Abstract
Importance
Cognitive decline is a leading cause of disability and death in old age but its neurobiological bases are not well understood.
Objective
To test the hypothesis that transactive response DNA-binding protein 43 (TDP-43) is related to late life cognitive decline.
Design
Longitudinal clinical-pathologic cohort study.
Setting
More than 40 Catholic groups across the United States.
Participants
A total of 130 older Catholic nuns, priests, and monks underwent annual clinical evaluations, including detailed cognitive testing, for a mean of 10.1 years prior to death. On neuropathologic examination, we collected semiquantitative measures of TDP-43 pathology, density of neuronal neurofibrillary tangles, area occupied by amyloid-beta plaques, and the presence of alpha-synuclein Lewy bodies from multiple brain regions. Gross and microscopic cerebral infarcts and hippocampal sclerosis were also identified.
Main Outcome Measure
Annual rate of change in a previously established composite measure of global cognition during a mean of 10.1 years of annual observation before death.
Results
TDP-43 pathology ranging from sparse to severe was identified in 46% of participants and was associated with amyloid plaques, tangles, and hippocampal sclerosis but not neocortical Lewy bodies or cerebral infarcts. After controlling for amyloid plaques, tangles, and hippocampal sclerosis, TDP-43 pathology was associated with more rapid cognitive decline and accounted for nearly as much of the variability in rates of global cognitive decline as did tangles. TDP-43 pathology had a distinct cognitive profile that differed from other neuropathologic processes (related to decline in episodic and working memory but not in other cognitive domains), and it was elevated in those who developed dementia but not in those with mild cognitive impairment.
Conclusion
The results suggest that TDP-43 is an important brain pathology underlying cognitive decline and dementia in old age.
INTRODUCTION
Transactive response DNA-binding protein 43 (TDP-43) is the primary protein aggregate in frontotemporal lobar degeneration and amyotrophic lateral sclerosis,1,2 but TDP-43 also forms pathologic aggregates in other proteinopathies such as Alzheimer’s disease (AD),3 suggesting that it may contribute to cognitive dysfunction in these disorders. TDP-43 immunoreactivity is related to older age,4,5 with nearly half of older controls in a recent study showing evidence of at least mild TDP-43 pathology,5 suggesting that it may play a more prominent role in late life cognitive decline than previously recognized. However, few studies of TDP-43 have included measurement of cognitive function4,6–9 and these have been based on a single point in time rather than direct assessment of change. In addition, TDP-43 is associated with other neuropathologic conditions such as AD3,8–10 and hippocampal sclerosis,4,9,10 and it is uncertain whether TDP-43 has an association with cognitive functioning that is independent of these other pathologic processes.9
The present study tests the hypothesis that TDP-43 pathology is related to cognitive decline in old age. Participants were older Catholic nuns, priests, and monks without dementia at study entry who completed annual cognitive testing for a mean of 10.1 years prior to death. On neuropathologic examination, immunohistochemical measures of TDP-43 pathology and other neurodegenerative markers were collected from multiple brain regions and the presence of infarcts and hippocampal sclerosis was determined. In analyses, we tested for the hypothesized association of TDP-43 with cognitive decline, examined whether other pathological conditions could account for the association, compared the cognitive profile of TDP-43 with profiles of other conditions, and assessed whether TDP-43 was elevated in mild cognitive impairment and dementia.
METHODS
Participants
We used data from persons in the Religious Orders Study, a longitudinal clinical-pathologic investigation of older Catholic nuns, priests, and brothers recruited from more than 40 groups across the United States.11,12 Eligibility required age > 55, absence of a prior dementia diagnosis, and agreement to annual clinical evaluations (begun in 1994 and continuing) and organ donation at death. All participants signed an informed consent and anatomic gift act. The project was approved by the institutional review board of Rush University Medical Center.
At the time of these analyses, 539 of 1081 study participants without baseline dementia had died, 505 of these (94%) had undergone a brain autopsy which had been completed in the first consecutive 490, of whom 463 had longitudinal cognitive data. Of these, TDP-43 data had been collected in 130. Compared to the 333 without TDP-43 data, the 130 with TDP-43 had more follow-up (10.1 years versus 8.7, χ2 [1] = 17.7, p<0.001) but did not differ in age, sex, education, global cognition (at baseline or proximate to death), postmortem interval, amyloid plaques, tangles, hippocampal sclerosis, neocortical Lewy bodies, or cerebral infarcts. They died at a mean age of 88.1 (SD = 7.5) after a mean of 10.1 years (SD = 3.1) of annual cognitive testing. They had completed a mean of 18.0 years of education (SD = 3.3) and 70.8% were women.
Assessment of Cognitive Function
Cognition was assessed annually with a battery of 20 tests that are described in Table 1. To minimize measurement error, we used composite cognitive measures in most analyses. We formed a composite measure of global cognition that used all 20 tests and, based in part on previous factor analyses in this13 and other14,15 cohorts, composite measures of episodic memory (based on 7 tests), semantic memory (4 tests), working memory (4 tests), perceptual speed (2 tests), and visuospatial ability (2 tests). Scores on individual tests were converted to z scores, using the baseline mean and standard deviation, and the z scores were averaged to yield each composite. We also analyzed change in the raw scores of each individual test.
Table 1.
Psychometric information on cognitive measuresa
| Cognitive Measure | Function Assessed | Items | Baseline Evaluation | Last Evaluation | Annual Change* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Evaluation | SD | Mean | SD | Change* | SE | p | |||
| Logical Memory Ia | Episodic memory | 25 | 11.8 | 3.7 | 8.6 | 5.7 | −0.078 | 0.013 | <0.001 |
| Logical Memory IIa | Episodic memory | 25 | 10.0 | 4.0 | 7.8 | 5.5 | −0.061 | 0.012 | <0.001 |
| Immediate story recall | Episodic memory | 12 | 9.8 | 1.8 | 7.2 | 3.5 | −0.123 | 0.015 | <0.001 |
| Delayed story recall | Episodic memory | 12 | 9.2 | 2.2 | 6.5 | 3.8 | −0.108 | 0.015 | <0.001 |
| Word List Memory | Episodic memory | 30 | 17.5 | 4.0 | 14.0 | 7.0 | −0.091 | 0.016 | <0.001 |
| Word List Recall | Episodic memory | 10 | 5.4 | 2.1 | 3.8 | 3.0 | −0.070 | 0.013 | <0.001 |
| Word List Recognition | Episodic memory | 10 | 9.6 | 1.2 | 8.1 | 3.1 | −0.110 | 0.023 | <0.001 |
| Vocabulary test | Semantic memory | 15 | 10.6 | 3.3 | 8.7 | 4.5 | −0.053 | 0.011 | <0.001 |
| Reading test | Semantic memory | 20 | 18.2 | 2.4 | 17.0 | 4.0 | −0.036 | 0.015 | 0.013 |
| Verbal Fluency | Semantic memory | na | 33.5 | 8.6 | 21.1 | 11.9 | −0.127 | 0.012 | <0.001 |
| Boston Naming Test | Semantic memory | 20 | 18.0 | 1.9 | 15.7 | 4.5 | −0.101 | 0.016 | <0.001 |
| Digit Span Forward | Working memory | 12 | 8.4 | 2.0 | 7.1 | 2.8 | −0.046 | 0.013 | <0.001 |
| Digit Span Backward | Working memory | 12 | 6.1 | 1.9 | 4.5 | 2.6 | −0.066 | 0.012 | <0.001 |
| Digit ordering | Working memory | 16 | 6.8 | 2.7 | 5.5 | 2.5 | −0.051 | 0.009 | <0.001 |
| Alpha span | Working memory | 14 | 4.8 | 1.6 | 2.8 | 1.9 | −0.099 | 0.011 | <0.001 |
| Symbol Digit Modalities Test | Perceptual speed | 110 | 38.4 | 10.0 | 23.6 | 14.8 | −0.125 | 0.011 | <0.001 |
| Number Comparison | Perceptual speed | 48 | 24.2 | 6.5 | 16.3 | 8.9 | −0.106 | 0.012 | <0.001 |
| Line Orientation | Visuospatial ability | 15 | 9.7 | 3.2 | 7.7 | 3.8 | −0.066 | 0.010 | <0.001 |
| Standard Progressive Matrices | Visuospatial ability | 17 | 9.8 | 3.2 | 7.8 | 3.4 | −0.052 | 0.009 | <0.001 |
| Mini-Mental State Examination | Global cognition | 30 | 28.3 | 1.6 | 21.0 | 9.4 | −0.215 | 0.030 | <0.001 |
From 20 separate mixed-effects models adjusted for age at death.
Clinical Evaluation
The annual evaluations also included a medical history and neurological examination.11–13 After each evaluation, an experienced clinician diagnosed dementia and mild cognitive impairment. Classification of dementia required a history of cognitive decline and impairment in at least 2 cognitive domains.16 To maintain uniformity in the diagnostic process, we used an algorithm to rate impairment in 5 cognitive domains (orientation, attention, memory, language, perception) based on educational level and scores on 11 of the cognitive tests. 17 After review of all cognitive data, a neuropsychologist agreed or disagreed with each algorithmic rating and supplied new ratings in the event of disagreements. The diagnosis of mild cognitive impairment required impairment in 1 or more cognitive domains in the absence of dementia.17 Upon death, all clinical data were reviewed by a board-certified neurologist blinded to all pathologic data and final diagnoses of mild cognitive impairment and dementia were made.
Neuropathologic Examination
The brain was removed a median of 5.5 hours (interquartile range = 5.4) after death which occurred a median of 7.7 months (interquartile range = 7.0) following the last clinical evaluation. One cerebral hemisphere, one cerebellar hemisphere, and the brainstem were fixed in 4% paraformaldehyde for at least 72 hours. The brain was cut coronally into 1-cm slabs and all slabs were examined for gross infarcts. A standard protocol was followed for tissue preservation, tissue sectioning, and quantification of pathologic data by examiners blinded to all clinical data.18,19 We used hemotoxylin and eosin to identify microinfarcts (i.e., visible on microscopic but not gross inspection) in 9 regions in one hemisphere, as previously described.20 In analyses, chronic gross and microscopic infarcts were each treated as present or absent.
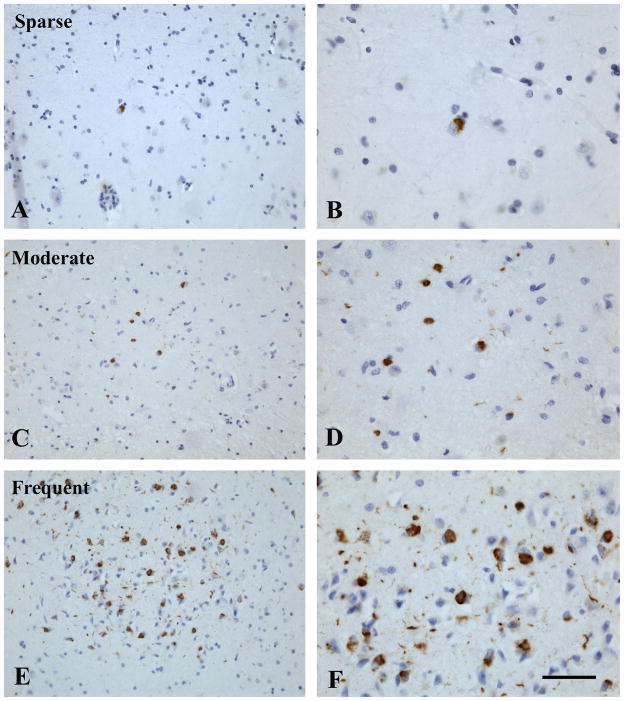
Based on prior research,1,21–26 we investigated TDP-43 pathology in 6 brain regions: amygdala (and periamygdalar region when available), hippocampus CA1/subiculum, dentate gyrus, entorhinal cortex, midfrontal cortex, and middle temporal cortex. Immunostaining was done on 6 μm sections using monoclonal antibodies to phosphorylated TDP-43 (pS409/410;1:100)27 which stain the pathologically phosphorylated TDP-43 proteins in the inclusions seen in amyotrophic lateral sclerosis, frontotemporal lobar degeneration, and other neurodegenerative diseases but not the normal nuclear TDP-43. Each region of interest was reviewed for the presence, severity, and location of TDP-43 cytoplasmic inclusions (both neuronal and glial) and was rated on a 6-point scale based on the number of inclusions in a 0.25mm2 area of greatest density within that region (none, sparse [1–2 inclusions], sparse to moderate [3–5 inclusions], moderate [6–12 inclusions], moderate to severe [13–19 inclusions], severe [20 or more inclusions]) (Figure 1).
Figure 1.
TDP-43 immunoreactive inclusions in the entorhinal cortex. TDP-43 immunoreactive cellular inclusions (brown) and hematoxylin counter stain in the entorhinal cortex from 3 cases: case 1 with sparse (A and B), case 2 with moderate (C and D), and case 3 with frequent (E and F) inclusions. Left panel (A, C, E) scale bar = 100 μm; Right panel (B, D, F) scale bar = 50 μm.
An anti-paired helical filaments-tau antibody clone AT8 (ThermoScientific, Rockford, IL USA; 1:2000) and computer assisted sampling28 were used to measure density of tau-immunoreactive neurofibrillary tangles in at least 2 sections from 8 limbic and neocortical regions (entorhinal cortex, CA1/subiculum, anterior cingulate cortex, dorsolateral prefrontal cortex, superior frontal cortex, inferior temporal cortex, inferior parietal cortex, primary visual cortex). The raw scores in each section and region were averaged to yield a composite measure of tangle density/mm2, as previously described.26
Beta amyloid-immunoreactive plaques were assessed in the 8 regions examined for tau using a monoclonal antibody (1:50; Beta-Amyloid, Clone 6F/3D, Dako, North America) with diaminobenzidine as the reporter with 2.5% nickel sulphate to enhance contrast. Computer assisted sampling and image analysis were used to quantify the percent of each area occupied by beta-amyloid-immunoreactive pixels. Regional measures were averaged to yield a composite measure of amyloid burden.28
Lewy bodies were identified in the substantia nigra, 2 limbic sites (entorhinal cortex, anterior cingulate cortex), and 3 neocortical sites (midfrontal cortex, superior or middle temporal cortex, inferior parietal cortex) using a monoclonal antibody to alpha-synuclein (Zymed LB 509; 1:50).18 We used a modified version29,30 of the staging criteria of McKeith et al31 to classify Lewy body disease as nigral, limbic, or neocortical. Neocortical disease required Lewy bodies in frontal, temporal, or parietal cortex and was usually accompanied by nigral and limbic Lewy bodies.
Statistical Analysis
To assess agreement among TDP-43 raters, we calculated the component of variance due to rater as a fraction of the total variance for each brain region. The dimensionality of the regional TDP-43 measures was assessed in a principal-components analysis. We used the LOESS procedure32 to determine whether TDP-43 had a linear relation to cognitive slope and then used linear mixed-effects models to estimate the association of TDP-43 with level of global cognition and annual rate of change. Each model included terms for time (in years from death), age at death, TDP-43, and the interactions of time with age and TDP-43. We repeated the analysis with additional pathologic measures and their interactions with time and then with multiple cognitive outcomes. Clinical diagnosis proximate to death, with a mild cognitive impairment reference group contrasted with no cognitive impairment and dementia groups, was regressed on TDP-43 in a polychotomous logit model33 adjusted for age at death and AD pathology (but not hippocampal sclerosis which was present in only one person without dementia).
RESULTS
As shown in Table 2, the regional measures of TDP-43 were positively skewed, with no TDP-43 pathology in 54% and levels ranging from sparse to severe in the remaining 46%. TDP-43 pathology was most common in the amygdala (45%), less common in the entorhinal cortex (22%) and hippocampus (16–21%), and least common in the neocortex (5–10%). None of those with neocortical TDP-43 pathology had degeneration of the frontal or temporal lobes plus layer 2 spongiform changes compatible with a diagnosis of Frontotemporal Lobar Degeneration with TDP-43 inclusions.34 The intraclass correlation coefficients of the regional measures, based on independent ratings of an approximate 8% subset of slides by 3 individuals, indicate good interrater reliability (Table 2). In a principal-components analysis, the regional TDP-43 measures loaded on a single factor that accounted for 63.3% of the variance (Table 2). Therefore, we summed the regional measures to create an index of the severity and extent of TDP-43 pathology (mean=3.00, SD=5.19, skewness = 2.00, range: 0–21). The 60 persons (46%) with at least some evidence of pathology on this measure were older at death than unaffected persons (eTable 2).
Table 2.
Descriptive information on regional measures of TDP-43
| Brain region | N | Frequency of TDP-43 scores
|
Mean | SD | Skewness | Intraclass correlationa | Factor loadingb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||||||
| Amygdala | 130 | 55.4 | 18.5 | 1.5 | 9.2 | 5.4 | 10.0 | 1.21 | 1.73 | 1.22 | 0.90 | 0.78 |
| Enthorhinal cortex | 128 | 78.1 | 5.5 | 4.7 | 4.7 | 3.1 | 3.9 | 0.61 | 1.33 | 2.20 | 0.97 | 0.94 |
| CA1/subiculum | 127 | 78.7 | 3.9 | 7.9 | 8.7 | 0.0 | 0.8 | 0.50 | 1.05 | 2.02 | 0.84 | 0.81 |
| Dentate gyrus | 125 | 83.2 | 4.8 | 4.0 | 5.6 | 1.6 | 0.8 | 0.40 | 1.01 | 2.63 | 0.94 | 0.87 |
| Middle temporal gyrus | 129 | 89.9 | 3.1 | 3.1 | 2.3 | 0.8 | 0.8 | 0.23 | 0.80 | 3.91 | 0.89 | 0.78 |
| Midfrontal gyrus | 128 | 94.5 | 3.9 | 0.8 | 0.0 | 0.0 | 0.8 | 0.09 | 0.51 | 7.81 | 0.96 | 0.54 |
Indicator of interrater reliability.
From a principal-components analysis.
Beta-amyloid plaque burden ranged from 0 to 14.87 (n=130, mean = 2.72, SD = 2.89, skewness = 1.23), tau tangle density ranged from 0 to 61.55 (n=130, mean = 5.05, SD = 7.46, skewness = 4.10), 39.2% had at least 1 chronic gross infarct, 28.5% had at least 1 chronic microinfarct, 7.7% had neocortical Lewy bodies, and 9.2% had hippocampal sclerosis. The presence of TDP-43 pathology was associated with higher amyloid plaque burden, tangle density, and likelihood of hippocampal sclerosis but not with likelihood of gross infarcts, microinfarcts, or neocortical Lewy bodies (eTable 1).
TDP-43 and Global Cognitive Decline
Figure 2A, which shows rate of global cognitive decline plotted by level of TDP-43 fit with a LOESS, suggests that the association of TDP-43 with rate of cognitive decline is approximately linear and supports the use of linear mixed-effects models. In the initial analysis, there was a mean decline of 0.073-unit per year in the global cognitive measure (SE = 0.012, p < 0.001). Higher levels of TDP-43 inclusions were associated with lower level of global cognition (estimate = −0.090, SE=0.021, p<0.001) and more rapid global cognitive decline (estimate = −0.010, SE=0.002, p<0.001).
Figure 2.
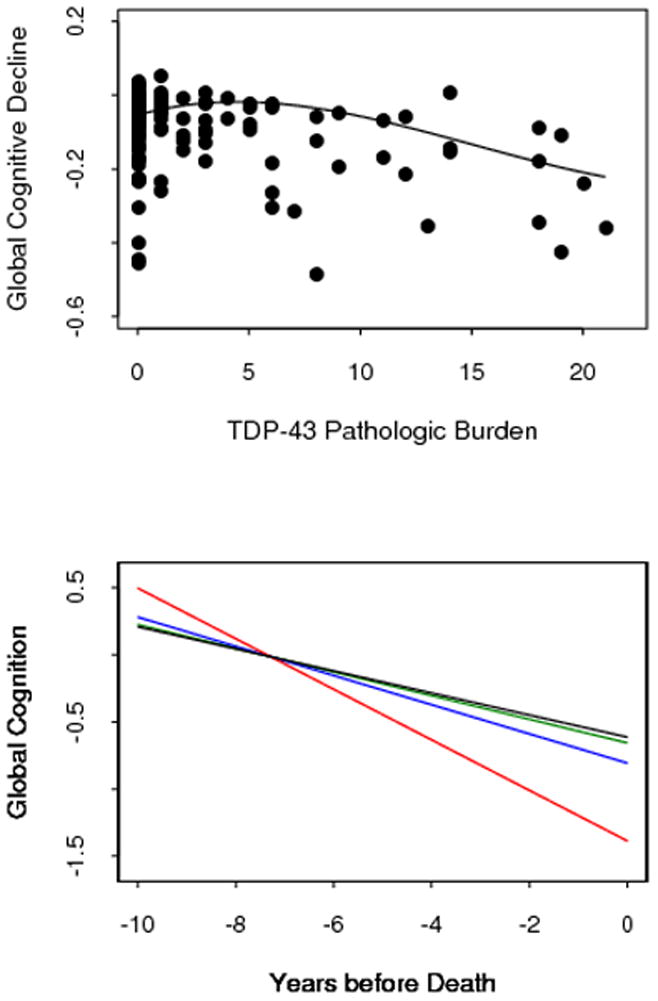
Relation of TDP-43 pathologic burden to rate of cognitive decline. A. Individual rates of global cognitive decline, adjusted for age at death, plotted by level of TDP-43 pathology, fitted with a locally reweighted linear smooth function. B. Ten year paths of global cognitive decline in typical participants with no TDP-43 pathology (black line) and with low (green line, 10th percentile), moderate (blue line, 50th percentile), or high (red line, 90th percentile) levels of TDP-43 pathology, adjusted for age at death, amyloid, tangles, and hippocampal sclerosis.
Because TDP-43 was associated with amyloid plaques, tangles, and hippocampal sclerosis, we repeated the analysis with terms added for these pathologic measures. In this model, TDP-43 (estimate = −0.006, SE=0.002, p=0.007) and tangles (estimate = −0.007, SE = 0.002, p<0.001) were each related to more rapid cognitive decline with no association for amyloid (estimate = −0002, SE = 0.004, p =0.599) or hippocampal sclerosis (estimate = −0.048, SE = 0.037, p = 0.197). Figure 2B, which is based on this analysis, suggests a dose response relationship with higher levels of TDP-43 pathology associated with increasingly rapid cognitive decline.
In further analyses, we compared the impact of TDP-43 and tangles on cognitive aging. Relative to a model adjusted for age at death, TDP-43 accounted for an additional 21% of the variance in rates of cognitive decline versus 26% for tangles. With tangles in the model, TDP-43 accounted for an additional 12% of the variance versus 16% for tangles with TDP-43 in the model.
TDP-43 and Decline in Cognitive Domains
To determine whether TDP-43 was related to decline in some cognitive functions but not others, we assessed change in 5 different cognitive domains (Table 3). Higher level of TDP-43 pathology was associated with more rapid decline in episodic memory and working memory but not with decline in other domains. In contrast, tangle density was associated with decline in all domains, amyloid plaque burden was not related to decline in any domain, and hippocampal sclerosis was associated with decline in semantic memory but not other domains.
Table 3.
Association of pathologic measure with annual rate of change in different cognitive domainsa
| TDP-43 Pathology | Tangle Density | Amyloid Plaques | Hippocampal Sclerosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Episodic memory | −0.010 | 0.002 | <0.001 | −0.006 | 0.002 | 0.001 | −0.003 | 0.004 | 0.459 | −0.018 | 0.040 | 0.655 |
| Semantic memory | −0.003 | 0.002 | 0.143 | −0.006 | 0.002 | <0.001 | −0.005 | 0.004 | 0.177 | −0.082 | 0.036 | 0.024 |
| Working memory | −0.004 | 0.002 | 0.021 | −0.005 | 0.002 | <0.001 | 0.001 | 0.004 | 0.782 | −0.007 | 0.032 | 0.820 |
| Perceptual speed | −0.003 | 0.002 | 0.186 | −0.007 | 0.002 | <0.001 | −0.005 | 0.004 | 0.182 | −0.057 | 0.035 | 0.103 |
| Visuospatial ability | −0.001 | 0.002 | 0.761 | −0.004 | 0.001 | 0.009 | −0.003 | 0.003 | 0.396 | −0.037 | 0.028 | 0.195 |
From 5 separate mixed-effects models adjusted for age at death. Results show the shift in the mean annual rate of cognitive change with each 1-unit of a given pathologic measure.
We conducted additional analyses with the individual tests as outcomes instead of composite measures. For each test, Table 1 shows the mean score at baseline, the mean score proximate to death, and the estimated annual rate of change. In the analyses (eTable 2), TDP-43 was related to decline on all 7 episodic memory tests, the Boston Naming Test, Digit Span Forward, and the Mini-Mental State Examination. Tangle density was associated with decline on 15 tests, amyloid plaque burden with decline on 2 tests (immediate story recall, Boston Naming Test), and hippocampal sclerosis with decline on 3 tests (vocabulary test, verbal fluency, Standard Progressive Matrices).
TDP-43 and Clinical Diagnoses
Proximate to death, 38(29%) participants had no evidence of cognitive impairment, 39(30%) had mild cognitive impairment, and 53(41%) had dementia. To determine whether TDP-43 was differentially related to cognition along the spectrum from intact function to dementia, we examined its relation to clinical diagnosis. In an analysis that included terms for age at death, amyloid plaques, and tangles, higher level of TDP-43 pathology was associated with higher likelihood of dementia relative to mild cognitive impairment (χ2 [1] = 5.35, p = 0.021) but not with likelihood of mild cognitive impairment relative to no cognitive impairment (χ2 [1] = 0.17, p = 0.677). By contrast, tangles had a nearly significant association with mild cognitive impairment relative to no cognitive impairment (χ2 [1] = 2.97, p = 0.085) but did not differentiate mild cognitive impairment from dementia (χ2 [1] = 0.28, p = 0.594) and amyloid plaques were not related to diagnosis.
DISCUSSION
A cohort of 130 older persons without dementia at study entry underwent annual cognitive testing for a mean of about 10 years during which more than 40% developed dementia. On neuropathologic examination, TDP-43 pathology was identified in nearly half of the participants, and it accounted for nearly as much of the variability in rates of cognitive decline as did AD pathology. The findings suggest that TDP-43 is an important pathology underlying late life cognitive decline and dementia.
In previous research, TDP-43 pathology has been associated with lower level of global cognition4,6,8 and higher likelihood of dementia.9 The present results extend these observations in 2 important ways. First, the association of TDP-43 with lower level of global cognition persisted after controlling for age, AD pathology, and hippocampal sclerosis, supporting the idea that its association with cognitive impairment is relatively independent of these other pathologic processes. Second, after controlling for this association, age, and other pathologies, TDP-43 was associated with more rapid cognitive decline. That it accounted for nearly as much of the variability in cognitive decline as neurofibrillary tangles suggests that TDP-43 pathology plays a substantial role in late life loss of cognition.
The manner in which TDP-43 pathology leads to impaired neuronal function is not known. Multiple pathways activated by TDP-43 aggregation may be involved, but the downstream consequences of TDP-43 phosphorylation, aggregation, cleavage, mislocalization and clearance from the nucleus are still unclear.35 The clearance of normal TDP-43 from the nucleus may represent a loss of normal TDP-43 that leads to neurodegeneration, or, alternatively, the retention of TDP-43 in cytoplasmic aggregates could sequest diverse RNAs and thereby lead to neurodegeneration through a toxic gain of function.35
Whether TDP-43 pathology has a characteristic cognitive profile is not known. Few TDP-43 studies have assessed multiple cognitive functions4,7–9 and results have been inconsistent. For example, TDP-43 was associated with impairment of semantic memory but not episodic memory in 1 study4 whereas another study reported the opposite pattern.8 This inconsistency may reflect several factors, including reliance on cross-sectional cognitive data and the potentially confounding effects of other pathologic processes on cognition. In the present study, TDP-43 was associated with impairment and decline in multiple cognitive domains. After adjustment for AD pathology and hippocampal sclerosis, however, TDP-43 pathology was related to decline in episodic and working memory but not to decline in other cognitive domains. The cognitive profile associated with TDP-43 pathology differed from the cognitive profiles associated with AD pathology and hippocampal sclerosis. Consistent with prior research,1,21–26 TDP-43 pathology was most common in the medial temporal lobe which may account for its robust association with episodic memory dysfunction.
In this cohort, TDP-43 pathology was increased in persons with dementia but not in those with mild cognitive impairment. This suggests that TDP-43 may be more strongly related to progression of cognitive symptoms than to their initial development, or that it is a separate age related process that is age shifted, occurring in most instances after the onset of other age related neurodegenerative processes. In contrast, the pathologic processes traditionally associated with late life dementia (e.g., tangles, Lewy bodies, cerebral infarcts) appear to account for more of the variability in incipient cognitive decline than in later acceleration of decline.36–39
Study strengths and limitations should be noted. A uniform clinical evaluation and established criteria were used to clinically classify individuals. Participation in annual follow-up and autopsy was high, minimizing risk of bias due to selective attrition. The availability of a mean of approximately 10 years of annual cognitive assessments with psychometrically established measures allowed us to reliably estimate individual trajectories of cognitive change. Because of the selected nature and relatively small size of the group studied, it will be important to replicate these findings. In addition, use of a nonphosphorylated epitope rather than a phosphorylated antibody probably resulted in underestimation of the burden of synucleinopathy.
Supplementary Material
Acknowledgments
The authors thank the many Catholic clergy members who have participated in the Religious Orders Study; Karen Skisk, MS, for overall coordination of pathologic data collection; Srbani Mondal for immunohistochemical staining; Virginia Kriho for TDP-43 data collection; Traci Colvin, MPH, for coordinating the clinical study; Woojeong Bang, MS, for statistical programming; and John Gibbons, MS, and Greg Klein MS, for data management. This research was supported by National Institute on Aging grants R01AG42210, P30AG10161, R01AG15819, R01AG10124, R01AG32953 and by the Illinois Department of Public Health.
Footnotes
The authors have nothing to disclose. Dr. Wilson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Lei Yu, Email: lei_yu@rush.edu.
John Q. Trojanowski, Email: trojanow@mail.med.upenn.edu.
Er-Yun Chen, Email: er-yun_chen@rush.edu.
Patricia A. Boyle, Email: patricia_boyle@rush.edu.
David A. Bennett, Email: dbennett@rush.edu.
Julie A. Schneider, Email: julie_a_schneider@rush.edu.
References
- 1.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 3.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geser F, Robinson JL, Malunda JA, et al. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 2010;67:1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathologic changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease, and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay C, St-Amour I, Schneider JA, et al. Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer Disease. J Neuropathol Exp Neurol. 2011;70:788–798. doi: 10.1097/NEN.0b013e31822c62cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson JL, Geser F, Corrada MM, et al. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain. 2011;134:3708–3715. doi: 10.1093/brain/awr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropsychol Cogn. 2004;11:280–303. [Google Scholar]
- 12.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religous orders study. Curr Alzheimer Res. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 14.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Aggarwal NT, Barnes LL, et al. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66:767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Li JL, Li Y, et al. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older perons without cognitive impairment. Neurology. 2008;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Boyle PA, Levine SR, et al. Emotional neglect in childhood and cerebral infarction in older age. Neurology. 2012;79:1534–1539. doi: 10.1212/WNL.0b013e31826e25bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 23.Geser F, Prvulovic D, O’Dwyer L, et al. On the development of markers for pathological TDP-43 in amyotrophic lateral sclerosis with and without dementia. Prog Neurobiol. 2011;95:649–662. doi: 10.1016/j.pneurobio.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120:43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Kadokura A, Yamazaki T, Lemere CA, et al. Regional distribution of TDP-43 inclusions in Alzheimer disease(AD) brains: their relation to common AD pathology. Neuropathol. 2009;29:566–573. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 27.Neumann M, Kwong LK, Lee E, et al. Phosphorylation of S409/410 in TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropath. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett DA, Schneider JA, Wilson RS, et al. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer’s disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Schneider JA, Arnold SE, et al. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36:367–373. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of consortium on DLB International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 32.Cleveland WS, Grosse E. Computational methods for local regression. Statistics and Computing. 1991;1:47–62. [Google Scholar]
- 33.Agresti A. Categorical Data Analysis. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 34.Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosological criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EB, Lee VMY, Trojanowski TQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci. 2011;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson RS, Leurgans SE, Boyle PA, et al. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson RS, Segawa E, Hizel LP, et al. Terminal dedifferentiation of cognitive abilities. Neurology. 2012;78:1116–1122. doi: 10.1212/WNL.0b013e31824f7ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson RS, Segawa E, Buchman AS, et al. Terminal decline in motor function. Psychol Aging. 2012 May 21; doi: 10.1037/a0028182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common pathologies. Ann Neurol. doi: 10.1002/ana.23964. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.