Abstract
Background
Preclinical drug screens identified disulfiram as a potent in vitro inhibitor of prostate cancer cell growth. Although many mechanisms for its anticancer activity have been proposed, tumor suppressor gene re-expression through promoter demethylation emerged as one of the more plausible.
Methods
We conducted an open-label, dose escalation trial of disulfiram in men with non-metastatic recurrent prostate cancer after local therapy. Dose escalation occurred if a demethylating “response” [i.e. ≥10% decrease in peripheral blood mononuclear cell (PBMC) global 5meC content] was observed in <3 patients in cohort 1. Cohort 1 and 2 received disulfiram 250 mg and 500 mg daily respectively. The primary endpoint was the proportion of subjects with a demethylation response. Secondary endpoints included rate of PSA progression at 6 months, changes in PSA doubling time and safety/tolerability.
Results
Changes in global 5meC content were observed in 2 of 9 patients (22.2%) in cohort 1 and 3 of 10 (30.0%) in cohort 2. Only 5 subjects were on trial for ≥6 months, all were in cohort 1 and all had PSA progression by 6 months. No changes in PSA kinetics were observed in either cohort. Disulfiram was poorly tolerated with 6 patients experiencing grade 3 AEs (3 per cohort). Three of the responders displayed pre-treatment instability in their 5meC content.
Conclusions
A minority of patients had transient global PBMC demethylation changes. Instability in 5meC may limit the reproducibility of these findings, limiting our ability to confirm our hypothesis. Given the toxicities and no clinical benefits, further development of disulfiram should not be pursued in this population.
Keywords: Disulfiram, prostate cancer, epigenetics, demethylation, hypomethylation
Introduction
Epigenetic changes in prostate cancer (PCa) are recognized as occurring at the earliest phase of carcinogenic transformation.1 Further, alterations in the epigenome persist and evolve during invasion, metastasis and progression.2–4 One of the most recognized epigenetic alterations, methylation of cytosines in gene promoter regions, can lead to tumor suppressor gene silencing and in turn contribute to the cancer phenotype.1,5 DNA methyltransferases (DNMTs) constitute the group of enzymes responsible for maintaining these CpG methylation marks, and have been the primary target of drugs developed as demethylating agents.1 Two nucleoside DNMT inhibitors, azacitidine and decitabine, are currently approved for the treatment of myelodysplastic syndrome. Their extensive incorporation into DNA may in theory lead to increased toxicity and carcinogenesis.6–8
Preclinical compound screens revealed that disulfiram potently inhibits PCa cell line growth.8,9 Given that disulfiram is a known thiol-reactive compound, we hypothesized that it may inhibit DNMTs, which use a catalytic cysteine residue in the methyltransferase reaction.10 Indeed, preclinical work by our group established that disulfiram was able to inhibit DNMT1 activity and could lead to DNA demethylation in PCa cells; manifested as global reductions in 5-methyl cytosine (5meC) content, decreased methylation in APC and RARB gene promoters, and subsequent gene re-expression.8 There have been a multitude of additional proposed mechanisms for disulfiram’s anti-tumor activity, including that the disulfiram analogue pyrrolidine dithiocarbamate (PDTC) may be able to chelate copper, possibly inhibiting proteasomes or exerting an antiangiogenic effect.11–22
To evaluate disulfiram’s potential as an epigenetic therapy, we conducted a translational pilot trial (registration ID: NCT01118741) through the Department of Defense Prostate Cancer Clinical Trials Consortium. Our primary objective was to evaluate if disulfiram produced demethylating changes in men with biochemically recurrent PCa through quantifying changes in global 5meC DNA content in peripheral blood mononuclear cells (PBMC).23
Methods
Inclusion criteria
Eligible patients were ≥18 years old, previously treated with local therapy (e.g. radiation or surgery) for histologically proven prostatic adenocarcinoma and subsequently developed biochemically recurrent disease (confirmed rising PSA of ≥1 ng/mL at least 2 weeks apart). Biochemically recurrent patients were the focus given that this population would be less likely to experience serious morbidity as a result of disease progression should disulfiram lack an anti-prostate cancer effect. Subjects were required to have adequate bone marrow, renal, and liver function and no evidence of metastasis. All previous local therapies must have been discontinued at least four weeks prior to enrollment. Patients were allowed to have received prior systemic therapies. Those treated with hormonal therapy were allowed to enroll if their treatment was discontinued >6 months prior and had testosterone recovery. Patients were also required to have an Eastern Cooperative Oncology Group performance status of ≤2. Participants had to agree not to drink alcohol during the study and for 14 days after its completion. Participants signed an Institutional Review Board-approved consent form.
Treatment plan
This was an open-label, prospective, multicenter, clinical trial evaluating disulfiram in men with biochemically recurrent PCa. Two cohorts were planned with doses selected based on the FDA approved doses for disulfiram. A dose escalation occurred based on whether a demethylating effect (i.e. ≥10% decrease in global 5meC content from PBMC) was observed at the initial dose. Cohorts 1 and 2 received disulfiram 250 mg and 500 mg daily respectively. If a demethylating response was observed in <3 out of the nine subjects initially enrolled to cohort 1, then cohort 2 was opened. If there was evidence for a demethylating response in ≥3 subjects in cohort 1 or 2, then that cohort would expand to treat 17 total patients (figure 1). Toxicity of disulfiram was also taken into account, with dose escalation or cohort expansion only occurring if grade 3–4 adverse events (AEs) were observed in <3 subjects out of the first 9 patients enrolled to cohort 1 or 2. Toxicity was defined using the National Cancer Institute (NCI) guidelines in the Common Terminology Criteria for Adverse Events Version 3.0.
Figure 1.
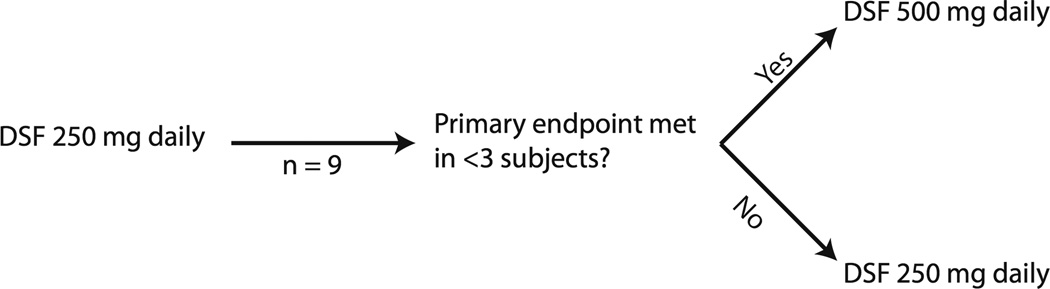
Treatment scheme.
Global methylation assay
Blood samples were drawn serially at the time of screening, on day 1/cycle 1 and weeks 4, 8, 16 and 24. Samples were stored at 4°C or on ice prior to PBMC DNA extraction. Samples not drawn at the primary site (i.e. Johns Hopkins) were shipped overnight to the primary site for processing. DNA samples were batched and stored at −70°C. Global DNA methylation status was determined on PBMC DNA using a High Pressure Liquid Chromatography-Tandem Mass Spectrometry (HPLC/MS) assay as described previously.3,8,23 The overall 5meC content (as percentage of total 2’-deoxycytidine content) in genomic DNA was compared before and after treatment. Given slight variations in the assay between batches, all batches were normalized to the same mean.
Pharmacokinetics
Disulfiram plasma concentrations were not measured due to stability issues.24 Plasma samples were obtained at baseline and prior to disulfiram administration [minimum concentration (Cmin)] at week 4 to measure the metabolite, S-Methyl N,N-diethyldithiocarbamate (MeDDC). Briefly, 0.1ml plasma was extracted with acetonitrile precipitation. MeDDC separated on a Waters X-Terra MS C18 (50 × 2.1mm, 3.5µm; Milford, MA, USA) column with 0.1% formic acid in acetonitrile/2mM ammonium acetate (70:30,v/v) using isocratic flow of 0.2ml/min for 5min. MeDDC was quantitated over the range of 10–2000ng/mL. Since disulfiram was administered in the evening for multiple patients, the reported Cmin was corrected to the actual/theoretical Cmin at 24 hours utilizing the following formula:
Where k as the elimination rate constant based on a reported T1/2 of 6.3 hour, and t was the difference between the time of the Cmin,reported sample and a 24 hour Cmin.24 If the Cmin sample was below the lower limit of quantitation (BLQ) or calculated to be BLQ, a concentration of 1/2 the lower limit of quantitation (5ng/mL) was utilized in the calculations and subsequent statistics.
Ceruloplasmin
Peripheral blood for determining ceruloplasmin levels were drawn at the same time points as the DNA methylation samples. The ceruloplasmin assay used is a Clinical Laboratory Improvement Amendments certified lab test. It was performed through participating sites’ pathology departments.
Endpoints
The primary endpoint of this study was the proportion of patients achieving a demethylation response (≥10% decrease from baseline in total 5meC global gene DNA content as assessed from PBMC) at a given dose level. The average of the screening and day 1/cycle 1 5meC content levels was used as a baseline. Our lab has found that the global 5meC content assay being used is reproducible, with an average percent difference between replicates of 2.2% [95% confidence interval (CI) 0.4–4%]. Given that the upper bound of the 95% CI for percent difference between replicates was 4%, a ≥10% decrease in 5meC was felt unlikely to occur by chance.
Secondary endpoints included PSA progression at 6 months (confirmed PSA >50% above baseline and ≥2 ng/mL above nadir), changes in PSA kinetics, safety, pharmacokinetic/pharmacodynamic (PK/PD) correlates, and ceruloplasmin changes.
Adverse events were assessed by the lead site on an ongoing basis, with quarterly reviews of the clinical data at a minimum. Severe AEs were reviewed as they were reported. These were assessed by the medical monitor and their decision regarding the safety of continuing the study was shared with the investigators. The study could be terminated based on the assessment of either the lead site, study sponsor, or the principal investigator.
Statistical analysis
The dose escalation scheme was designed using a Simon two-stage approach (figure 1). A study cohort of 17 would have yielded 95% power to detect a 60% demethylating response rate compared to a null rate of 20%, with a one-sided alpha error of 0.05. The probability of stopping the trial with only nine patients in either cohort was 2.5% assuming that the true response rate was 60%. The probability of stopping the trial if the true demethylating response rate was 20% in either cohort was 73.8%.
Results
Patients
Between June, 2010 and August, 2011 a total of 19 patients were screened and enrolled in this study – nine in cohort 1 and ten in cohort 2. Three patients in cohort 1 received prior hormonal therapy whereas none in cohort 2 did. Otherwise, baseline characteristics between cohorts were well matched (table 1).
Table 1.
Patient demographics.
| Disulfiram 250 mg (N = 9) |
Disulfiram 500 mg (N = 10) |
Combined (N = 19) | |
|---|---|---|---|
| Age, y [median (min, max)] | 68 (57, 77) | 62.5 (56, 67) | 65 (56, 77) |
| Race [caucasian, n (%)] | 9 (100%) | 9 (90%) | 18 (94.7%) |
| Prior hormonal therapy, n(%) | 3 (33.3%) | 0 (0%) | 3 (15.8%) |
| Characteristics at diagnosis | |||
| Gleason score, median (min, max) | 7 (6, 9) | 7.5 (6, 9) | 7 (6, 9) |
| PSA | 5.4 (0.2, 28) | 5.6 (2.2, 20) | 5.4 (0.2, 28) |
Note: all prior hormonal therapies were given intermittently for the purpose of palliation.
Demethylating response
Two patients out of 9(22.2%) in cohort 1 had a demethylation response. Cohort 2 was therefore opened, and 10 patients were accrued at that dose level. Three of the first 9(33.3%) patients enrolled in cohort 2 were responders, and while this met our threshold for cohort expansion, toxicities at this dose level were, in the opinion of the investigators, too toxic to allow accrual beyond the 10th patient. There were no differences in the demethylating response rate observed between cohorts during any of the treatment cycles. Demethylating responses were generally transient, persisting for one or two cycles only (table 2).
Table 2.
Summary of subjects exhibiting a demethylating response to disulfiram.
| Demethylation Response (1=yes, 0=no, X=missing) | |||||
|---|---|---|---|---|---|
| Dose (mg) | Number of cycles received |
C2 | C3 | C5 | EOS |
| 500 | 4 | 1 | 1 | X | 0 |
| 500 | 5 | 1 | 0 | 0 | 0 |
| 500 | 6 | 0 | 1 | 0 | 0 |
| 250 | 2 | 1 | X | X | X |
| 250 | 9 | 0 | 0 | 0 | 1 |
Samples for a given cycle were drawn on day 1 of said cycle. The end of study (EOS) sample was obtained at the time a subject came off study. Missing values were due to subjects coming off trial early.
Toxicity
While there were no grade 4 AEs, the drug was in general poorly tolerated at the higher dose. Six patients experienced one grade 3 AE (3 per cohort) (table 3). Other common grade 1 and 2 AEs included fatigue, GI toxicity, ataxia/dizziness, and constitutional symptoms. In contrast to the low-dose cohort where the most common reason for coming off study was PSA or radiographic progression (n=6), those in the high-dose cohort most often came off study due to toxicity (n=5). Toxicities leading to patient withdrawal in the high-dose cohort included grade 3 diarrhea (n=1), grade 2 fatigue (n=2), grade 2 neuropathy (n=1) and unspecified toxicity (n=1). Overall, there was a trend toward fewer median number of completed cycles in the high-dose compared to the low-dose cohort (5 vs 9 cycles,P=0.12), presumably due to excess toxicity (table 3). Upon this trial’s termination, two subjects in the low-dose cohort and four in the high-dose cohort had ongoing AEs deemed at least possibly due to disulfiram. These included: hearing loss, fatigue, taste alteration, tremor, sensory neuropathy, muscle weakness, speech impairment and pain.
Table 3.
Reason for study discontinuation and toxicity summary.
| Disulfiram 250 mg | Disulfiram 500 mg | |
|---|---|---|
| Reason off study | ||
| Toxicity | 1 (11) | 5 (50) |
| PSA progression | 4 (44) | 2 (20) |
| Radiographic progression | 2 (22) | 0 |
| Patient decision | 1 (11) | 3 (30) |
| Median number of cycles completed* | 9 | 5 |
| Grade 3 or higher AE | ||
| Double vision | 1 (11)** | |
| Hearing loss | 1 (11) | |
| LFT abnormality | 1 (11) | |
| Diarrhea | 1 (10)** | |
| Constipation | 1 (10) | |
| Ataxia | 1 (10) |
Difference in median number of completed cycles trended towards statistical significance (P = 0.12).
Resulted in subject coming off study.
Global Methylation Assay
To assess the stability of global 5meC DNA content over time we obtained two serial blood samples prior to initiating therapy. These samples were obtained in all but one patient in cohort 2. The median time between pre-treatment samples was 6.5 days (range,2–76 days), with a median absolute change in 5meC content of 2.11% (range,0.84–12.55%). There were two subjects who had ≥10% decrease in 5meC content and an additional subject who had a ≥10% increase in 5meC content prior to receiving disulfiram. The median absolute change in 5meC content for these individuals was 12.38% occurring after a median of 6 days. These subjects represented the three responders from cohort 2.
PSA endpoints
When stratified by dose level, no differences in PSA-based outcomes were observed (table 4). Additionally, there was no difference in PSA-based endpoints for those demonstrating a demethylation response compared to those who did not. Only five subjects remained on trial for ≥6 months and therefore qualified for PSA progression assessment as defined per protocol. All were in cohort 1 and all had PSA progression by 6 months. Treating physicians were encouraged not to remove their patients from the study solely for a rising PSA. As such, the median time that the five subjects who remained on protocol for ≥6 months was 9.6 months (range,7.5–15.9 months).
Table 4.
PSA kinetics summary.
| Disulfiram 250 mg (N=9) |
Disulfiram 500 mg (N=10) |
P | |
|---|---|---|---|
| Pre-treatment PSA velocity (ng/ml/year) - median (range) | 1.3 (0.6, 15.8) | 2.3 (0.6, 3.7) | 0.963 |
| Post-treatment PSA velocity (ng/ml/year) - median (range) | 1.1 (0.5, 11.6) | 2.4 (0, 6.8) | 0.963 |
| Change in pre- to post-treatment PSA velocity (ng/ml/year) - median (range) | −0.4 (−4.2, 0.8) | −0.2 (−2.4, 1.8) | 0.536 |
| Pre-treatment PSADT (days) - median (range) | 192.2 (16, 445.8) | 108.5 (68.1, 402.2) | 0.963 |
| Post-treatment PSADT (days) - median (range) | 233.1 (21.9, 540.4) | 99.4 (−5279.7, 543.2) | 0.423 |
| Change in pre- to post-treatment PSADT - median (range) | 22.5 (−193.1, 136.9) | −39.7 (−5388.2, 338.4) | 0.536 |
PK/PD
The 12 patients enrolled at the primary study site (Johns Hopkins) had their plasma disulfiram concentrations indirectly measured via its metabolite MeDDC. The MeDDC Cmin,actual was only detectable in two patients, 1 out of 9 at 250 mg and 1 out of 3 at 500 mg. MeDDC Cmin,actual was significantly higher for the responders (n=4) compared to non-responders (n=7) (13.2 and 14.5ng/mL in 2 patients vs. undetectable,P=0.05). No other significant correlations with MeDDC Cmin,actual were determined. This data should be interpreted with caution given that MeDDC Cmin,actual was only detectable in a minority of patients.
Ceruloplasmin levels
All of the patients enrolled in the trial had at least two ceruloplasmin levels obtained. None of those enrolled had a significant change in ceruloplasmin level between treatment cycles. Median ceruloplasmin level for the entire cohort remained stable between treatment cycles as well.
Conclusions
Disulfiram may lead to transient demethylating changes in a subset of patients, with those who achieve higher disulfiram metabolite (MeDDC) levels more often displaying evidence of demethylation. We also found that disulfiram’s use at high-doses was significantly limited due to toxicity. Future studies to develop less toxic non-nucleoside analog DNMT inhibitors may be warranted.
We evaluated disulfiram’s ability to achieve our proposed target biologic effect, global DNA demethylation. By relying on PBMC 5meC content, a translational pharmacodynamic endpoint which measures global DNA methylation, we hoped to avoid the pitfalls associated with PSA-based endpoints in the rising PSA window.3,8,23,25 Only transient demethylating changes in a minority of patients were observed, and while the obvious explanation for this lack of a robust response is that disulfiram is a poor DNMT inhibitor, other possibilities exist. For instance, a recent report has shown that while most display very little alteration in their 5meC content over time, a subset of individuals, typically in familial clusters, demonstrate dynamic change in their 5meC content.26 Given that three individuals in this study exhibited ≥10% changes in 5meC content prior to receiving disulfiram speaks to the high likelihood that some of the observed demethylating responses may have been a result of intrinsic variability in DNA methylation, and not an effect of the study drug. The issue of 5meC content stability in PBMC over time, particularly in men with PCa, as well as the correlation between tumoral and PBMC methylation and the clinical significance of changes in global methylation warrant further study prior to utilizing 5meC content as an endpoint in future trials. It is also uncertain whether disulfiram’s parental compound or one of its intermediate metabolites may be a more effective DNMT inhibitor and/or anti-cancer agent. Disulfiram’s known complex metabolic processing may explain the differences observed between the promising pre-clinical data and the modest effects seen in this trial.24
The possibility of disulfiram exerting an anti-tumor effect through a mechanism other than DNA demethylation was also explored. The copper chelator ATN-224 has demonstrated mixed results in regard to its ability to cause favorable PSA changes in men with PCa, with one out of two trials demonstrating an effect on PSA.27,28 Given that the disulfiram metabolite PDTC has been proposed to exert an anti-cancer effect through copper chelation, we sought to explore this as an alternative mechanism for disulfiram’s preclinical anti-cancer activity. We did not observe any changes in ceruloplasmin levels in those enrolled on this study, implying that PDTC does not impact copper levels. Whether intratumoral copper-PDTC complexes were formed was not directly addressed.
No changes in PSA-based outcomes were observed on this trial. The Prostate Cancer Working Group has pointed out that considerable variation in PSA and PSA kinetics can occur during trials evaluating therapeutic interventions in men in the rising PSA clinical state, and have emphasized the need to define intervention-specific PSA-based outcome at the outset of a study.25 In the case of this trial, our pre-defined PSA-based outcome was the rate of progression at 6 months. While it is difficult to make definitive statements regarding the effect of disulfiram on PSA given that only five patients remained on trial ≥6 months, the fact that these five subjects all progressed has dampened our enthusiasm for disulfiram in this setting.
The fact that we observed five declines and no increases ≥10% in 5meC content speaks to the possibility that disulfiram may be a weak demethylating agent; however, given potential issues with intrinsic 5meC content variability in this patient population the hypothesis of this study cannot be confirmed. This lack of robust activity coupled with the high rates of toxicity seen at the higher dose leaves us unable to recommend future study of disulfiram in this patient population. While this study provides limited insights regarding pharmacokinetic correlations given that only 2/12 subjects had detectable plasma MeDDC levels, given that these levels were elevated in the ‘responders’, may indicate that inconsistent disulfiram pharmacokinetics may have been partly responsible for the low observed rate of demethylation.24 If developed, newer non-nucleoside DNMT inhibitors with improved pharmacokinetic properties and less toxic side effect profiles, perhaps based off disulfiram or one of its metabolite’s parent structures, should be tested in men with prostate cancer. At this point, however, the utility of DNA demethylation as a therapeutic strategy for patients with prostate cancer remains to be proven.
Acknowledgments
Support: NIH/NCI CA58236, Commonwealth Foundation; David H. Koch Charitable Foundation; DOD #W81XWH-09-1-0149; DOD; Cancer Center Core Grant P30CA006973. This research was supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 RR025005) and the Shared Instrument Grant (1S10RR026824-01). The project described was supported in part by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
References
- 1.Nelson WG, De Marzo AM, Yegnasubramanian S. Epigenetic alterations in human prostate cancers. Endocrinology. 2009;150:3991–4002. doi: 10.1210/en.2009-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 3.Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, et al. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008;68:8954–8967. doi: 10.1158/0008-5472.CAN-07-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aryee MJ, Liu W, Engelmann JC, Nuhn P, Gurel M, Haffner MC, et al. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci Transl Med. 2013;5:169ra10. doi: 10.1126/scitranslmed.3005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 6.Mack GS. Epigenetic cancer therapy makes headway. J Natl Cancer Inst. 2006;98:1443–1444. doi: 10.1093/jnci/djj447. [DOI] [PubMed] [Google Scholar]
- 7.Muller CI, Ruter B, Koeffler HP, Lubbert M. DNA hypermethylation of myeloid cells, a novel therapeutic target in MDS and AML. Curr Pharm Biotechnol. 2006;7:315–321. doi: 10.2174/138920106778521523. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Haffner MC, Zhang Y, Lee BH, Brennen WN, Britton J, et al. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate. 2011;71:333–343. doi: 10.1002/pros.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iljin K, Ketola K, Vainio P, Halonen P, Kohonen P, Fey V, et al. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res. 2009;15:6070–6078. doi: 10.1158/1078-0432.CCR-09-1035. [DOI] [PubMed] [Google Scholar]
- 10.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem. 2002;3:274–293. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Marikovsky M, Nevo N, Vadai E, Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. Int J Cancer. 2002;97:34–41. doi: 10.1002/ijc.1565. [DOI] [PubMed] [Google Scholar]
- 12.Shian SG, Kao YR, Wu FY, Wu CW. Inhibition of invasion and angiogenesis by zinc-chelating agent disulfiram. Mol Pharmacol. 2003;64:1076–1084. doi: 10.1124/mol.64.5.1076. [DOI] [PubMed] [Google Scholar]
- 13.Yakisich JS, Siden A, Eneroth P, Cruz M. Disulfiram is a potent in vitro inhibitor of DNA topoisomerases. Biochem Biophys Res Commun. 2001;289:586–590. doi: 10.1006/bbrc.2001.6027. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, McLeod HL, Cassidy J. Disulfiram-mediated inhibition of NF-kappaB activity enhances cytotoxicity of 5-fluorouracil in human colorectal cancer cell lines. Int J Cancer. 2003;104:504–511. doi: 10.1002/ijc.10972. [DOI] [PubMed] [Google Scholar]
- 15.Liu GY, Frank N, Bartsch H, Lin JK. Induction of apoptosis by thiuramdisulfides, the reactive metabolites of dithiocarbamates, through coordinative modulation of NFkappaB, c-fos/c-jun, and p53 proteins. Mol Carcinog. 1998;22:235–246. doi: 10.1002/(sici)1098-2744(199808)22:4<235::aid-mc5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Kim CH, Kim JH, Moon SJ, Hsu CY, Seo JT, Ahn YS. Biphasic effects of dithiocarbamates on the activity of nuclear factor-kappaB. Eur J Pharmacol. 2000;392:133–136. doi: 10.1016/s0014-2999(00)00109-6. [DOI] [PubMed] [Google Scholar]
- 17.Cho HJ, Lee TS, Park JB, Park KK, Choe JY, Sin DI, et al. Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol. 2007;40:1069–1076. doi: 10.5483/bmbrep.2007.40.6.1069. [DOI] [PubMed] [Google Scholar]
- 18.Cen D, Gonzalez RI, Buckmeier JA, Kahlon RS, Tohidian NB, Meyskens FL., Jr Disulfiram induces apoptosis in human melanoma cells: a redox-related process. Mol Cancer Ther. 2002;1:197–204. [PubMed] [Google Scholar]
- 19.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Peng F, Cui QC, Daniel KG, Orlu S, Liu J, et al. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front Biosci. 2005;10:2932–2939. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 22.Lovborg H, Oberg F, Rickardson L, Gullbo J, Nygren P, Larsson R. Inhibition of proteasome activity, nuclear factor-KappaB translocation and cell survival by the antialcoholism drug disulfiram. Int J Cancer. 2006;118:1577–1580. doi: 10.1002/ijc.21534. [DOI] [PubMed] [Google Scholar]
- 23.Huang WY, Su LJ, Hayes RB, Moore LE, Katki HA, Berndt SI, et al. Prospective study of genomic hypomethylation of leukocyte DNA and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:2014–2021. doi: 10.1158/1055-9965.EPI-12-0700-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 1992;369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Eisenberger M, D'Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 26.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–2883. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry NL, Dunn R, Merjaver S, Pan Q, Pienta KJ, Brewer G, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71:168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Beer TM, Ryan CJ, Mathew P, Wilding G, Morris M, et al. A randomized, phase II study of ATN-224 in patients with biochemically relapsed, hormone-naive prostate cancer: A DOD/PCF Prostate Cancer Clinical Trials Consortium trial. J Clin Oncol. 2009;27:15s. (suppl abstr 5135). [Google Scholar]
- 29.Keizman D, Zahurak M, Sinibaldi V, Carducci M, Denmeade S, Drake C, et al. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clin Cancer Res. 2010;16:5269–5276. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]


