Abstract
Background
Previous mouse studies suggest that decreasing dietary fat content can slow prostate cancer (PCa) growth. To our knowledge, no study has yet compared the effect of multiple different fats on PCa progression. We sought to systematically compare the effect of fish oil, olive oil, corn oil, and animal fat on PCa progression.
Methods
A total of 96 male SCID mice were injected with LAPC-4 human PCa cells. Two weeks following injection, mice were randomized to a fish oil, olive oil, corn oil, or animal fat-based Western diet (35% kcals from fat). Animals were euthanized when tumors reached 1,000mm3. Serum was collected at sacrifice and assayed for PSA, insulin, IGF-1, IGFBP-3, and PGE-2 levels. Tumors were also assayed for PGE-2 and COX-2 levels and global gene expression analyzed using Affymetrix microarrays.
Results
Mice weights and tumor volumes were equivalent across groups at randomization. Overall, fish oil consumption was associated with improved survival, relative to other dietary groups (p=0.014). On gene expression analyses, the fish oil group had decreased signal in pathways related to mitochondrial physiology and insulin synthesis/secretion.
Conclusions
In this xenograft model, we found that consuming a diet in which fish oil was the only fat source slowed tumor growth and improved survival, compared to mice consuming diets composed of olive oil, corn oil, or animal fat. While prior studies showed that the amount of fat is important for PCa growth, the current study suggests that type of dietary fat consumed may also be important.
Keywords: prostatic neoplasms, diet, fat
INTRODUCTION
Prostate cancer (PCa) is the second-leading cause of cancer-related death among men in Western society.(1, 2) Geographical differences in incidence rates suggest that environmental factors play a significant role in the progression and development of PCa. As diet varies significantly across nationalities, and is an easily-modified, low-cost intervention, many prior studies have queried dietary contributions to PCa development and outcome. Consumption of high amounts of dietary fat is a commonly identified factor in the development and progression of PCa.(3-6) Indeed, per capita total fat consumption is strongly correlated with national PCa mortality in some datasets.(7) In contrast, the multicenter European Prospective Investigation into Cancer (EPIC) study, which enrolled 142,520 men, found no association between dietary fat intake and PCa risk.(8)
When specific types of fat are examined, population-based studies suggest that consumption of fatty fish reduces PCa incidence.(9, 10) Similarly, the 12-year prospective Health Professionals Follow-Up Study showed that higher consumption of fish was strongly associated with a reduced risk of metastatic PCa.(11) Olive oil has also been touted as potentially decreasing PCa risk. Indeed, a randomized trial showed that a Mediterranean diet (high in both ω-3 fatty acids and olive oil-derived fats) may protect against many forms of cancer, including prostate.(12) On the contrary, other studies have suggested that increased consumption of ω-6 fatty acids(13) and animal fats (saturated fat)(14) can increase the risk of PCa.
Similarly, in vivo animal studies have suggested that increased dietary fat promotes PCa growth.(15-17) However, the vast majority of these studies have focused on the amount of dietary fat and neglected to examine the type of fat consumed. Of major dietary fat sources, fish oil is the most heavily studied. Previous mouse xenograft studies have suggested that diets high in fish oil (an ω-3 fatty acid), relative to diets with a greater proportion of corn oil (an ω-6 fatty acid), may slow tumor growth and prolong survival.(18, 19) Prior studies by our group have suggested that decreasing dietary saturated fat levels (animal fats, such as milk fat and lard) does not prolong survival in either intact or castrated mouse PCa xenograft models.(20, 21) However, to our knowledge, never before has a head-to-head trial of the effect of multiple types of dietary fat on tumor progression and PCa survival been undertaken. As such, we sought to systematically examine this question and perhaps identify mechanisms by which more “prostate-healthy” fats exert their effects. We hypothesized that fish oil and olive oil-based diets would slow tumor progression relative to the “less healthy” corn oil and animal fat-based diets.
MATERIALS & METHODS
Cell Culture
LAPC-4 human PCa cells were a generous gift from William J. Aronson, UCLA School of Medicine. This cell line was developed at UCLA by direct transfer of cancer cells from a patient with advanced prostatic adenocarcinoma. LAPC-4 produces prostate-specific antigen, has a wild-type androgen receptor, and shows features of hormone-dependent growth and metastasis.(22)This cell line has been frequently used to model localized, androgen-sensitive disease.(23-25) We specifically chose this cell line for its hormone dependence, as we aim to model the effect of dietary intervention on early stage disease, which is typically androgen-sensitive. Cells were maintained in Iscove’s modified medium with 10% fetal bovine serum and supplemented with the synthetic androgen R1881 at 1nM. Cells were grown in 5% CO2 at 37°C and harvested by trypsinization at ~80% confluence in log phase growth.
Animal Studies
After approval from the Duke University Institutional Animal Care and Use Committee, 100 male SCID (CB.17 scid/scid) mice, aged 8 weeks, were purchased from Taconic Farms (Hudson, NY). Animals were housed five-to-a-cage and fed an ad libitum diet of standard mouse chow (20% protein, 9% fat, 71% carbohydrate kcals) for a one-week acclimation period. Following acclimation, mice were injected in the flank with 1×105 LAPC-4 cells in 0.1mL of Matrigel (Becton Dickinson, Franklin Lakes, NJ) and returned to group housing with ad lib feeding using standard mouse chow. Eleven days after injection, mice were transitioned to single housing. Given the importance of energy balance in modulating tumor growth, all mice were housed one per cage for the duration of the study in order to permit precise measurement of caloric intake.(26)
Given that changes in housing can be stressful, we waited three additional days before randomizing the mice to the four diets arms at two weeks post-injection. We elected to randomize the mice at two-weeks following injection in order to ensure that we were studying the effect of diet on PCa progression rather than disease initiation. It was our goal to model treatment of early stage disease in this study, rather than prevention, and at 14-days after injection, it is likely that all tumor xenografts had taken and were metabolically active.
Mice were randomized to one of four diets (all 16% protein, 35% fat, 49% carbohydrate kcals), which differed only in their dietary fat source. Fat sources were fish oil, olive oil, corn oil, and animal fat (lard/milk fat). The diets were prepared by TestDiet (Indianapolis, IN) (Table 1). Primary fatty acid compositions were 30–40% ω-3 for fish oil, 65–80% oleic acid for olive oil, 54% ω-6 for corn oil, and 56% saturated fat for the animal fat (Table 2). In a pilot study (data not shown), we determined that fish oil-fed mice consumed fewer calories on average than the other groups. As such, the fish oil group was fed ad libitum and mice in the other groups were fed via a modified paired-feeding protocol to maintain isocaloric intake between the groups.(20) Mice were weighed twice weekly to ensure equal body weights across groups.
Table 1.
Ingredients of Experimental Diets*
| Grams | % of Energy | |
|---|---|---|
|
| ||
| FAT ** | 17.0 | 35.0 |
|
| ||
| PROTEIN | 19.7 | 15.8 |
| Casein | 19.4 | |
| DL-methionine | 0.3 | |
|
| ||
| CARBOHYDRATE | 53.0 | 49.2 |
| Dextrin | 5.0 | |
| Maltodextrin 10 | 10.0 | |
| Sucrose | 38.0 | |
|
| ||
| Cholesterol | 0.15 | 0.0 |
| AIN-76 mineral mix | 3.5 | |
| AIN-76 vitamin mix | 1.0 | |
| Cellulose | 5.0 | |
| Calcium carbonate | 0.4 | |
| Choline bitartrate | 0.2 | |
|
| ||
| TOTAL | 172.65 | 100% |
Based upon amount of food needed to deliver 760 kcal of energy
Type of fat varies by diet. All diets were formulated with a single fat source, with no contamination by other sources of fat.
Table 2.
Fatty Acid Profiles by Diet
| Carbon Atoms | Formal Name | Corn Oil (%) | Fish Oil (%) | Olive Oil (%) | Animal Fat (%) |
|---|---|---|---|---|---|
| C4:0 | Butyric | <0.06 | – | – | 1.04 |
| C6:0 | Caproic | <0.06 | – | – | 1.21 |
| C7:0 | Heptanoic | – | – | – | <0.1 |
| C8:0 | Caprylic | <0.06 | – | – | 0.9 |
| C9:0 | Nonanoic | – | – | – | <0.1 |
| C10:0 | Capric | <0.06 | – | – | 2.25 |
| C11:0 | Undecanoic | – | – | – | 0.23 |
| C12:0 | Lauric | <0.06 | – | – | 2.72 |
| C13:0 | Tridecanoic | – | – | – | 0.15 |
| C14:0 | Myristic | <0.06 | 6.85 | 0.01 | 9.47 |
| C14:1n5 | Myristoleic | <0.06 | – | – | 0.660 |
| C15:0 | Pentandecanoic | <0.06 | 0.46 | – | 0.95 |
| C15:1n5 | 10-Pentadecenoic | <0.06 | – | – | <0.1 |
| C16:0 | Palmitic | 10.3 | 14.8 | 11.9 | 28.2 |
| C16:1n7 | Palmitoleic | 0.12 | 9.74 | – | 1.51 |
| C17:0 | Margaric | 0.08 | 0.38 | 0.1 | 0.64 |
| C17:1n7 | Margaroleic | <0.06 | – | 0.1 | <0.1 |
| C16:2 | Hexadecadianoic | – | 1.62 | – | <0.1 |
| C18:0 | Stearic | 1.98 | 2.55 | 3.4 | 13.8 |
| C18:1n9T | Elaidic | 0.08 | – | – | 2.96 |
| C16:3n4 | Hexadecatrianoic | – | 1.51 | – | <0.1 |
| C18:1n9C | Oleic | – | 9.58 | 77.2 | 22.8 |
| C18:1n7C | Vaccenic | – | – | – | 0.94 |
| C18:1 | Other cis isomers | – | – | – | 1.4 |
| C19:0 | Nonadecanoic | – | – | – | 0.24 |
| C18:2 | Other trans isomers | 0.40 | – | – | 0.94 |
| C16:4n1 | Hexadecatetraenoic | – | 1.53 | – | <0.10 |
| C18:2n6 | Linoleic | 53.7 | 1.93 | 3.9 | 3.16 |
| C20:0 | Arachidic | 0.40 | 0.17 | 0.3 | 0.16 |
| C18:3 | Trans isomers | <0.06 | – | – | <0.1 |
| C13:3n6 | Gamma Linolenic | – | – | – | <0.1 |
| C18:3 | Octadecatrienoic | – | – | – | <0.1 |
| C20:1n11 | Eicosenoic | – | 1.48 | 0.2 | <0.1 |
| C18:3n3 | Linolenic | – | 1.48 | 0.7 | 0.34 |
| C21:0 | Heneicosanoic | – | – | – | <0.1 |
| C18:4n3 | Octadecatetraenoic | – | 3.09 | – | <0.1 |
| C20:2n6 | Eicosadienoic | <0.06 | 0.18 | – | <0.1 |
| C22:0 | Behenic | 0.12 | 0.10 | 0.1 | <0.1 |
| C20:3n6 | Homo-gama-linolenic | – | – | – | 0.12 |
| C22:1n11 | Docosaenoic | – | – | – | <0.1 |
| C22:1n9 | Erucic | <0.06 | 0.33 | – | <0.1 |
| C20:3n3 | Eicosatrienoic | <0.06 | 0.37 | – | <0.1 |
| C20:4n6 | Arachidonic | <0.06 | 2.09 | – | 0.12 |
| C23:0 | Tricosanoic | – | – | – | <0.1 |
| C20:4n3 | Eicosatetraenoic | – | – | – | <0.1 |
| C22:2n6 | Docosadienoic | – | – | – | <0.1 |
| C24:0 | Lignoceric | 0.17 | 0.60 | trace | <0.1 |
| C20:5n3 | Eicosapentaenoic | <0.06 | 14.16 | – | <0.1 |
| C21:5 | Norvonic | – | – | – | <0.1 |
| C22:5n6 | Heneicosapentaenoic | – | 0.76 | – | <0.1 |
| C22:5n3 | Docosapentaenoic | <0.06 | 2.82 | – | <0.1 |
| C22:6n3 | Docosahexaenoic | <0.06 | 12.2 | – | <0.1 |
| C22:4 | Adrenic | – | 0.24 | – | – |
| C24:1 | Selacholeic | – | 0.22 | – | – |
| Others | – | – | – | 2.7 |
When tumors became palpable, their dimensions were measured using digital caliper. Tumor volumes, measured twice weekly, were calculated using the formula: width x height x length x 0.5236.(27) At 3 weeks post-randomization to the diets (5 weeks post-tumor injection), mice were bled via the facial vein and blood glucose measured using a handheld Accu-Chek Active glucometer (Roche Diagnostics, Indianapolis, IN). Glucose measurements were taken immediately prior to feeding, which likely reflected a partially-fasted state, as the majority of the animals consumed all of their prescribed diet prior to food allocation each day. However, we did not remove the diet from the animal’s cages at any given time before assessing glucose levels, so we cannot comment on how long the animals were fasted prior assessment.
Animals were euthanized using a lethal dose of pentobarbital when tumors reached 1,000mm3 or when the health of the animal appeared compromised per institutional criteria (ruffled fur, hunched posture, lethargy, weight loss, etc). Serum was obtained via cardiac puncture. All tumor tissue was surgically excised from the subcutaneous flank pockets in which it was growing. Serum and tumor samples were snap frozen at -80°C for subsequent analysis.
Serum from the 8 median surviving mice from each group (total 32 mice) was assayed for levels of murine insulin, insulin-like growth factor (IGF)-1, IGF-1 binding protein (IGFBP)-3, and prostaglandin E-2 (PGE-2) using mouse-specific enzyme-linked immunoassays (ELISA) (Linco, Billerica, MA; R&D Systems, Minneapolis, MN; and Neogen, Lexington, KY; respectively). Serum was also assessed for human prostate-specific antigen (PSA) produced by the LAPC-4 xenograft using ELISA (Abazyme, Needham, MA).
Western blot analysis was conducted on the xenograft tumor tissue of the median surviving 6 mice from each group (total 24 mice). Tissue lysate was first prepared, using the QProteome Mammalian Protein Preparation kit (Qiagen, Valencia, CA). A total of 1mL cell lysis buffer was added to 50-60μg of prostate xenograft tumor tissue and processed using a mechanical tissue homogenizer. Homogenates were centrifuged at 10,000rpm for 20min to clarify the lysates, and total protein concentration was determined using the BCA Protein Assay Reagent (Thermo Fisher Scientific, Rockford, IL). All lysates were stored at -80°C until further analysis.
To conduct the Western blot analysis, denatured samples of tissue homogenates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent immunoblotting using the Fast Western Blot kit (Pierce-Thermo Scientific, Rockford, IL) to determine expression of target proteins. The primary antibody used for immunoblotting was anti-COX-2 (160112) from Cayman Chemical (Ann Arbor, MI). Given the need to run 24 samples, and given that the gels hold 12 samples, we ran 3 mice per group x 4 groups on each gel, requiring a total of 2 gels to be analyzed. These were repeated twice. Densitometric analysis was performed using the ImageJ software (National Institutes of Health, Bethesda, MD).
RNA isolation and microarray analysis
Total RNA from the excised xenograft tumors of each of the 6 median-surviving mice per group was purified with TriZol (Invitrogen, Grand Island, NY) and verified to be intact by Bioanalyzer. Extracted RNA was then hybridized to Affymetrix (Santa Clara, CA) Human genome 133A 2.0 arrays using a standard protocol described previously.(28) All data have been deposited into .cel files (GEO (GSE40654)).
Statistical Analysis
The primary end-point was survival, defined as time from randomization to sacrifice, which was examined using the log-rank test. Graphically, survival was represented using Kaplan-Meier curves. Comparisons of secondary outcomes including body weight, tumor volume, serum levels of PSA, IGF-axis hormones, glucose, and PGE-2; and tumor COX-2 levels were determined using the Kruskal-Wallis test.
For microarray analyses, all .cel files were normalized by RMA (Expression Console, Affymetrix, Santa Clara, CA). Given the distinct biological response of the fish oil, we compared differences in biological pathways between the 6 median surviving fish oil-fed mice vs. the 6 median surviving mice from each of the other dietary groups combined (18 samples total). Data were analyzed with Gene Set Enrichment Analysis (GSEA) using the Broad public server to identify biological pathways affected by dietary fat source.(29)
All statistical analyses were performed using STATA 10.0 (Stata Corp., College Station, TX) with p≤0.05 considered statistically significant.
RESULTS
Body Weight
At randomization, mouse weights were equivalent across arms (p=0.99). A paired-feeding protocol allowed for isocaloric feeding between groups. Despite careful titration of caloric intake, mouse body weights did differ significantly across groups at a small number of timepoints (Figure 1). Of note, during the first 20 days, fish oil-fed mice were slightly lighter than the other groups (typically <1 gram difference in body weight), with no appreciable differences during the next 20 days. By day 40, fish oil-fed mice were the heaviest and remained so for the majority of subsequent timepoints until only a few mice were still alive, near day 95. Overall, all mice consumed their prescribed diets without observed toxicity in any group.
Figure 1.
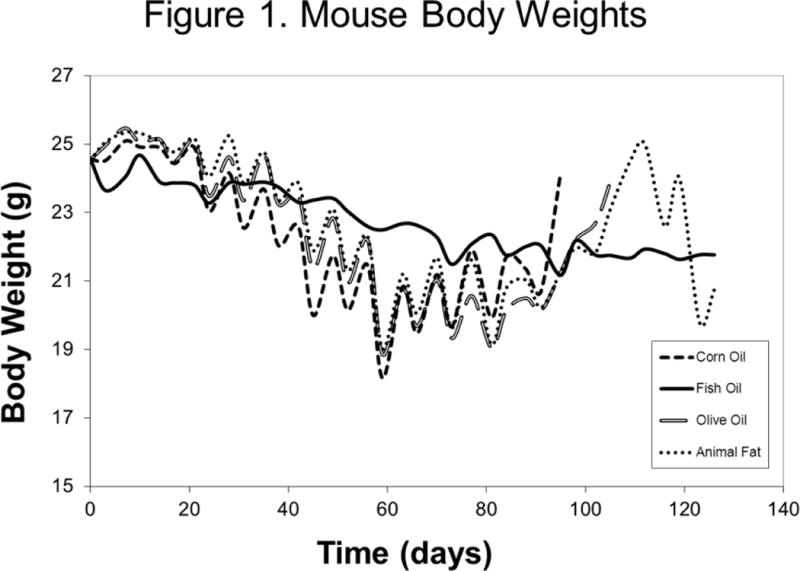
Median Mouse Body Weights by Treatment Arm from the Day of Tumor Inoculation
Tumor Growth and Survival
Median tumor volumes at randomization (day 14) were equivalent across all four groups (p=0.99, Figure 2). As the study progressed, disparities in tumor growth became apparent. From approximately day 28 (14 days after randomization), fish oil-fed mice demonstrated significantly smaller tumors than mice in each of the other dietary groups. This trend continued throughout the duration of the study. While fish oil-fed mice had smaller tumors at every time point, these differences only reached statistical significance at certain, but not all, time points. Of the 96 mice included in the study, 11 (12%) required sacrifice prior to their tumors reaching 1,000mm3 due to apparent compromise in overall health. Of these, 2 came from the corn oil group, 3 from fish oil, 3 from olive oil, and 3 from the animal fat group.
Figure 2.
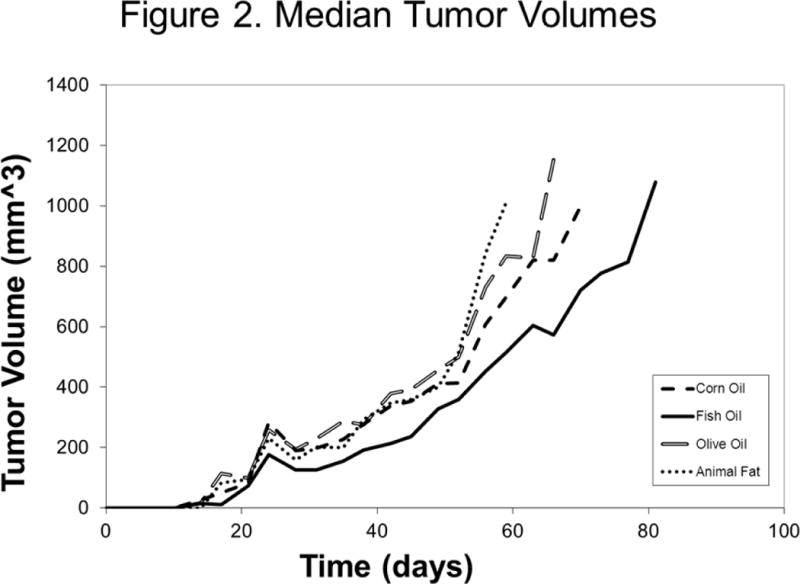
Median Tumor Volumes by Treatment Arm from the Day of Tumor Inoculation
Overall, there were trends toward an association between diet and survival, though this did not reach statistical significance (p=0.093, log rank test, Figure 3). When examined in two-way analyses, mice in the fish oil group survived longer than mice in any other group (p=0.014 for fish vs. corn, p=0.017 for fish vs. olive, p=0.090 for fish vs. animal fat). After combining the three non-fish oil groups, which all had similar survival times, fish oil was associated with a significant improvement in survival versus non-fish oil diets (p=0.014, log-rank test, Figure 4).
Figure 3.
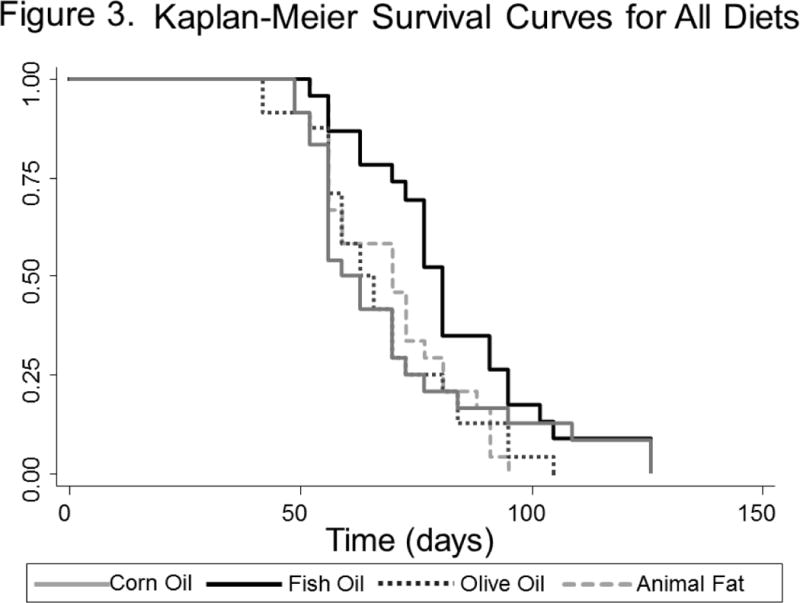
Kaplan-Meier Survival Curve by Treatment Arm from the Day of Tumor Inoculation – All Diets
Figure 4.
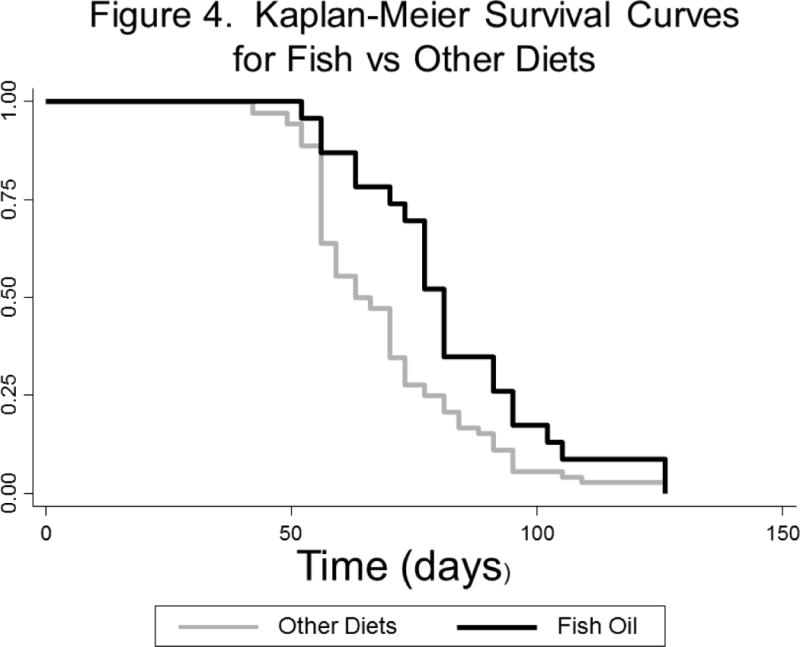
Kaplan-Meier Survival Curve by Treatment Arm from the Day of Tumor Inoculation –Fish-oil diet versus other diets combined
Serum & Tissue Analyses
Serum glucose at the time of sacrifice differed significantly among groups, with the fish oil group having the highest levels (p=0.03, Supplementary Figure 1). Median serum PSA at sacrifice was 38.7 (IQR 33.1–64.2), 44.0 (34.6–48.4), 59.6 (43.6–78.5), and 53.0 (41.0–73.8) ng/mL for the corn, fish, olive, and animal fat groups, respectively; these values did not differ significantly (p=0.24). Similarly, we saw no significant difference in serum IGF-axis parameters among groups (insulin p=0.28, IGF-1 p=0.18, IGFBP-3 p=0.18, IGF-1/IGFBP-3 ratio p=0.33; Figure 5). Serum PGE-2 levels were also similar (p=0.40), and there was no significant difference in tumor COX-2 expression across dietary groups (p=0.31).
Figure 5.
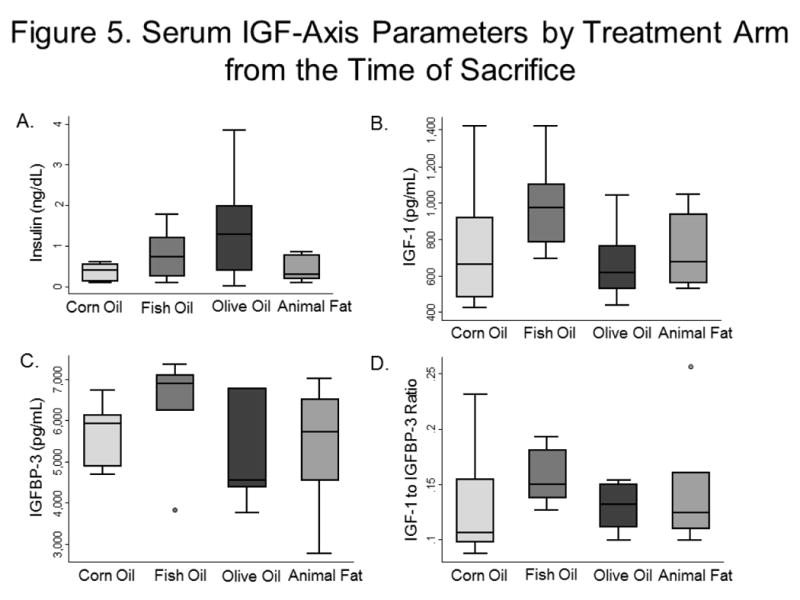
Serum IGF-Axis Parameters by Treatment Arm from the Time of Sacrifice
5a. Insulin
5b. IGF-1
5c. IGFBP-3
5d. IGF-1 to IGFBP-3 Ratio
Gene Expression Profiles and GSEA Analysis
To understand the mechanisms underlying the slower growth of xenograft tumors in mice consuming the fish oil diet, we performed global gene expression analysis using Affymetrix U133 A2 arrays to analyze 6 xenograft tumors excised from each of the four treatment groups, for a total of 24 samples. The microarray data were normalized by RMA, and the differences in the pathway composition between the 6 fish oil and 18 non-fish oil diet samples were compared using Gene Set Enrichment Analysis (GSEA).(29) The GSEA applied a Kolmogorov-Smirnov statistic to determine whether specific biological processes (represented as 4850 gene sets in the Molecular Signature Database (MSigDB) http://www.broadinstitute.org/gsea/msigdb/collections.jsp#C2) were enriched or depleted in tumors of mice taking fish oil (Figure 6a). This analysis revealed 41 gene sets which were depleted in fish oil samples with a false discovery rate (FDR) of <25%. No gene set was enriched in the fish oil samples. Among the gene sets most significantly depleted in the fish oil group, two biological processes were particularly prominent: 1) regulation of insulin synthesis and secretion (e.g. gene sets related to reactome insulin synthesis, glucose regulation of insulin secretion, and diabetes pathways) (Figure 6b) and 2) mitochondrial activity (e.g. gene sets related to the mitochondrial gene module and mitochondrial oxidative phosphorylation) (Figure 6c).
Figure 6.

Enrichment Plots of Six Represented Gene Sets Significantly Depleted in Tumors of Fish-Oil-Fed Mice
6a. Schematic of the Molecular Signature Database (MSigDB)
6b. Insulin synthesis and secretion pathways (three gene sets)
6c. Mitochondrial pathways (three gene sets)
DISCUSSION
Epidemiologic studies suggest that increased dietary fat consumption has a negative effect on PCa outcomes; however, it is unclear to what extent the type of dietary fat consumed influences disease initiation and progression. Indeed, both population-based(9-11) and xenograft(18, 19) studies have suggested that fish oil consumption decreases PCa risk. Other studies have shown that, when using a corn oil-based diet, decreasing total dietary fat increases survival in a xenograft model.(16, 17) In contrast, another study demonstrated no improvement in PCa outcome when the amount of dietary fat was decreased using a saturated fat-based diet.(20, 21) This raised the hypothesis that, in addition to the amount of dietary fat being important, the type of fat mattered too. Thus, we sought to systematically examine the effects of various types of fat on PCa progression. We found that male mice xenografted with LAPC-4 tumors fed a diet in which the dietary fat source was fish oil outlived mice fed diets composed of corn oil, olive oil, or animal fat; survival was similar across the non-fish oil groups. Gene expression analyses revealed decreased signal related to mitochondrial and insulin synthesis/secretion pathways in the fish oil-fed mice relative to other groups, suggesting these pathways may be important for mediating the anti-PCa activity of our fish oil-based diet.
Numerous but not all population-based studies have demonstrated a correlation between high dietary fat intake and PCa initiation/progression.(7, 8, 30-34) However, these studies comment only on the amount, not the type, of dietary fat consumed; most of these studies assessed people eating a primarily “Western” diet high in fats from vegetable oils, meat, and dairy sources, all thought to promote PCa development and progression.
Thus, it is noteworthy that other studies have shown both a diet high in cold-water fish(9-11) and the Mediterranean diet(35) (rich in plant foods, fish, and olive oil) to decrease PCa risk. Moreover, a prospective phase II randomized trial of men undergoing radical prostatectomy showed that a low-fat diet with fish oil supplementation reduced tumor Ki67 expression compared to Western diet controls.(36) While promising, future studies are required to apply this intervention in a longer-term setting. Though it is difficult to isolate single dietary components in dietary intervention trials, long-term interventions modifying dietary fat have been undertaken previously and proved to be feasible.(37)
Given the challenges of studying dietary effects in humans, studies using animal models offer significant insight in this area. In a mouse xenograft model, high consumption of ω-3 fatty acids (the primary fatty component of fish oil) has been shown to impair tumor cell proliferation, increase apoptosis, and reduce tumor mass.(18, 19, 38) These findings align with the results of the current study, in which fish oil-fed mice demonstrated prolonged survival relative to mice consuming diets in which the fat source was olive oil, corn oil, or animal fat.
The beneficial effects of fish oil are thought to stem from the ability of ω-3 fatty acids to suppress the production of arachidonic acid, which, in turn, suppresses production of arachidonic acid-derived eicosanoids, in particular prostaglandins and thromboxanes. Prostaglandins produced from arachidonic acid (specifically, PGE-2) tend to be pro-inflammatory and pro-proliferative in contrast to prostaglandins derived from ω-3 fatty acids, which tend to be less favorable for the growth and development of cancer cells.(39) Moreover, incorporation of ω-3 fatty acids has been shown to suppress production of COX-2, which further diminishes production of pro-inflammatory prostaglandins.(40)
In the current study, however, we found no significant difference in serum PGE-2 and tumor COX-2 levels across the study groups. This may be due to the relatively small sample size or perhaps argues for an alternative mechanism underlying the positive effect seen with the fish oil diet. Alternatively, using samples at the time of sacrifice when all mice have large tumors, we may have missed the window of time wherein there were differences in PGE-2 and/or COX-2. However, in support of the idea that fish oil may alter PCa biology independent of COX-2 and PGE-2, we note a recent pre-prostatectomy randomized trial which showed that fish oil supplementation lowered tumor Ki67 levels without changes in COX-2 or PGE-2 levels.(36) Therefore, as we noted that prior dietary studies from our group appeared to be “work” via altering IGF/insulin hormone levels, we evaluated the insulin/IGF-axis. However, again, no significant differences were noted.
Given the lack of corroborative data to support our two leading hypotheses for why fish-oil fed mice survived the longest, we turned to gene expression analysis. Using this approach, we found significant downregulation of pathways related to insulin synthesis and secretion as well as mitochondrial activity in tumors from mice fed fish oil. Though serum insulin levels were not different between the fish oil arm and other dietary groups, such a comparison may not fully reflect insulin activity within the tumor microenvironment. Thus, while we cannot easily explain how a fish oil diet may have altered the tumor microenvironment to specifically inhibit insulin signaling, the gene expression data are nonetheless hypothesis-generating, suggesting the existence of such a difference in insulin signaling between the groups. Indeed, this may have contributed to prolonged survival in the fish oil-fed mice given that insulin is a potent direct mitogen for PCa.(41, 42) Moreover, a recent study found that insulin increases de novo steroidogenesis, including androgen production, in PCa cells.(43) Notably, cholesterol—the backbone of steroid synthesis—must be imported into the mitochondria for conversion to pregnenolone before steroidogenesis/androgen synthesis can occur. Thus, the results revealed in our gene expression analyses, namely decreased insulin signaling and decreased mitochondrial activity, appear to fit with these external findings. Ultimately, further studies are needed to better elucidate the exact mechanisms through which a fish-oil based diet slows PCa xenograft growth.
This study must be viewed in light of its limitations. First, as with any single animal model, further studies in other models are needed before any generalizable conclusions can be drawn. More broadly, the use of an animal model is itself a limitation, given that to what degree animal studies model human prostate cancer is unclear and ultimately these results need to be validated in human studies. Second, our study utilized a simplified diet in which all dietary fat was provided by a single source, which is unlikely to be reproduced in humans given the varied and complex nature of human dietary consumption. Moreover, evolving understandings of ethnic variations in bioavailability of dietary fatty acid metabolites must be considered(44); as such, it is possible that these interventions would require targeting to specific racial/ethnic groups for maximal efficacy. Third, we did observe higher blood glucose in fish oil-fed mice at the time of sacrifice. This likely represents a slight overfeeding of fish oil-fed mice relative to other groups, an assessment supported by the observation that mice in the fish oil group were slightly but significantly heavier at time of sacrifice than mice in other groups. However, given that increased caloric intake and body mass are generally positively associated with larger and more aggressive PCa tumors, the prolonged survival of the fish oil-fed mice in our study actually argues even more strongly for the beneficial effects of this diet.(27, 45, 46)
CONCLUSION
We found that in a mouse PCa xenograft model, mice fed a diet in which fish oil provided the sole source of fat outlived mice fed diets composed of corn oil, olive oil, or animal fat; survival was similar across the non-fish oil groups. On gene expression analysis, the fish oil-fed group demonstrated downregulation of pathways related to mitochondrial activity and insulin synthesis/secretion, suggesting the hypothesis that these pathways may be important in producing the benefits of the fish oil diet. The results of our study suggest that increased dietary intake of fish oil may slow PCa progression. Further research is needed to characterize the utility of this potentially low-cost, low-risk intervention in human populations as well as to better understand the potential mechanisms through which this intervention slows PCa progression.
Supplementary Material
Supp Figure 1. Serum Glucose Levels by Treatment Arm from the Time of Sacrifice
Acknowledgments
Jodi Antonelli1,2, Jean-Alfred Thomas 2nd1,2, and Tameika E. Phillips1,2
Supported by the Department of Veterans Affairs; Division of Urology, Department of Surgery, Duke University; Prostate Cancer Foundation; NIH Training Grant 1 TL1 RR024126; and NIH 5R01 CA131235-03. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Conflict of Interest:
None of the authors have any conflict of interest, financial or otherwise, to disclose.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–49. doi: 10.3322/caac.20006. PubMed PMID: 19474385. Epub 2009/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000 Jan 1;85(1):60–7. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. PubMed PMID: 10585584. [DOI] [PubMed] [Google Scholar]
- 3.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999 Mar 3;91(5):414–28. doi: 10.1093/jnci/91.5.414. PubMed PMID: 10070940. [DOI] [PubMed] [Google Scholar]
- 4.Villeneuve PJ, Johnson KC, Kreiger N, Mao Y. Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. The Canadian Cancer Registries Epidemiology Research Group Cancer Causes Control. 1999 Oct;10(5):355–67. doi: 10.1023/a:1008958103865. PubMed PMID: 10530605. Epub 1999/10/26. eng. [DOI] [PubMed] [Google Scholar]
- 5.Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev. 2001;23(1):72–81. doi: 10.1093/oxfordjournals.epirev.a000798. PubMed PMID: 11588857. Epub 2001/10/09. eng. [DOI] [PubMed] [Google Scholar]
- 6.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005 Nov 10;23(32):8152–60. doi: 10.1200/JCO.2005.03.1492. PubMed PMID: 16278466. [DOI] [PubMed] [Google Scholar]
- 7.Clinton SK, Giovannucci E. Diet, nutrition, and prostate cancer. Annu Rev Nutr. 1998;18:413–40. doi: 10.1146/annurev.nutr.18.1.413. PubMed PMID: 9706231. Epub 1998/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 8.Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008 May;87(5):1405–13. doi: 10.1093/ajcn/87.5.1405. PubMed PMID: 18469265. Epub 2008/05/13. eng. [DOI] [PubMed] [Google Scholar]
- 9.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999 Dec;81(7):1238–42. doi: 10.1038/sj.bjc.6690835. PubMed PMID: 10584888. Epub 1999/12/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001 Jun 2;357(9270):1764–6. doi: 10.1016/S0140-6736(00)04889-3. PubMed PMID: 11403817. Epub 2001/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 11.Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003 Jan;12(1):64–7. PubMed PMID: 12540506. Epub 2003/01/24. eng. [PubMed] [Google Scholar]
- 12.de Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998 Jun 8;158(11):1181–7. doi: 10.1001/archinte.158.11.1181. PubMed PMID: 9625397. Epub 1998/06/13. eng. [DOI] [PubMed] [Google Scholar]
- 13.Godley PA, Campbell MK, Gallagher P, Martinson FE, Mohler JL, Sandler RS. Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiol Biomarkers Prev. 1996 Nov;5(11):889–95. PubMed PMID: 8922296. Epub 1996/11/01. eng. [PubMed] [Google Scholar]
- 14.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989 Aug 1;64(3):598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. PubMed PMID: 2743254. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87(19):1456–62. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 16.Ngo TH, Barnard RJ, Anton T, Tran C, Elashoff D, Heber D, et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004 Feb 15;64(4):1252–4. doi: 10.1158/0008-5472.can-03-3830. PubMed PMID: 14973081. [DOI] [PubMed] [Google Scholar]
- 17.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003 Jul;9(7):2734–43. PubMed PMID: 12855654. [PubMed] [Google Scholar]
- 18.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006 Aug 1;12(15):4662–70. doi: 10.1158/1078-0432.CCR-06-0459. PubMed PMID: 16899616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007 Jul;117(7):1866–75. doi: 10.1172/JCI31494. PubMed PMID: 17607361. Epub 2007/07/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008 Jan 1;68(1):11–9. doi: 10.1002/pros.20683. PubMed PMID: 17999389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd JC, Antonelli JA, Phillips TE, Masko EM, Thomas JA, Poulton SH, et al. Effect of isocaloric low fat diet on prostate cancer xenograft progression in a hormone deprivation model. J Urol. Apr;183(4):1619–24. doi: 10.1016/j.juro.2009.12.003. PubMed PMID: 20172549. Epub 2010/02/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nature medicine. 1997 Apr;3(4):402–8. doi: 10.1038/nm0497-402. PubMed PMID: 9095173. [DOI] [PubMed] [Google Scholar]
- 23.Gravina GL, Marampon F, Giusti I, Carosa E, Di Sante S, Ricevuto E, et al. Differential effects of PXD101 (belinostat) on androgen-dependent and androgen-independent prostate cancer models. International journal of oncology. 2012 Mar;40(3):711–20. doi: 10.3892/ijo.2011.1270. PubMed PMID: 22134754. [DOI] [PubMed] [Google Scholar]
- 24.Emonds KM, Swinnen JV, Lerut E, Koole M, Mortelmans L, Mottaghy FM. Evaluation of androgen-induced effects on the uptake of [18F]FDG, [11C]choline and [11C]acetate in an androgen-sensitive and androgen-independent prostate cancer xenograft model. EJNMMI research. 2013;3(1):31. doi: 10.1186/2191-219X-3-31. Pubmed Pmid: 23618081. Pubmed Central Pmcid: 3640969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas R, Sharifi N. SOD mimetics: a novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer. Molecular cancer therapeutics. 2012 Jan;11(1):87–97. doi: 10.1158/1535-7163.MCT-11-0540. PubMed PMID: 22172488. Pubmed Central PMCID: 3256291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee P, Sotnikov AV, Mangian HJ, Zhou JR, Visek WJ, Clinton SK. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J Natl Cancer Inst. 1999 Mar 17;91(6):512–23. doi: 10.1093/jnci/91.6.512. PubMed PMID: 10088621. [DOI] [PubMed] [Google Scholar]
- 27.Thomas JA, 2nd, Antonelli JA, Lloyd JC, Masko EM, Poulton SH, Phillips TE, et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate cancer and prostatic diseases. 2010 Dec;13(4):350–5. doi: 10.1038/pcan.2010.24. PubMed PMID: 20733612. Epub 2010/08/25. eng. [DOI] [PubMed] [Google Scholar]
- 28.Chen JL, Merl D, Peterson CW, Wu J, Liu PY, Yin H, et al. Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS genetics. 2010 Sep;6(9) doi: 10.1371/journal.pgen.1001093. PubMed PMID: 20844768. Pubmed Central PMCID: 2937306. Epub 2010/09/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005 Oct 25;102(43):15545–50. doi: 10.1073/pnas.0506580102. PubMed PMID: 16199517. Pubmed Central PMCID: 1239896. Epub 2005/10/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolonel LN. Nutrition and prostate cancer. Cancer Causes Control. 1996 Jan;7(1):83–44. doi: 10.1007/BF00115640. PubMed PMID: 8850437. Epub 1996/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 31.Lee MM, Wang RT, Hsing AW, Gu FL, Wang T, Spitz M. Case-control study of diet and prostate cancer in China. Cancer Causes Control. 1998 Dec;9(6):545–52. doi: 10.1023/a:1008840105531. PubMed PMID: 10189039. Epub 1999/04/03. eng. [DOI] [PubMed] [Google Scholar]
- 32.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995 May 3;87(9):652–61. doi: 10.1093/jnci/87.9.652. PubMed PMID: 7752270. [DOI] [PubMed] [Google Scholar]
- 33.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007 Sep 15;121(6):1339–45. doi: 10.1002/ijc.22805. PubMed PMID: 17487838. Epub 2007/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 34.Ramon JM, Bou R, Romea S, Alkiza ME, Jacas M, Ribes J, et al. Dietary fat intake and prostate cancer risk: a case-control study in Spain. Cancer Causes Control. 2000 Sep;11(8):679–85. doi: 10.1023/a:1008924116552. PubMed PMID: 11065004. Epub 2000/11/07. eng. [DOI] [PubMed] [Google Scholar]
- 35.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000 Sep;9(9):869–73. PubMed PMID: 11008902. Epub 2000/09/29. eng. [PubMed] [Google Scholar]
- 36.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2011 Dec;4(12):2062–71. doi: 10.1158/1940-6207.CAPR-11-0298. PubMed PMID: 22027686. Pubmed Central PMCID: 3232341. Epub 2011/10/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Aronson WJ, Arteaga JR, Hong K, Thames G, Henning SM, et al. Feasibility of a low-fat/high-fiber diet intervention with soy supplementation in prostate cancer patients after prostatectomy. European journal of clinical nutrition. 2008 Apr;62(4):526–36. doi: 10.1038/sj.ejcn.1602743. PubMed PMID: 17392697. Epub 2007/03/30. eng. [DOI] [PubMed] [Google Scholar]
- 38.Connolly JM, Coleman M, Rose DP. Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutrition and cancer. 1997;29(2):114–9. doi: 10.1080/01635589709514611. PubMed PMID: 9427973. Epub 1997/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 39.Hardman WE. (n-3) fatty acids and cancer therapy. The Journal of nutrition. 2004 Dec;134(12 Suppl):3427S–30S. doi: 10.1093/jn/134.12.3427S. PubMed PMID: 15570049. Epub 2004/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 40.Needleman P, Raz A, Minkes MS, Ferrendelli JA, Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proceedings of the National Academy of Sciences of the United States of America. 1979 Feb;76(2):944–8. doi: 10.1073/pnas.76.2.944. PubMed PMID: 218223. Pubmed Central PMCID: 383100. Epub 1979/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKeehan WL, Adams PS, Rosser MP. Direct mitogenic effects of insulin, epidermal growth factor, glucocorticoid, cholera toxin, unknown pituitary factors and possibly prolactin, but not androgen, on normal rat prostate epithelial cells in serum-free, primary cell culture. Cancer Res. 1984 May;44(5):1998–2010. PubMed PMID: 6370422. [PubMed] [Google Scholar]
- 42.Hsing AW, Chua S, Jr, Gao YT, Gentzschein E, Chang L, Deng J, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001 May 16;93(10):783–9. doi: 10.1093/jnci/93.10.783. PubMed PMID: 11353789. [DOI] [PubMed] [Google Scholar]
- 43.Lubik AA, Gunter JH, Hendy SC, Locke JA, Adomat HH, Thompson V, et al. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011 Sep 1;71(17):5754–64. doi: 10.1158/0008-5472.CAN-10-2470. PubMed PMID: 21747118. Epub 2011/07/13. eng. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong P, Kelley DS, Newman JW, Staggers FE, Sr, Hartiala J, Allayee H, et al. Arachidonate 5-lipoxygenase gene variants affect response to fish oil supplementation by healthy African Americans. The Journal of nutrition. 2012 Aug;142(8):1417–28. doi: 10.3945/jn.112.159814. PubMed PMID: 22739369. Pubmed Central PMCID: 3397335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. PubMed PMID: 12711737. [DOI] [PubMed] [Google Scholar]
- 46.Whitley BM, Moreira DM, Thomas JA, Aronson WJ, Terris MK, Presti JC, Jr, et al. Preoperative weight change and risk of adverse outcome following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. Prostate cancer and prostatic diseases. 2011 Dec;14(4):361–6. doi: 10.1038/pcan.2011.42. PubMed PMID: 21894174. Epub 2011/09/07. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Figure 1. Serum Glucose Levels by Treatment Arm from the Time of Sacrifice


