovery rate of 95%. Building on this approach, Huang and Gascoyne et al proposed DEP-FFF as a continuous cell fractionation process that did not require intermittent activation and deactivation of an electrical field 150. Gupta and Davis et al presented ApoStream™, the first commercial instrument for continuous flow DEP-FFF enrichment of CTCs (Fig. 2H) 62. They reported a capture efficiency of over 70% and viability greater than 97% from cell lines spiked in 7.5 mL of whole blood after an initial Ficoll gradient centrifugation step. Preliminary efforts have been undertaken to demonstrate the application of DEP-FFF to clinical patient samples 119.
The unique application of DEP-FFF to cell electrical properties may enable the capture of a population of CTCs that would not be obtained by other physical enrichment approaches. Testing with cell lines has demonstrated excellent viability and minimal disruption to captured cells. However, an initial centrifugation enrichment step is required and whole blood can not be processed directly. One hour is required to process mononuclear cells obtained from 7.5 mL of blood after Ficoll enrichment. The capture purity is expected to be less than 1%, though this can be significantly improved with additional enrichment stages at the risk of reduced capture efficiency.
4. Perspectives
Any enrichment technique applied to CTCs will be biased according to its principle of enrichment. It is therefore likely that different approaches will result in the enrichment of different CTCs. These CTC populations might not completely overlap. There is currently no universal marker that may be used to enrich or detect all CTCs. There is a need for comparative studies between technologies to learn more about the variety of cells being captured with the different enrichment methods.
It is expected that other proposed CTC enrichment and detection systems will apply the same criteria used by the current gold standard, CellSearch® for comparison. However, this is complicated by the fact that there are no standardized antibodies for CTC detection. CK consists of many different types and isoforms, and their expression levels vary widely among different cancer types 151. Moreover, given the variable expression of markers during EMT and within CSCs, the current definition of a CTC in terms of immunocytochemistry may not include all clinically relevant tumor cells. For certain cancer types, there are known tissue- or organ-specific markers, such as prostate-specific antigen (PSA) for prostate cancer, carcinoembryonic antigen (CEA) for colon cancer, cancer antigen-125 (CA-125) for ovarian cancer, and human epidermal growth factor receptor 2 (HER-2) for breast cancer, etc. These may be used to identify CTCs of that specific cancer type.
It is important not to lose sight of the overall clinical purpose of CTC enrichment. Since the ultimate goal of CTC analysis is to improve cancer diagnosis and treatment monitoring, it is important to demonstrate a correlation with disease progression and patient outcome. Promising new approaches such as enrichment by acoustophoresis 152, 153 and other emerging technologies must be evaluated with patient samples to determine clinical relevance. The clinical relevance of any enriched CTC subpopulation must eventually be established through large-scale clinical trials. To date, this has only been achieved with the US FDA clearance of CellSearch® in breast, prostate, and colorectal cancers.
It is desirable to obtain CTCs that are not disturbed by the enrichment process, and that maintain their viability. Enrichment approaches that allow rapid isolation in a single step could avoid phenotypic alterations to CTCs that would complicate RNA and protein expression analyses. The proliferation and primary culture of viable CTCs has the potential to significantly advance our understanding of CTC biology, as well as enable the evaluation of therapeutic efficacies ex vivo.
One of the most promising CTC applications is treatment monitoring, where patient blood samples are obtained periodically before, during and after cancer treatment for CTC detection and analysis to determine if the treatment is effective. Conventional treatment monitoring is performed mainly with medical imaging techniques. However, CTC analysis might be a more sensitive and accurate technology and potentially provide more useful information 13, 154. So far, CTC enrichment and detection has been mostly limited to metastatic solid tumors. However, there is recent evidence that CTCs at even lower concentrations might exist in early stage cancer patients 155, 156. If the sensitivity and sample processing capability of CTC analysis can be improved, it is possible that CTC technologies can be used for early cancer detection.
Recent studies have confirmed a high degree of heterogeneity among CTCs. The resolution of CTC analysis must improve beyond enumeration and analysis of CTCs as a population, and should allow for evaluation on a single cell level. This will allow the characterization of clinically important rare cell phenotypes including CSCs and CTCs in various stages of EMT.
Acknowledgements
We thank the Penn State Materials Research Institute, Nanofabrication laboratory and Microscopy and Cytometry facility, and Penn State Hershey Cancer Institute for their support. This work is partially supported by the Pennsylvania State University start-up fund and the National Cancer Institute of the National Institutes of Health under Award Number R21CA161835 and DP2CA174508. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CTC
circulating tumor cell
- FDA
Food and Drug Administration
- EpCAM
epithelial cell adhesion molecule
- CK
cytokeratins
- EMT
epithelial to mesenchymal transition
- MET
mesenchymal to epithelial transition
- CSC
cancer stem cell
- AFM
atomic force microscopy
- ROT
electrorotation
- DEP
dielectrophoresis
- FFF
field-flow fractionation
References
- 1.Weinberg RA. The biology of cancer. New York: Garland Science, Talyor & Francis Group, LLC; 2007. [Google Scholar]
- 2.Fidler IJ. Timeline - The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Reviews Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep Nature of Metastatic Inefficiency : Dormancy of Solitary Cells after Successful Extravasation andLimited Survival of Early Micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.Alix-Panabières C, Pantel K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clinical Chemistry. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 6.Lacy A, Delgado S, Garcia-Valdecasas J, Castells A, Pique J, Grande L, Fuster J, Targarona E, Pera M, Visa J. Port site metastases and recurrence after laparoscopic colectomy. Surgical endoscopy. 1998;12:1039–1042. doi: 10.1007/s004649900776. [DOI] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. Journal of Clinical Oncology. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 8.Ushijima K. Treatment for recurrent ovarian cancer—at first relapse. Journal of oncology. 2009 doi: 10.1155/2010/497429. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–149. [Google Scholar]
- 10.Carey RW, Taft PD, Bennett JM, Kaufman S. Carcinocythemia (carcinoma cell leukemia): An acute leukemia-like picture due to metastatic carcinoma cells. The American journal of medicine. 1976;60:273–278. doi: 10.1016/0002-9343(76)90437-x. [DOI] [PubMed] [Google Scholar]
- 11.Myerowitz RL, Edwards PA, Sartiano GP. Carcinocythemia (carcinoma cell leukemia) due to metastatic carcinoma of the breast. Report of a case. Cancer. 1977;40:3107–3111. doi: 10.1002/1097-0142(197712)40:6<3107::aid-cncr2820400653>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Gallivan MV, Lokich JJ. Carcinocythemia (carcinoma cell leukemia). Report of two cases with English literature review. Cancer. 1984;53:1100–1102. doi: 10.1002/1097-0142(19840301)53:5<1100::aid-cncr2820530514>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LWWM. Circulating Tumor Cells at Each Follow-up Time Point during Therapy of Metastatic Breast Cancer Patients Predict Progression-Free and Overall Survival. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 14.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clinical Cancer Research. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LWMM, Meropol NJ. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, Hayes DF. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 17.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450 doi: 10.1038/nature06385. 1235-U10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber DA, Toner M. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proceedings of the National Academy of Sciences. 2010 doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh K-H, Yu W, Xiao W, Davis MM, Pease RF, Mindrinos MN, Jeffrey SS, Davis RW. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proceedings of the National Academy of Sciences. 2009;106:3970–3975. doi: 10.1073/pnas.0813188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Liu K, Liu J, Yu ZTF, Xu X, Zhao L, Lee T, Lee EK, Reiss J, Lee Y-K, Chung LWK, Huang J, Rettig M, Seligson D, Duraiswamy KN, Shen CKF, Tseng H-R. Highly Efficient Capture of Circulating Tumor Cells by Using Nanostructured Silicon Substrates with Integrated Chaotic Micromixers. Angewandte Chemie International Edition. 2011;50:3084–3088. doi: 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes AD, Mattison J, Western LT, Powderly JD, Greene BT, King MR. Microtube Device for Selectin-Mediated Capture of Viable Circulating Tumor Cells from Blood. Clinical Chemistry. 2012;58:846–853. doi: 10.1373/clinchem.2011.176669. [DOI] [PubMed] [Google Scholar]
- 22.Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Jansen NMH. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. International Journal of Oncology. 2012;41:1241. doi: 10.3892/ijo.2012.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Research. 2008:10. doi: 10.1186/bcr2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu C-L, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. I solation and Characterization of Circulating Tumor Cells from Patients with Localized and Metastatic Prostate Cancer. Science Translational Medicine. 2010;2:25ra–23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirby BJ, Jodari M, Loftus MS, Gakhar G, Pratt ED, Chanel-Vos C, Gleghorn JP, Santana SM, Liu H, Smith JP, Navarro VN, Tagawa ST, Bander NH, Nanus DM, Giannakakou P. Functional Characterization of Circulating Tumor Cells with a Prostate-Cancer-Specific Microfluidic Device. Plos One. 2012;7:e35976. doi: 10.1371/journal.pone.0035976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecot CV, Bischoff FZ, Mayer JA, Wong KL, Pham T, Bottsford-Miller J, Stone RL, Lin YG, Jaladurgam P, Roh JW. A novel platform for detection of CK+ and CK− CTCs. Cancer discovery. 2011;1:580–586. doi: 10.1158/2159-8290.CD-11-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara O, Tong X, Zborowski M, Chalmers JJ. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through immunomagnetic cell separation. Experimental Hematology. 2004;32:891–904. doi: 10.1016/j.exphem.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Lang JC, Balasubramanian P, Jatana KR, Schuller D, Agrawal A, Zborowski M, Chalmers JJ. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnology and Bioengineering. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T, Wallwiener M. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nature Biotechnology. 2013 doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 31.Riethdorf S, Fritsche H, Muller V, Rau T, Schindibeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clinical Cancer Research. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 32.Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak HF. Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 34.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, de Herreros AG. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature cell biology. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 35.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow EK-H. Implication of Cancer Stem Cells in Cancer Drug Development and Drug Delivery. Journal of Laboratory Automation. 2013;18:6–11. doi: 10.1177/2211068212454739. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 38.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. 1994 doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 39.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proceedings of the National Academy of Sciences. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 42.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic stem-like neural precursors from human glioblastoma. Cancer research. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 43.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly J, Chandra D, Zhou J, Claypool K. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 44.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, MacLaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proceedings of the National Academy of Sciences. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer research. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 46.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 47.Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating Tumor Cells and Circulating Tumor DNA. Annual Review of Medicine. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- 48.Attard G, de Bono JS. Utilizing circulating tumor cells: challenges and pitfalls. Current Opinion in Genetics & Development. 2011;21:50–58. doi: 10.1016/j.gde.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Mocellin S, Keilholz U, Rossi CR, Nitti D. Circulating tumor cells: the 'leukemic phase' of solid cancers. Trends in Molecular Medicine. 2006;12:130–139. doi: 10.1016/j.molmed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Pantel K, Alix-Panabieres C. The clinical significance of circulating tumor cells. Nat Clin Prac Oncol. 2007;4:62–63. doi: 10.1038/ncponc0737. [DOI] [PubMed] [Google Scholar]
- 51.Hayes DF, Smerage J. Is there a role for circulating tumor cells in the management of breast cancer? Clinical Cancer Research. 2008;14:3646–3650. doi: 10.1158/1078-0432.CCR-07-4481. [DOI] [PubMed] [Google Scholar]
- 52.Arya SK, Lim B, Rahman ARA. Enrichment Detection and Clinical Significance of Circulating Tumor Cell. Lab On A Chip. 2013 doi: 10.1039/c3lc00009e. [DOI] [PubMed] [Google Scholar]
- 53.Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Research. 2011;13:228. doi: 10.1186/bcr2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attard G, de Bono JS. Utilizing circulating tumor cells: challenges and pitfalls. Curr Opin Genet Dev. 2011;21:50–8. doi: 10.1016/j.gde.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends in Molecular Medicine. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Letters. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 57.den Toonder J. Circulating tumor cells: the Grand Challenge. Lab On A Chip. 2011;11:375–377. doi: 10.1039/c0lc90100h. [DOI] [PubMed] [Google Scholar]
- 58.Li P, Stratton ZS, Dao M, Ritz J, Huang TJ. Probing circulating tumor cells in microfluidics. Lab On A Chip. 2013;13:602–609. doi: 10.1039/c2lc90148j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joosse SA, Pantel K. Biologic Challenges in the Detection of Circulating Tumor Cells. Cancer Res. 2013;73:8–11. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Li J, Sun Y. Microfluidic approaches for cancer cell detection, characterization and separation. Lab On A Chip. 2012;12:1753–1767. doi: 10.1039/c2lc21273k. [DOI] [PubMed] [Google Scholar]
- 61.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta V, Jafferji I, Garza M, Melnikova VO, Hasegawa DK, Pethig R, Davis DW. ApoStream™, a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics. 2012;6:024133. doi: 10.1063/1.4731647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker FF, Wang X-B, Huang Y, Pethig R, Vykoukal J, Gascoyne P. Separation of human breast cancer cells from blood by differential dielectric affinity. Proceedings of the National Academy of Sciences. 1995;92:860–864. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA, Means RT. Wintrobe's Clinical Hematology. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 65.Schmid-Schonbein G, Shih Y, Chien S. Morphometry of human leukocytes. Blood. 1980;56:866–875. [PubMed] [Google Scholar]
- 66.Shapiro HM, Schildkraut ER, Curbelo R, Laird CW, Turner RB, Hirschfeld T. Combined Blood-Cell Counting And Classification With Fluorochrome Stains And Flow Instrumentation. Journal Of Histochemistry & Cytochemistry. 1976;24:396–411. doi: 10.1177/24.1.56391. [DOI] [PubMed] [Google Scholar]
- 67.Ruban GI, Kosmacheva SM, Goncharova NV, Van Bockstaele D, Loiko VA. Investigation of morphometric parameters for granulocytes and lymphocytes as applied to a solution of direct and inverse light-scattering problems. J Biomed Opt. 2007;12:044017. doi: 10.1117/1.2753466. [DOI] [PubMed] [Google Scholar]
- 68.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M, Lacour B, Brechot C, Paterlini-Brechot P. Isolation by size of epithelial tumor cells - A new method for the immunomorphological and molecular characterization of circulating tumor cells. American Journal Of Pathology. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gascoyne PRC, Shim S, Noshari J, Becker FF, Stemke-Hale K. Correlations between the dielectric properties and exterior morphology of cells revealed by dielectrophoretic field-flow fractionation. ELECTROPHORESIS. 2012 doi: 10.1002/elps.201200496. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, Voigt K, Lazar D, Nieva J, Bazhenova L, Ko AH, Korn WM, Schram E, Coward M, Yang X, Metzner T, Lamy R, Honnatti M, Yoshioka C, Kunken J, Petrova Y, Sok D, Nelson D, Kuhn P. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Physical Biology. 2012;9:016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyskens FL, Thomson SP, Moon TE. Quantitation of the Number of Cells within Tumor Colonies in Semisolid Medium and Their Growth as Oblate Spheroids. Cancer Research. 1984;44:271–277. [PubMed] [Google Scholar]
- 72.Vollmer RT. The effect of cell size on the pathologic diagnosis of small and large cell carcinomas of the lung. Cancer. 1982;50:1380–1383. doi: 10.1002/1097-0142(19821001)50:7<1380::aid-cncr2820500725>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 73.Lazar DC, Cho EH, Luttgen MS, Metzner TJ, Uson ML, Torrey M, Gross ME, Kuhn P. Fluid biopsy for solid tumors: a patient’s companion for lifelong characterization of their disease. Future Oncology. 2012;8:989. doi: 10.2217/fon.12.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB, Lerner RA, Bruce RH. A rare-cell detector for cancer. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2004;101:10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nieva J, Wendel M, Luttgen MS, Marrinucci D, Bazhenova L, Kolatkar A, Santala R, Whittenberger B, Burke J, Torrey M, Bethel K, Kuhn P. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Physical Biology. 2012;9:016004. doi: 10.1088/1478-3975/9/1/016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Giorgi V, Pinzani P, Salvianti F, Panelos J, Paglierani M, Janowska A, Grazzini M, Wechsler J, Orlando C, Santucci M. Application of a filtration-and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. Journal of Investigative Dermatology. 2010;130:2440–2447. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 77.Peeters DJ, Van den Eynden GG, van Dam PJ, Prove A, Benoy IH, van Dam PJ, Vermeulen PB, Pauwels P, Peeters M, Van Laere SJ, Dirix LY. Circulating tumour cells in the central and the peripheral venous compartment in patients with metastatic breast cancer. Br J Cancer. 2011;104:1472–7. doi: 10.1038/bjc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Carlo D. A Mechanical biomarker of cell state in medicine. Journal of Laboratory Automation. 2012;17:32–42. doi: 10.1177/2211068211431630. [DOI] [PubMed] [Google Scholar]
- 79.Shim S, Stemke-Hale K, Tsimberidou AM, Noshari J, Anderson TE, Gascoyne PR. Antibody-independent isolation of circulating tumor cells by continuous-flow dielectrophoresis. Biomicrofluidics. 2013;7:011807. doi: 10.1063/1.4774304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jen C-P, Chang H-H. A handheld preconcentrator for the rapid collection of cancerous cells using dielectrophoresis generated by circular microelectrodes in stepping electric fields. Biomicrofluidics. 2011;5:034101. doi: 10.1063/1.3609263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Svoboda K, Block SM. Biological Applications of Optical Forces. Annual Review of Biophysics and Biomolecular Structure. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- 82.Thoumine O, Ott A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. Journal of cell science. 1997;110(Pt 17):2109–16. doi: 10.1242/jcs.110.17.2109. [DOI] [PubMed] [Google Scholar]
- 83.Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151–60. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proceedings of the National Academy of Sciences. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nat Mater. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 86.Vliet K, Bao G, Suresh S. The biomechanics toolbox: experimental approaches for living cells and biomolecules. Acta Materialia. 2003;51:5881–5905. [Google Scholar]
- 87.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomaterialia. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenbluth MJ, Lam WA, Fletcher DA. Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophys J. 2006;90:2994–3003. doi: 10.1529/biophysj.105.067496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuznetsova TG, Starodubtseva MN, Yegorenkov NI, Chizhik SA, Zhdanov RI. Atomic force microscopy probing of cell elasticity. Micron. 2007;38:824–833. doi: 10.1016/j.micron.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 90.Lee YJ, Patel D, Park S. Local rheology of human neutrophils investigated using atomic force microscopy. Int J Biol Sci. 2011;7:102–11. doi: 10.7150/ijbs.7.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohamed H, Murray M, Turner JN, Caggana M. Isolation of tumor cells using size and deformation. Journal of Chromatography A. 2009;1216:8289–8295. doi: 10.1016/j.chroma.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 92.Li QS, Lee GY, Ong CN, Lim CT. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008;374:609–13. doi: 10.1016/j.bbrc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 93.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Käs J, Ulvick S, Bilby C. Optical Deformability as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic Competence. Biophysical Journal. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lincoln B, Erickson HM, Schinkinger S, Wottawah F, Mitchell D, Ulvick S, Bilby C, Guck J. Deformability-based flow cytometry. Cytometry . Part A : the journal of the International Society for Analytical Cytology. 2004;59:203–9. doi: 10.1002/cyto.a.20050. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W, Kai K, Choi DS, Iwamoto T, Nguyen YH, Wong H, Landis MD, Ueno NT, Chang J, Qin L. Microfluidics separation reveals the stem-cell-like deformability of tumor-initiating cells. Proceedings of the National Academy of Sciences. 2012;109:18707–18712. doi: 10.1073/pnas.1209893109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nature Nanotechnology. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 97.Cross SE, Jin Y-S, Rao J, Gimzewski JK. Applicability of AFM in cancer detection. Nat Nano. 2009;4:72–73. doi: 10.1038/nnano.2009.004. [DOI] [PubMed] [Google Scholar]
- 98.Weitz J, Kienle P, Lacroix J, Willeke F, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clinical Cancer Research. 1998;4:343–348. [PubMed] [Google Scholar]
- 99.Gossett DR, Henry T, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao J, Clark AT, Di Carlo C. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proceedings of the National Academy of Sciences. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ochalek T, Nordt FJ, Tullberg K, Burger MM. Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res. 1988;48:5124–8. [PubMed] [Google Scholar]
- 101.Liu Z, Huang F, Du J, Shu W, Feng H, Xu X, Chen Y. Rapid isolation of cancer cells using microfluidic deterministic lateral displacement structure. Biomicrofluidics. 2013;7:011801. doi: 10.1063/1.4774308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. British Journal of Cancer. 2011;105:847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weiss L, Zeigel R. Cell surface negativity and the binding of positively charged particles. Journal of Cellular Physiology. 1971;77:179–185. doi: 10.1002/jcp.1040770208. [DOI] [PubMed] [Google Scholar]
- 104.Mehrishi JN, Bauer J. Electrophoresis of cells and the biological relevance of surface charge. ELECTROPHORESIS. 2002;23:1984–1994. doi: 10.1002/1522-2683(200207)23:13<1984::AID-ELPS1984>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 105.Pethig R. Review Article---Dielectrophoresis: Status of the theory, technology and applications. Biomicrofluidics. 2010;4:022811–35. doi: 10.1063/1.3456626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asami K. Characterization of biological cells by dielectric spectroscopy. Journal of Non-Crystalline Solids. 2002;305:268–277. [Google Scholar]
- 107.Cheung K, Gawad S, Renaud P. Impedance spectroscopy flow cytometry: on-chip label-free cell differentiation. Cytometry . Part A : the journal of the International Society for Analytical Cytology. 2005;65:124–32. doi: 10.1002/cyto.a.20141. [DOI] [PubMed] [Google Scholar]
- 108.Gawad S, Schild L, Renaud P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab On A Chip. 2001;1:76–82. doi: 10.1039/b103933b. [DOI] [PubMed] [Google Scholar]
- 109.Sohn LL, Saleh OA, Facer GR, Beavis AJ, Allan RS, Notterman DA. Capacitance cytometry: Measuring biological cells one by one. Proceedings of the National Academy of Sciences. 2000;97:10687–10690. doi: 10.1073/pnas.200361297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun T, Holmes D, Gawad S, Green NG, Morgan H. High speed multi-frequency impedance analysis of single particles in a microfluidic cytometer using maximum length sequences. Lab on a chip. 2007;7:1034–1040. doi: 10.1039/b703546b. [DOI] [PubMed] [Google Scholar]
- 111.Jones TB. Basic theory of dielectrophoresis and electrorotation. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2003;22:33–42. doi: 10.1109/memb.2003.1304999. [DOI] [PubMed] [Google Scholar]
- 112.Chan KL, Morgan H, Morgan E, Cameron IT, Thomas MR. Measurements of the dielectric properties of peripheral blood mononuclear cells and trophoblast cells using AC electrokinetic techniques. Biochim Biophys Acta. 2000;1500:313–22. doi: 10.1016/s0925-4439(99)00115-5. [DOI] [PubMed] [Google Scholar]
- 113.Borgatti M, Bianchi N, Mancini I, Feriotto G, Gambari R. New trends in non-invasive prenatal diagnosis: Applications of dielectrophoresis-based Lab-on-a-chip platforms to the identification and manipulation of rare cells (Review) International Journal of Molecular Medicine. 2008;21:3–12. [PubMed] [Google Scholar]
- 114.Gagnon ZR. Cellular dielectrophoresis: Applications to the characterization, manipulation separation and patterning of cells. ELECTROPHORESIS. 2011;32:2466–2487. doi: 10.1002/elps.201100060. [DOI] [PubMed] [Google Scholar]
- 115.Pethig R. Dielectrophoresis: Using inhomogeneous AC electrical fields to separate and manipulate cells. Critical Reviews in Biotechnology. 1996;16:331–348. [Google Scholar]
- 116.Han A, Yang L, Frazier AB. Quantification of the heterogeneity in breast cancer cell lines using whole-cell impedance spectroscopy. Clin Cancer Res. 2007;13:139–43. doi: 10.1158/1078-0432.CCR-06-1346. [DOI] [PubMed] [Google Scholar]
- 117.Han S-I, Joo Y-D, Han K-H. An electrorotation technique for measuring the dielectric properties of cells with simultaneous use of negative quadrupolar dielectrophoresis and electrorotation. Analyst. 2013;138:1529–1537. doi: 10.1039/c3an36261b. [DOI] [PubMed] [Google Scholar]
- 118.Shim S, Gascoyne P, Noshari J, Hale KS. Dynamic physical properties of dissociated tumor cells revealed by dielectrophoretic field-flow fractionation. Integrative biology : quantitative biosciences from nano to macro. 2011;3:850–62. doi: 10.1039/c1ib00032b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shim S, Stemke-Hale K, Noshari J, Becker FF, Gascoyne PRC. Dielectrophoresis has broad applicability to marker-free isolation of tumor cells from blood by microfluidic systems. Biomicrofluidics. 2013;7:011808–12. doi: 10.1063/1.4774307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seal S. Silicone flotation: A simple quantitative method for the isolation of free floating cancer cells from the blood. Cancer. 1959;12:590–595. doi: 10.1002/1097-0142(195905/06)12:3<590::aid-cncr2820120318>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 121.Rosenberg R, Gertler R, Friederichs J, Fuehrer K, Dahm M, Phelps R, Thorban S, Nekarda H, Siewert JR. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49:150–158. doi: 10.1002/cyto.10161. [DOI] [PubMed] [Google Scholar]
- 122.Müller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, Jänicke F, Pantel K. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases heterogeneous response to systemic therapy and low proliferative activity. Clinical Cancer Research. 2005;11:3678–3685. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- 123.Balic M, Dandachi N, Hofmann G, Samonigg H, Loibner H, Obwaller A, van der Kooi A, Tibbe AGJ, Doyle GV, Terstappen L, Bauernhofer T. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry Part B-Clinical Cytometry. 2005;68B:25–30. doi: 10.1002/cyto.b.20065. [DOI] [PubMed] [Google Scholar]
- 124.Fleischer RL, Price PB, Symes EM. Novel Filter for Biological Materials. Science. 1964;143:249–250. doi: 10.1126/science.143.3603.249. [DOI] [PubMed] [Google Scholar]
- 125.Fleischer RL, Alter HW, Walker RM, Furman SC, Price PB. Particle Track Etching. Science. 1972;178:255. doi: 10.1126/science.178.4058.255. [DOI] [PubMed] [Google Scholar]
- 126.Seal SH. A sieve for the isolation of cancer cells and other large cells from the blood. Cancer. 1964;17:637–642. doi: 10.1002/1097-0142(196405)17:5<637::aid-cncr2820170512>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 127.Vona G, Estepa L, Beroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, Brechot C, Paterlini-Brechot P. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39:792–797. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- 128.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clinical Cancer Research. 2011;17:827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 129.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105:1338–1341. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen CL, Mahalingam D, Osmulski P, Jadhav RR, Wang CM, Leach RJ, Chang TC, Weitman SD, Kumar AP, Sun L. Single cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT related genes in metastatic prostate cancer. The Prostate. 2012 doi: 10.1002/pros.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hofman VJ, Ilie MI, Bonnetaud C, Selva E, Long E, Molina T, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E. Cytopathologic Detection of Circulating Tumor Cells Using the Isolation by Size of Epithelial Tumor Cell Method Promises and Pitfalls. American journal of clinical pathology. 2011;135:146–156. doi: 10.1309/AJCP9X8OZBEIQVVI. [DOI] [PubMed] [Google Scholar]
- 132.Zheng S, Lin HK, Liu J-Q, Balic M, Datar R, Cote RJ, Tai Y-C. Membrane microfilter device for selective capture electrolysis and genomic analysis of human circulating tumor cells. Journal of Chromatography A. 2007;1162:154–161. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 133.Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC, Cote RJ. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010;16:5011–5018. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hosokawa M, Hayata T, Fukuda Y, Arakaki A, Yoshino T, Tanaka T, Matsunaga T. Size-Selective Microcavity Array for Rapid and Efficient Detection of Circulating Tumor Cells. Analytical Chemistry. 2010;82:6629–6635. doi: 10.1021/ac101222x. [DOI] [PubMed] [Google Scholar]
- 135.Lim LS, Hu M, Huang MC, Cheong WC, Gan ATL, Looi XL, Leong SM, Koay ES-C, Li M-H. Microsieve lab-chip device for rapid enumeration and fluorescence in situ hybridization of circulating tumor cells. Lab On A Chip. 2012;12:4388–4396. doi: 10.1039/c2lc20750h. [DOI] [PubMed] [Google Scholar]
- 136.Kuo JS, Zhao YX, Schiro PG, Ng LY, Lim DSW, Shelby JP, Chiu DT. Deformability considerations in filtration of biological cells. Lab On A Chip. 2010;10:837–842. doi: 10.1039/b922301k. [DOI] [PubMed] [Google Scholar]
- 137.Zheng S, Lin HK, Lu B, Williams A, Datar RH, Cote RJ, Tai YC. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomedical Microdevices. 2011;13:203. doi: 10.1007/s10544-010-9485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Desitter I, Guerrouahen B, Benali-Furet N, Wechsler J, Jänne P, Kuang Y, Yanagita M, Wang L, Berkowitz J, Distel R. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Research. 2011;31:427. [PubMed] [Google Scholar]
- 139.Xu T, Lu B, Tai Y-C, Goldkorn A. A Cancer Detection Platform Which Measures Telomerase Activity from Live Circulating Tumor Cells Captured on a Microfilter. Cancer Research. 2010;70:6420–6426. doi: 10.1158/0008-5472.CAN-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mohamed H, McCurdy LD, Szarowski DH, Duva S, Turner JN, Caggana M. Development of a rare cell fractionation device: application for cancer detection. NanoBioscience, IEEE Transactions on. 2004;3:251–256. doi: 10.1109/tnb.2004.837903. [DOI] [PubMed] [Google Scholar]
- 141.Tan S, Yobas L, Lee G, Ong C, Lim C. Microdevice for the isolation and enumeration of cancer cells from blood. Biomedical Microdevices. 2009;11:883–892. doi: 10.1007/s10544-009-9305-9. [DOI] [PubMed] [Google Scholar]
- 142.Tan SJ, Lakshmi RL, Chen PF, Lim WT, Yobas L, Lim CT. Versatile label free biochip for the detection of circulating tumor cells from peripheral blood in cancer patients. Biosensors & Bioelectronics. 2010;26:1701–1705. doi: 10.1016/j.bios.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 143.Bhagat AAS, Hou HW, Li LD, Lim CT, Han J. Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation. Lab On A Chip. 2011;11:1870–1878. doi: 10.1039/c0lc00633e. [DOI] [PubMed] [Google Scholar]
- 144.Hur SC, Mach AJ, Di Carlo D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics. 2011:5. doi: 10.1063/1.3576780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hur SC, Henderson-MacLennan NK, McCabe ER, Di Carlo D. Deformability-based cell classification and enrichment using inertial microfluidics. Lab On A Chip. 2011;11:912–920. doi: 10.1039/c0lc00595a. [DOI] [PubMed] [Google Scholar]
- 146.Sun J, Li M, Liu C, Zhang Y, Liu D, Liu W, Hu G, Jiang X. Double spiral microchannel for label-free tumor cell separation and enrichment. Lab On A Chip. 2012;12:3952–3960. doi: 10.1039/c2lc40679a. [DOI] [PubMed] [Google Scholar]
- 147.Sun J, Liu C, Li M, Wang J, Xianyu Y, Hu G, Jiang X. Size-based hydrodynamic rare tumor cell separation in curved microfluidic channels. Biomicrofluidics. 2013;7:011802. doi: 10.1063/1.4774311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS-W, Lim W-T, Han J, Bhagat AAS, Lim CT. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013:3. doi: 10.1038/srep01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Becker F, Wang X-B, Huang Y, Pethig R, Vykoukal J, Gascoyne P. The removal of human leukaemia cells from blood using interdigitated microelectrodes. Journal of Physics D: Applied Physics. 1994;27:2659. [Google Scholar]
- 150.Huang Y, Wang X-B, Becker FF, Gascoyne P. Introducing dielectrophoresis as a new force field for field-flow fractionation. Biophysical Journal. 1997;73:1118–1129. doi: 10.1016/S0006-3495(97)78144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins - patterns of expression in normal epithelia tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 152.Augustsson P, Magnusson C, Grenvall C, et al. Extraction of circulating tumor cells from blood using acoustophoresis. Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences; Oct 3–7 2010; Groningen, The Netherlands. 2010. pp. 1592–1594. [Google Scholar]
- 153.Augustsson P, Magnusson C, Nordin M, Lilja H, Laurell T. Microfluidic Label-Free Enrichment of Prostate Cancer Cells in Blood Based on Acoustophoresis. Analytical Chemistry. 2012;84:7954–7962. doi: 10.1021/ac301723s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen L, Hayes DF. Circulating tumor cells versus imaging - Predicting overall survival in metastatic breast cancer. Clinical Cancer Research. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 155.Nakagawa T, Martinez SR, Goto Y, Koyanagi K, Kitago M, Shingai T, Elashoff DA, Ye X, Singer FR, Giuliano AE. Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clinical Cancer Research. 2007;13:4105–4110. doi: 10.1158/1078-0432.CCR-07-0419. [DOI] [PubMed] [Google Scholar]
- 156.Wendel M, Bazhenova L, Boshuizen R, Kolatkar A, Honnatti M, Cho EH, Marrinucci D, Sandhu A, Perricone A, Thistlethwaite P, Bethel K, Nieva J, Heuvel Mv. d, Kuhn P. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Physical Biology. 2012;9:016005. doi: 10.1088/1478-3967/9/1/016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu B, Xu T, Zheng S, et al. Parylene membrane slot filter for the capture analysis and culture of viable circulating tumor cells. Proceedings of the 23rd International Conference on Micro Electro Mechanical Systems, IEEE; Jan 25–28 2010; Hong Kong, China. pp. 935–938. [Google Scholar]