Abstract
Heterocyclic amines (HCAs) are mutagenic compounds generated when meats are cooked at high temperature and for long duration. The findings from previous studies on the relation between HCAs and breast cancer are inconsistent, possibly due to genetic variations in the enzymes metabolizing HCAs.
To evaluate whether the associations of intakes of estimated HCAs, meat-derived mutagenicity (MDM), and red meat with risk of postmenopausal breast cancer were modified by N-acetyltransferase 2 (NAT2) acetylator genotype or cytochrome P450 1A2 -164 A/C (CYP1A2) polymorphism, we conducted a nested case-control study with 579 cases and 981 controls within a prospective cohort, the Nurses’ Health Study (NHS). HCAs and MDM intakes were derived using a cooking method questionnaire administered in 1996.
NAT2 acetylator genotype, the CYP1A2 polymorphism, and intakes of HCAs, MDM, and red meat were not associated with risk of postmenopausal breast cancer. There was also no interaction between NAT2 acetylator genotype or CYP1A2 polymorphism and HCAs and MDM and red meat intake in relation to breast cancer.
These results do not support the hypothesis that genetic polymorphisms of xenobiotic enzymes involved in the metabolism of HCAs may modify the associations between intakes of red meat or meat-related mutagens and breast cancer risk.
Keywords: NAT2, CYP1A2, heterocyclic amines, breast cancer, epidemiology
Introduction
Heterocyclic amines (HCAs) are carcinogenic compounds generated from the cooking of meats at high temperature and for long duration (1, 2). Even though evidence from animal studies has pointed towards a possible role of HCAs in breast carcinogenesis (3, 4), findings from previous studies on HCAs and breast cancer are inconsistent (5, 6).
One possible reason for these inconsistencies is genetic variation in the xenobiotic enzymes involved in the metabolism of HCAs (7, 8). HCAs are metabolically activated primarily through N-oxidation by cytochrome P450 1A2 (CYP1A2), a phase I xenobiotic metabolizing enzyme, and are further O-acetylated by N-acetyltransferase 2 (NAT 2), a phase II xenobiotic metabolizing enzyme (9, 10). Previous epidemiological studies that have examined the interactions between either HCAs or red meat intake and NAT2 acetylation status with regard to breast cancer risk have reported inconsistent results (11–16).
However, to the best of our knowledge, no epidemiological study has evaluated the interactions between HCAs intake and CYP1A2 genetic polymorphisms on risk of breast cancer.
Some studies suggest that the effect of certain risk factors for breast cancer, including red meat intake, may differ by hormone receptor status (17, 18). Heterocyclic amines can be estrogenic and may stimulate estrogen receptor (ER)-dependent gene expression and the expression of progesterone receptor (PR) in vitro (19, 20). Few studies examined the association between HCA intake and breast cancer risk by hormone receptor status (13).
Thus, we evaluated whether NAT2 and CYP1A2 genetic polymorphisms influence the associations between HCAs intake, meat-derived mutagenicity (MDM) and red meat intake, and postmenopausal breast cancer risk in a case-control study nested within the prospective Nurses’ Health Study (NHS). We also examined the associations by hormone receptor status.
Materials and Methods
Study Population
The NHS was founded in 1976 and includes 121,700 female registered nurses who responded to a mailed questionnaire. Every two years participants are mailed follow-up questionnaires (FFQs) inquiring about disease diagnoses and lifestyle factors. Food frequency questionnaires were added to the follow-up survey in 1980 and repeated every 2–4 years since then. Questions on cooking methods, doneness, and outside appearance of several meats were added to the 1996 questionnaire. Blood samples were collected from 32,826 (27%) of the cohort members between 1989 and 1990. More detailed information on the NHS cohort is provided elsewhere (21). Study investigators confirmed breast cancer cases through review of medical records and pathology reports.
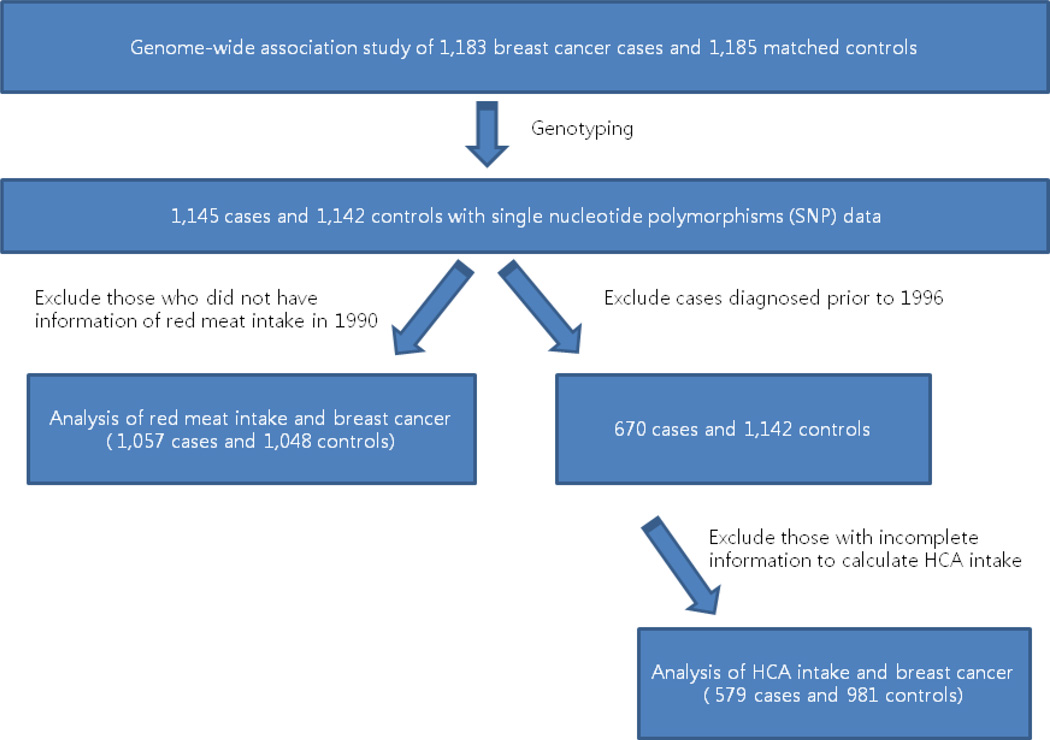
We included breast cancer cases and controls from a genome-wide association study (GWAS) of breast cancer in the NHS (22). Briefly, the GWAS included 1,183 women with postmenopausal invasive breast cancer who provided blood samples and were diagnosed between 1990 and 2004. The study selected 1,185 controls (1:1 matching), who were postmenopausal women not diagnosed with breast cancer during follow-up until June 1, 2004. The controls were matched with the cases by year of birth and post-menopausal hormone use at the time of blood collection. After genotyping, a total of 1,145 cases and 1,142 controls were included in the genome-wide association analysis of 528,173 single nucleotide polymorphisms (SNPs) (Figure 1).
Figure 1.
Diagram of study population
Assessment of Diet, Cooking Method and HCAs Intake
Dietary intake data were collected in 1980, 1984, 1986, 1990, and 1994 using self-administered semi-quantitative FFQs. In 1980, an FFQ with about 60 food items was sent to the participants. An expanded FFQ with about 130 food items has been sent to participants in 1984, 1986, and every 4 years thereafter. The validity and reproducibility of these FFQs have been reported on elsewhere (23, 24).
The cooking method questionnaire administered in 1996 was designed to estimate the intake of HCAs in the NHS. The questions were based on results from a pilot study which used a detailed cooking questionnaire to identify specific questions related to cooking methods that would best predict HCAs intake in the cohort (25).
On this questionnaire, participants were asked about frequency of intake (i.e., never, < 1/month, 1/month, 2–3/month, 1/week, 2–3/week, and 4+/week) and doneness (i.e., depending on type of meat: lightly browned, medium browned, well browned, and blackened/charred) of cooked meats and fish (i.e., pan-fried, broiled and grilled chicken, broiled fish, roast beef, pan-fried steak, grilled or barbecued steak, and homemade beef gravy).
HCAs intakes were calculated from the data provided on the 1996 cooking method questionnaire and dietary data in 1994 using the “CHARRED Database” created by Sinha and colleagues from the National Cancer Institute (26, 27). The mutagenic activity of meat samples was assessed by the Ames/Salmonella test (28, 29). Intakes of MeIQx (2–amino-3,8-dimethylimidazo [4,5,-f] quinoxaline), PhIP (2–amino-1-methyl-6-phenylimidazo [4,5-b] pyridine), and DiMeIQx (2–amino-3,4,8-trimethylimidazo [4,5,-f]) were derived using a method described in more detail in (30). Intakes of those compounds and MDM were calculated by multiplying the HCAs or MDM values from the CHARRED Database (ng/gram meat for HCAs and revertant colonies/gram meat for MDM) with standard portion sizes from the CHARRED Database and frequency of intake of the relevant cooked meat item obtained from the 1996 cooking method questionnaire (31). More detail regarding the calculation of HCAs intake in our cohort is reported elsewhere (6). We assessed total red meat intake as cumulative average intake up to 1990.
Genotype classification of NAT2 and CYP1A2
We extracted SNPs data on CYP1A2 and NAT2 from our previous GWAS of breast cancer (22). Determination of NAT2 acetylator status was based on imputed SNPs information, including NAT2 I114T (rs1801280), R197Q (rs1799930), and G286E (rs1799931) (32). The imputation R2 for rs1801280, rs1799930, and rs1799931 were 0.9956, 0.9922, and 0.9557, respectively. The NAT*4 allele was defined as wild type, or the absence of amino acid changes due to the three polymorphisms. Individuals with two slow acetylator alleles were classified as NAT2 slow acetylators, whereas individuals with zero or one slow acetylator allele were classified as rapid acetylators. We also examined the CYP1A2 164A/C polymorphism (CYP1A2*1F; rs762551), which was also imputed (imputation R2=0.9988) (33). Genotypes of NAT2 were classified into slow and fast acetylators. For CYP1A2, due to small sample size of homozygous variant carriers, we combined individuals carrying variant allele (AC or CC), and used the homozygous wild type (AA) as the reference group.
Exclusion Criteria
For the analysis of HCAs and MDM, because HCA and MDM were calculated from cooking method questionnaires in 1996, breast cancer cases diagnosed after 1996 were only included (670 for cases and 1142 for controls) to conduct a prospective analysis (Figure 1). We further excluded participants following the exclusion criteria used in our previous report of HCA (6); Participants were excluded if they left more than 70 food items (50%) blank on the 1994 FFQs (n=80; 25 cases and 55 controls) and left the entire cooking method section blank on the 1996 questionnaire (n=23; 6 cases and 17 controls). Then, we also excluded participants (n=133; 48 cases and 85 controls) for whom HCAs/MDM could not be calculated because they had not reported frequency of cooked meat intake, even though they had reported information on doneness of meat for each meat item, and vice versa. After implementation of our exclusion criteria, a total of 579 breast cancer cases and 981 controls formed our study population. Our analysis population for red meat intake was 1,057 cases and 1,048 controls after excluding those who did not have information on red meat intake in 1990 (n=182; 88 cases and 94 controls), to conduct a prospective analysis (Figure 1).
For the cases, we extracted information on hormone receptor status [ER and PR] from the pathology reports. A total of 535 cases are available in the HCAs/MDM analysis with hormone receptor status. Among them, 369 were ER+/PR+, 79 were ER−/PR−, 74 were ER+/PR−, and 13 were ER−/PR+. A total of 914 cases are available in the red meat analysis with hormone receptor status. Among them, 614 were ER+/PR+, 143 were ER−/PR−, 133 were ER+/PR−, and 24 were ER−/PR+.
The study was approved by the Human Subjects Committee of the Brigham and Women’s Hospital.
Statistical Analysis
Multivariate unconditional logistic regression was used to assess the association between breast cancer risk and xenobiotic enzyme genotype and intake of HCAs, MDM, and red meat. Intakes were divided into tertiles based on the distribution among controls. We conducted analysis for associations stratified by genotype of NAT2 and CYP1A2. The reference group was the lowest-intake category for HCAs, MDM, and red meat. The multivariate models for HCAs and MDM were adjusted for the known and suspected risk factors for breast cancer, i.e., age, body mass index (BMI; kg/m2, continuous) at 18 yrs, weight change (continuous) in 1996 compared with aged 18yr, age at menarche (years ≤12, 13, ≥14), family history of breast cancer in mother or sister (yes, no), parity and age at first birth (nulliparous, 1–2 children and age at first birth <25 years, 1–2 children and age at first birth 25–<30 years, 1–2 children and age at first birth ≥30 years, 3+ children and age at first birth <25 years, 3+ children and age at first birth 25–<30 years, 3+ children and age at first birth ≥30 years), personal history of benign breast disease, smoking status (never, past, current) in 1996, postmenopausal hormone use (never, current, past) in 1996, and cumulative average energy and alcohol intake (continuous) until 1994. Multivariate models for red meat intake were adjusted for the same covariates as the above models, but in 1990. Energy and alcohol intake cumulative average were used until 1990. Multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. As a secondary analysis, we further adjusted for fruit and vegetable intakes, caffeine intake, and physical activity in the multivariate models. Tests for trend were conducted by using the median of each tertile of red meat or HCAs as a continuous variable in the model. For interaction tests, we conducted likelihood ratio tests using genotype as a categorical variable and median value for each tertile of intake of red meat or HCAs as a continuous variable. We evaluated total breast cancer risk and also examined breast cancer risk by hormone receptor status. All analyses were performed using SAS statistical software, UNIX version 9.1 (SAS Institute Inc, Cary, NC).
Results
The characteristics of cases and controls are presented in Table 1. Compared to controls, cases had lower age, higher alcohol intake, higher proportion of nulliparous women, and higher proportion of women with a family history of breast cancer or a history of benign breast disease.
Table 1.
The characteristics of breast cancer cases and control subjects in a nested case-control study in the Nurses’ Health Study in 1996
| Characteristics | Cases (n=579) |
Controls (n=981) |
P -value |
|---|---|---|---|
| Mean | |||
| Age (years ) | 63.8 | 64.8 | 0.006 |
| BMI at 18yr (kg/m2) | 21.1 | 21.3 | 0.21 |
| Weight gain since age 18yr (kg) | 13.6 | 12.6 | 0.09 |
| Age at menarche (years) | 12.5 | 12.6 | 0.13 |
| Age at first birth (years) | 25.3 | 25.1 | 0.40 |
| Cumulative total calorie intake (g/day)† | 1740.8 | 1722.4 | 0.39 |
| Cumulative alcohol intake (g/day)† | 7.4 | 6.2 | 0.02 |
| Total red meat intake (servings/day)‡ | 1.1 | 1.0 | 0.16 |
| HCA intake (ng/day) | |||
| PhIP | 84.2 | 90.4 | 0.20 |
| MeIQx | 17.7 | 17.0 | 0.46 |
| DiMeIQx | 2.3 | 2.3 | 0.66 |
| Meat-derived mutagenicity (MDM) (revertant colonies/day) |
3403.0 | 3526.0 | 0.46 |
| Percent | |||
| Parity (%) | |||
| nulliparous | 8.8 | 5.1 | |
| parity, 1–2 children | 29.7 | 32.1 | 0.03 |
| parity, >=3 children | 59.6 | 61.5 | |
| Family history of breast cancer in mother or sister (%) |
22.8 | 18.0 | 0.02 |
| History of benign breast disease (%) | 61.3 | 54.4 | 0.008 |
| Smoking (%) | |||
| Never | 46.6 | 48.4 | |
| Past | 45.4 | 44.0 | 0.79 |
| Current | 7.9 | 7.7 |
T-test for continuous and Chi–Square test for categorical variables were conducted.
Mean daily intakes of nutrients are energy-adjusted
Total red meat intake (until 1990) : 1,057cases /1,048 controls
Abbreviations:
BMI=Body mass index
PhIP=2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine
MeIQx=2-amino-3,8-dimethylimidazo [4,5,-f] quinoxaline
DiMeIQx=2-amino-3,4,8-trimethylimidazo [4,5,-f]
NAT2 acetylator genotype, CYP1A2 164A/C polymorphism, and intakes of HCAs, MDM, and red meat were not associated with the risk of postmenopausal breast cancer (Table 2).
Table 2.
The association of CYP1A2, NAT2 genotype, HCAs, MDM, and red meat intake with postmenopausal breast cancer risk.
| Genotype | Cases/controls (n) | age-adjusted OR |
P for trend |
MV OR* | P for trend |
|
|---|---|---|---|---|---|---|
| CYP1A2 | A/A | 303/514 | Ref | Ref | ||
| A/C | 220/388 | 0.96 (0.77 – 1.20) | 0.95 (0.76 – 1.18) | |||
| C/C | 56/79 | 1.21 (0.83 – 1.75) | 0.32 | 1.18 (0.81 – 1.72) | 0.4 | |
| NAT2 | Slow | 350/611 | Ref | Ref | ||
| Fast | 229/370 | 1.07 (0.87–1.33) | 0.51 | 1.06 (0.86–1.32) | 0.58 | |
| Tertile (median) | ||||||
| PhIP (ng/day ) |
T1 (23.3) | 201/327 | Ref | Ref | ||
| T2 (61.4) | 194/327 | 0.92 (0.72 – 1.19) | 0.90 (0.70–1.17) | |||
| T3 (155.5) | 184/327 | 0.84 (0.65 – 1.09) | 0.19 | 0.79 (0.61–1.03) | 0.09 | |
| MeIQx (ng/day ) |
T1 (4.7) | 188/327 | Ref | Ref | ||
| T2 (13.0) | 193/327 | 1.01 (0.78 – 1.30) | 0.96 (0.74–1.24) | |||
| T3 (26.7) | 198/327 | 1.02 (0.79 – 1.31) | 0.92 | 0.90 (0.69–1.18) | 0.45 | |
| DiMeIQx (ng/day ) |
T1 (0.5) | 190/327 | Ref | Ref | ||
| T2 (1.6) | 203/328 | 1.04 (0.81 – 0.34) | 1.02 (0.79–1.32) | |||
| T3 (3.6) | 186/326 | 0.93 (0.72 – 1.20) | 0.50 | 0.85 (0.65–1.11) | 0.17 | |
| Meat-derived mutagenicity (revertant colonies/day) |
T1 (1162.6) | 214/327 | Ref | Ref | ||
| T2 (2611.2) | 179/327 | 0.81 (0.63 – 1.04) | 0.78 (0.60–1.01) | |||
| T3 (5589.4) | 186/327 | 0.83 (0.65 – 1.07) | 0.23 | 0.79 (0.61–1.03) | 0.14 | |
| Red meat (servings/day) |
T1 (0.6) | 344/349 | Ref | Ref | ||
| T2 (1.0) | 340/349 | 0.92 (0.75 – 1.14) | 0.95 (0.76–1.19) | |||
| T3 (1.5) | 373/350 | 0.91 (1.99 – 1.10) | 0.88 | 1.06 (0.83–1.36) | 0.59 | |
Multivariate (MV) models were adjusted for age in years (continuous), smoking status (never, past, current), BMI at 18yr(continuous), weight gain from 18yr (contrinuous), age at menarche (years <=12, 13, >=14), family history of breast cancer (yes, no), parity and age at first birth (nulliparous, 1–2 children and age at first birth <25 years, 1–2 children and age at first birth 25-<30 years, 1–2 children and age at first birth >=30 years, 3–4 children and age at first birth <25 years, 3–4 children and age at first birth 25-<30 years, 3–4 children and age at first birth >=30 years, 5–8 children and age at first birth <25 years, 5–8 children and age at first birth 25-<30 years), postmenopausal hormone use (never, current, past), history of benign breast disease (yes, no), total calorie and alcohol intake.
There were also no effects of the interactions between NAT2 acetylator genotype and intakes of HCAs, MDM, and red meat intake on breast cancer risk (Table 3).
Table 3.
The association between HCAs, MDM, and red meat intake and postmenopausal breast cancer stratified by NAT2 and CYP1A2 genotype
| Variable | Rank | NAT2 | CYP1A2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slow acetylator | Fast acetylator | AA | AC/CC | ||||||||||
| Tertile | Cases | MV OR* | p for trend |
Cases | MV OR* | p for trend |
Cases | MV OR* | P for trend |
Cases | MV OR* | p for trend |
|
| PhIP (ng/day ) |
T1 | 120 | Ref | 81 | Ref | 110 | Ref | 91 | Ref | ||||
| T2 | 122 | 0.99 (0.71–1.38) | 72 | 0.77 (0.50–1.18) | 102 | 0.85 (0.59–1.21) | 92 | 0.97(0.66–1.42) | |||||
| T3 | 108 | 0.85 (0.60–1.20) | 0.30 | 76 | 0.71 (0.46–1.09) | 0.16 | 91 | 0.72 (0.50–1.05) | 0.10 | 93 | 0.88 (0.60–1.30) | 0.50 | |
| p for interaction = 0.46 | p for interaction = 0.86 | ||||||||||||
| MeIQx (ng/day ) |
T1 | 120 | Ref | 68 | Ref | 114 | Ref | 74 | Ref | ||||
| T2 | 116 | 0.94 (0.67–1.32) | 77 | 1.00 (0.66–1.54) | 96 | 0.74 (0.52–1.06) | 97 | 1.37 (0.92–2.02) | |||||
| T3 | 114 | 0.82 (0.58–1.17) | 0.26 | 84 | 1.06 (0.68–1.65) | 0.79 | 93 | 0.65 (0.44–0.96) | 0.03 | 105 | 1.36 (0.91–2.03) | 0.20 | |
| p for interaction = 0.58 | p for interaction = 0.10 | ||||||||||||
| DiMeIQx (ng/day ) |
T1 | 123 | Ref | 67 | Ref | 112 | Ref | 78 | Ref | ||||
| T2 | 117 | 0.93 (0.66–1.29) | 86 | 1.20 (0.78–1.83) | 106 | 0.92 (0.64–1.31) | 97 | 1.20 (0.81–1.78) | |||||
| T3 | 110 | 0.77 (0.54–1.09) | 0.13 | 76 | 0.93 (0.60–1.45) | 0.55 | 85 | 0.66 (0.45–0.98) | 0.03 | 101 | 1.10 (0.74–1.63) | 0.81 | |
| p for interaction = 0.83 | p for interaction = 0.31 | ||||||||||||
| Meat-derived mutagenicity (MDM) (revertant colonies/day) |
T1 | 136 | Ref | 78 | Ref | 126 | Ref | 88 | Ref | ||||
| T2 | 105 | 0.73 (0.53–1.03) | 74 | 0.81 (0.53–1.24) | 87 | 0.66 (0.45–0.95) | 92 | 0.95 (0.65–1.38) | |||||
| T3 | 109 | 0.81 (0.58–1.13) | 0.32 | 77 | 0.74 (0.48–1.13) | 0.19 | 90 | 0.58 (0.40–0.83) | 0.006 | 96 | 1.13 (0.77–1.65) | 0.46 | |
| p for interaction = 0.58 | p for interaction = 0.05 | ||||||||||||
| Red meat (servings/day) |
T1 | 214 | Ref | 130 | Ref | 194 | Ref | 150 | Ref | ||||
| T2 | 208 | 0.93 (0.70–1.24) | 132 | 1.00 (0.69–1.43) | 170 | 0.73 (0.53–1.00) | 170 | 1.28 (0.93–1.77) | |||||
| T3 | 216 | 0.96 (0.69–1.32) | 0.81 | 157 | 1.24 (0.83–1.84) | 0.27 | 191 | 0.83 (0.58–1.18) | 0.38 | 182 | 1.44 (1.00–2.06) | 0.05 | |
| p for interaction = 0.57 | p for interaction = 0.39 | ||||||||||||
Multivariate (MV) models were adjusted for the same covariates as in Table 2.
MDM levels were inversely associated with breast cancer risk among CYP1A2 AA carriers (OR=0.58; 95% CI=0.40–0.83; p for trend=0.006) but were not associated with risk among women carrying the C allele of CYP1A2 (OR=1.13; 95% CI=0.77–1.65; p for trend=0.46, p for interaction =0.05). There were no interactions between the CYP1A2 genotype and HCAs and red meat intake in relation to breast cancer. Among those who have the C allele of CYP1A2, there was a positive association between red meat intake and the risk of breast cancer (highest vs. lowest tertile, OR=1.44; 1.00–2.06; p for trend=0.05).
As a secondary analysis, we further adjusted for fruit and vegetable intakes, caffeine intake, and physical activity in the multivariate models, but the results remained essentially unchanged (data not shown).
Furthermore, we evaluated the interactions between genotypes of the xenobiotic enzymes and intakes of HCAs, MDM, and red meat in relation to risk of hormone receptor positive breast cancer which made up the majority of breast cancers (Table 4). The results were overall similar to those for total breast cancer cases. The interaction between NAT2 genotype and intake of HCAs, MDM, and red meat had no effect on the risk of ER+/PR+ breast cancer. Similarly, the associations between red meat intake and ER+/PR+ breast cancer were not significantly modified by CYP1A2 polymorphism. MeIQx intake showed a significant interaction with CYP1A2 for ER+/PR+ breast cancer (highest vs. lowest tertile of MeIQx: OR=0.54 [95 % CI=0.34–0.85, p for trend=0.01] in AA genotype and OR= 1.43 [95% CI=0.90–2.27, p for trend=0.15] in AC/CC genotype; p for interaction = 0.03). Although the interaction between red meat intake and CYP1A2 genotype on the risk of ER+/PR+ breast cancer was not significant (p=0.19), red meat intake was positively associated with the risk of ER+/PR+ breast cancer among the AC/CC genotype (highest vs. lowest: OR= 1.56; 95%CI=1.03–2.37, p for trend=0.04).
Table 4.
The association between HCA intake and postmenopausal ER+/PR+ breast cancer by NAT2 or CYP1A2 genotype
| Variablem | Rank | NAT2 | CYP 1A2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slow acetylator | Fast acetylator | AA | AC/CC | ||||||||||
| Tertile | Cases | MV OR* | p for trend |
Cases | MV OR* | p for trend |
Cases | MV OR* | P for trend |
Cases | MV OR* | p for trend |
|
| PhIP (ng/day) |
T1 | 72 | Ref | 54 | Ref | 65 | Ref | 61 | Ref | ||||
| T2 | 73 | 1.00 (0.68–1.49) | 46 | 0.71 (0.43–1.17) | 56 | 0.78 (0.50–1.21) | 63 | 0.98 (0.63–1.51) | |||||
| T3 | 73 | 0.95 (0.63–1.42) | 0.76 | 51 | 0.69 (0.42–1.14) | 0.22 | 61 | 0.81 (0.52–1.26) | 0.46 | 63 | 0.86 (0.55–1.34) | 0.47 | |
| p for interaction = 0.27 | p for interaction = 0.69 | ||||||||||||
| MeIQx (ng/day ) |
T1 | 82 | Ref | 43 | Ref | 76 | Ref | 49 | Ref | ||||
| T2 | 64 | 0.76 (0.51–1.14) | 50 | 1.05 (0.64–1.74) | 52 | 0.62 (0.40–0.95) | 62 | 1.27 (0.80–2.00) | |||||
| T3 | 72 | 0.73 (0.48–1.09) | 0.15 | 58 | 1.15 (0.69–1.93) | 0.58 | 54 | 0.54 (0.34–0.85) | 0.01 | 76 | 1.43 (0.90–2.27) | 0.15 | |
| p for interaction = 0.34 | p for interaction = 0.03 | ||||||||||||
| DiMeIQx (ng/day ) |
T1 | 80 | Ref | 43 | Ref | 70 | Ref | 53 | Ref | ||||
| T2 | 69 | 0.81 (0.54–1.20) | 52 | 1.12 (0.68–1.84) | 57 | 0.77 (0.50–1.19) | 64 | 1.10 (0.70–1.73) | |||||
| T3 | 69 | 0.70 (0.46–1.05) | 0.10 | 56 | 1.07 (0.65–1.77) | 0.86 | 55 | 0.66 (0.42–1.04) | 0.09 | 70 | 1.07 (0.68–1.68) | 0.83 | |
| p for interaction = 0.42 | p for interaction = 0.40 | ||||||||||||
| Meat derived mutagenicity (MDM) (revertant colonies/day) |
T1 | 82 | Ref | 52 | Ref | 77 | Ref | 57 | Ref | ||||
| T2 | 67 | 0.76 (0.51–1.14) | 53 | 0.90 (0.56–1.45) | 51 | 0.64 (0.41–0.99) | 69 | 1.09 (0.71–1.67) | |||||
| T3 | 69 | 0.84(0.57–1.25) | 0.51 | 46 | 0.67 (0.41–1.10) | 0.10 | 54 | 0.57 (0.37–0.88) | 0.02 | 61 | 1.08 (0.69–1.68) | 0.80 | |
| p for interaction = 0.27 | p for interaction = 0.14 | ||||||||||||
| Red meat ( servings/day) |
T1 | 122 | Ref | 76 | Ref | 112 | Ref | 86 | Ref | ||||
| T2 | 111 | 0.86 (0.61–1.20) | 78 | 1.05 (0.69–1.61) | 88 | 0.67 (0.46–0.97) | 101 | 1.29 (0.88–1.88) | |||||
| T3 | 128 | 1.02 (0.70–1.50) | 0.84 | 99 | 1.32 (0.83–2.08) | 0.23 | 106 | 0.81 (0.54–1.23) | 0.42 | 121 | 1.56 (1.03–2.37) | 0.04 | |
| p for interaction = 0.53 | p for interaction = 0.19 | ||||||||||||
Multivariate (MV) models were adjusted for the same covariates as in Table 2.
We were not able to evaluate the interaction among participants with other ER/PR status because of the small sample sizes.
Discussion
The results from this study do not support the contention that NAT2 and CYP1A2 genotypes modify associations between intakes of red meat or meat-related mutagens and risk of breast cancer in postmenopausal women.
The possible involvement of the NAT2 enzyme in the activation and detoxification of carcinogens has been suggested in animal and in vitro studies (34). It is plausible that genetic polymorphisms of HCA-metabolizing enzymes can influence how HCAs affect breast cancer risk (10, 35–37). The NAT2 fast acetylator phenotype could lead to the formation of DNA-bound adducts from O-acetylated HCAs, which might be considered a carcinogenic process (9). Compared to rats with the slow acetylator genotype, those with the rapid acetylator genotype have higher O-acetylation in mammary epithelial cells (38). Also, among consumers of well-done meat, women with the fast NAT2 genotype had higher levels of PhIP-DNA adducts in breast tissue than women with the slow NAT2 genotype (39). However, two recent meta-analyses found that the NAT2 polymorphism was not associated with breast cancer risk (40, 41).
Another major enzyme related to the metabolism of HCAs is CYP1A2, whose inducibility and activity may be reduced by 164 A/C polymorphism, which has been associated with increased risk of colorectal adenoma and cancer (33, 42). However, in a recent meta-analysis of 7,580 breast cancer cases, no association between the polymorphism and breast cancer risk was detected (43).
Neither the NAT2 genotype nor the CYP1A2 polymorphism was associated with the risk of postmenopausal breast cancer in our study population, consistent with the results from previous meta-analyses (40, 41, 43).
We found no evidence of interaction between NAT2 genotype and intake of HCAs, MDM, and red meat in relation to risk of breast cancer. Our finding for red meat was consistent with the previous nested case-control studies in the NHS (14), Iowa Women’s Health Study (16), and population-based case-control studies with over 1000 cases (12, 13). In our study, 26% of the cases were also included in the previous study in the NHS (14). However, a recent nested case-control study within a Danish prospective cohort found a significant interaction between NAT2 genotype and red meat intake for postmenopausal breast cancer, and found higher risk among subjects with the fast acetylator genotype (11). The study used a comprehensive 192-item FFQ with a total of 63 foods and recipes in the FFQ to cover intake of individual meat items and mixed-dishes containing meat. For individual HCAs and MDM intake, few studies have examined the interaction with NAT2 genotype, and no interactions between either HCAs (12, 15) or MDM (12) and NAT2 genotype were found on breast cancer risk, consistent with our findings.
To our knowledge, no study has examined the interaction between CYP1A2 164 A/C polymorphisms and HCAs intake on breast cancer risk. Studies that have assessed the interaction between the polymorphisms and HCAs or MDM on colorectal adenoma or other cancers including colorectal, stomach and pancreas did not find any evidence of interactions (44–47). Similarly, there was no interaction between red meat intake and the polymorphism on the risk of colorectal polyps (47). We found a marginal interaction between the polymorphism and MDM intake with regard to the risk of breast cancer but it was mostly due to an inverse association between MDM intake and breast cancer among those with AA genotype, which was hard to interpret. Further investigation is warranted for this association.
One study examined interactions between NAT2 genotype and red meat intake in relation to breast cancer by ER and PR status and found no significant interactions (13). No previous studies have examined the possible role of hormone receptor status in the interactions between either HCAs or red meat and CYP1A2 polymorphisms on breast cancer risk. In our study, the results for ER+/PR+ breast cancer were similar to those for total breast cancer cases except that there was an interaction between MeIQx and CYP1A2 polymorphism. However, again, the interaction was mostly due to an inverse association between MeIQx intake and breast cancer among those with AA genotype, which is hard to interpret because we hypothesized positive association between HCA intake and breast cancer risk. For red meat intake, there was no interaction with CYP1A2 polymorphism on the risk of ER+/PR+ breast cancer, however, among those with CYP1A2 164 AC/CC genotype (slow metabolizer), we found a significant positive association between red meat intake and ER+/PR+ breast cancer. Red meat intake has been associated with hormone receptor positive breast cancer risk (18). Further large scale studies are warranted to evaluate these associations.
Even though our study had several strengths including its prospective design and the ability to adjust for multiple potential confounders, there were some limitations. First, we did not examine genetic variants of genes encoding other enzymes also involved in HCA metabolism such as CYP1A1, NAT1, sulfotransferase (SULT), and gluthathionine S-transferase (GST) (48). Second, our sample size was relatively small for some of the analyses, especially for those by hormone receptor status. Third, intake ranges of red meat as well as HCAs were somewhat limited, which might have contributed to the null findings. Fourth, our HCA intake estimate is likely to include the measurement error pertinent to any nutritional epidemiology study where estimates are based on memory and ability of individuals to recall usual intake over a given period. Other sources of possible misclassification of exposure include the lack of information on possible modifications to the cooking method used, such as microwaving meat before cooking (49), marinating meats (50), or frequency of flipping meats while cooking (51), which could alter HCAs or MDM levels. In addition, HCAs and MDM intakes were estimated using a one-time measurement, and we did not assess changes in intake over time. Finally, our questionnaire only uses information on meat cooking practices: cooking methods and doneness. We were not able to include pictures of different level of doneness in this cohort, which according to a recent study, may have improved our estimates (52). Several studies have examined hair, DNA adducts, protein adducts as potential biomarkers for long-term HCA intake; but a long-term biomarker has yet to be established (53–57). Therefore, to overcome the various issues of measurement error, it is crucial to develop good long-term biomarkers of HCAs.
In conclusion, the results from our study indicate that genetic polymorphisms of xenobiotic enzymes involved in the metabolism of HCAs, i.e., NAT2 and CYP1A2, may not modify associations between intakes of red meat or meat-related mutagens and risk of postmenopausal breast cancer.
ACKNOWLEDGEMENTS
The authors are indebted to Rong Tilney for computer support. In addition, we would like to thank the participants and staff of the Nurses' Health Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL,IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY
Grant: this work was supported by a grant from the American Institute for Cancer Research as well as grant CA 87969 from the National Cancer Institute, National Institutes of Health
References
- 1.Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, et al. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16:39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- 2.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyderwine EG. Mammary gland carcinogenesis by food-derived heterocyclic amines: Metabolism and additional factors influencing carcinogenesis by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Environ Mol Mutagen. 2002;39:165–170. doi: 10.1002/em.10053. [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Migita H, Ohgaki H, Sato S, Takayama S, et al. Induction of tumors in the Zymbal gland, oral cavity, colon, skin and mammary gland of F344 rats by a mutagenic compound, 2-amino-3, 4-dimethylimidazo[4, 5-f)quinoline. Carcinogenesis. 1989;10:601–603. doi: 10.1093/carcin/10.3.601. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, Lee S-A. Well-Done Meat Intake, Heterocyclic Amine Exposure, and Cancer Risk. Nutr Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu K, Sinha R, Holmes MD, Giovannucci E, Willett WC, et al. Meat Mutagens and Breast Cancer in Postmenopausal Women—A Cohort Analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1301–1310. doi: 10.1158/1055-9965.EPI-10-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 8.Turesky RJ. The Role of Genetic Polymorphisms in Metabolism of Carcinogenic Heterocyclic Aromatic Amines. Curr Drug Metab. 2004;5:169–180. doi: 10.2174/1389200043489036. [DOI] [PubMed] [Google Scholar]
- 9.Minchin RF, Reeves PT, Teitel CH, McManus ME, Mojarrabi B, et al. N- and O-acetylation of aromatic and heterocyclic amine carcinogens by human monomorphic and polymorphic acetyltransferases expressed in COS-1 cells. Biochem. Biophys. Res. Commun. 1992;185:839–844. doi: 10.1016/0006-291x(92)91703-s. [DOI] [PubMed] [Google Scholar]
- 10.Stillwell WG, Turesky RJ, Sinha R, Tannenbaum SR. N-Oxidative Metabolism of 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in Humans Excretion of the N2-Glucuronide Conjugate of 2-Hydroxyamino-MeIQx in Urine. Cancer Res. 1999;59:5154–5159. [PubMed] [Google Scholar]
- 11.Egeberg R, Olsen A, Autrup H, Christensen J, Stripp C, et al. Meat consumption, N-acetyl transferase 1 and 2 polymorphism and risk of breast cancer in Danish postmenopausal women. Eur J Cancer Prev. 2008;17:39–47. doi: 10.1097/CEJ.0b013e32809b4cdd. [DOI] [PubMed] [Google Scholar]
- 12.Mignone L, Giovannucci E, Newcomb P, Titus-Ernstoff L, Trentham-Dietz A, et al. Meat Consumption, Heterocyclic Amines, NAT2, and the Risk of Breast Cancer. Nutr Cancer. 2009;61:36–46. doi: 10.1080/01635580802348658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabstein S, Brüning T, Harth V, Fischer H-P, Haas S, et al. N-acetyltransferase 2, exposure to aromatic and heterocyclic amines, and receptor-defined breast cancer. Eur J Cancer Prev. 2010;19:100–109. doi: 10.1097/CEJ.0b013e328333fbb7. [DOI] [PubMed] [Google Scholar]
- 14.Gertig DM, Hankinson SE, Hough H, Spiegelman D, Colditz GA, et al. N-acetyl transferase 2 genotypes, meat intake and breast cancer risk. Int J Cancer. 1999;80:13–17. doi: 10.1002/(sici)1097-0215(19990105)80:1<13::aid-ijc3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Delfino RJ, Sinha R, Smith C, West J, White E, et al. Breast cancer, heterocyclic aromatic amines from meat and N-acetyltransferase 2 genotype. Carcinogenesis. 2000;21:607–615. doi: 10.1093/carcin/21.4.607. [DOI] [PubMed] [Google Scholar]
- 16.Deitz AC, Zheng W, Leff MA, Gross M, Wen W-Q, et al. N-Acetyltransferase-2 Genetic Polymorphism, Well-done Meat Intake, and Breast Cancer Risk among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2000;9:905–910. [PubMed] [Google Scholar]
- 17.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk Factors for Breast Cancer According to Estrogen and Progesterone Receptor Status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 18.Cho E, Chen WY, Hunter DJ, Stampfer MJ, Colditz GA, et al. Red Meat Intake and Risk of Breast Cancer Among Premenopausal Women. Arch Intern Med. 2006;166:2253–2259. doi: 10.1001/archinte.166.20.2253. [DOI] [PubMed] [Google Scholar]
- 19.Lauber SN, Ali S, Gooderham NJ. The cooked food derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine is a potent oestrogen: a mechanistic basis for its tissue-specific carcinogenicity. Carcinogenesis. 2004;25:2509–2517. doi: 10.1093/carcin/bgh268. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C, Shan L, Yu M, Snyderwine EG. Steroid hormone receptor expression and proliferation in rat mammary gland carcinomas induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Carcinogenesis. 2005;26:763–769. doi: 10.1093/carcin/bgi013. [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 24.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, et al. Food-Based Validation of a Dietary Questionnaire: The Effects of Week-to-Week Variation in Food Consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 25.Byrne C, Sinha R, Platz EA, Giovannucci E, Colditz GA, et al. Predictors of dietary heterocyclic amine intake in three prospective cohorts. Cancer Epidemiol Biomarkers Prev. 1998;7:523–529. [PubMed] [Google Scholar]
- 26.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 2002;506–507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 27.Institute NC. CHARRED (Computerized Heterocyclic Amine Database Resource for Research in Epidemiologic Disease) Available from: http://dceg.cancer.gov/neb/tools/charred.
- 28.Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975;31:347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 29.Knize MG, Sinha R, Rothman N, Brown ED, Salmon CP, et al. Heterocyclic amine content in fast-food meat products. Food Chem Toxicol. 1995;33:545–551. doi: 10.1016/0278-6915(95)00025-w. [DOI] [PubMed] [Google Scholar]
- 30.Gross GA, Gruter A. Quantitation of mutagenic/carcinogenic heterocyclic aromatic amines in food products. J Chromatogr. 1992;592:271–278. doi: 10.1016/0021-9673(92)85095-b. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute. CHARRED (Computerized Heterocyclic Amine Database Resource for Research in Epidemiologic Disease) Available from : http://wwwcharredcancergov.
- 32.Chan AT, Tranah GJ, Giovannucci EL, Willett WC, Hunter DJ, et al. Prospective study of N-acetyltransferase-2 genotypes, meat intake, smoking and risk of colorectal cancer. Int J Cancer. 2005;115:648–652. doi: 10.1002/ijc.20890. [DOI] [PubMed] [Google Scholar]
- 33.Moonen H, Engels L, Kleinjans J, Kok Td. The CYP1A2-164A-->C polymorphism (CYP1A2*1F) is associated with the risk for colorectal adenomas in humans. Cancer Lett. 2005;229:25–31. doi: 10.1016/j.canlet.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Boukouvala S, Fakis G. Arylamine N-Acetyltransferases: What We Learn from Genes and Genomes. Drug Metab Rev. 2005;37:511–564. doi: 10.1080/03602530500251204. [DOI] [PubMed] [Google Scholar]
- 35.Stillwell WG, Turesky RJ, Sinha R, Skipper PL, Tannenbaum SR. Biomonitoring of heterocyclic aromatic amine metabolites in human urine. Cancer Lett. 1999;143:145–148. doi: 10.1016/s0304-3835(99)00144-5. [DOI] [PubMed] [Google Scholar]
- 36.Felton JS, Knize MG, Salmon CP, Malfatti MA, Kulp KS. Human exposure to heterocyclic amine food mutagens/carcinogens: Relevance to breast cancer. Environ Mol Mutagen. 2002;39:112–118. doi: 10.1002/em.10070. [DOI] [PubMed] [Google Scholar]
- 37.Stillwell WG, Sinha R, Tannenbaum SR. Excretion of the N2-glucuronide conjugate of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine in urine and its relationship to CYP1A2 and NAT2 activity levels in humans. Carcinogenesis. 2002;23:831–838. doi: 10.1093/carcin/23.5.831. [DOI] [PubMed] [Google Scholar]
- 38.Jefferson FA, Xiao GH, Hein DW. 4-Aminobiphenyl Downregulation of NAT2 Acetylator Genotype–Dependent N- and O-acetylation of Aromatic and Heterocyclic Amine Carcinogens in Primary Mammary Epithelial Cell Cultures from Rapid and Slow Acetylator Rats. Toxicol Sci. 2009;107:293–297. doi: 10.1093/toxsci/kfn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Chang P, Bondy ML, Sahin AA, Singletary SE, et al. Detection of 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]-Pyridine-DNA Adducts in Normal Breast Tissues and Risk of Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:830–837. [PubMed] [Google Scholar]
- 40.Ochs-Balcom HM, Wiesner G, Elston RC. A Meta-Analysis of the Association of N-Acetyltransferase 2 Gene (NAT2) Variants with Breast Cancer. Am J Epidemiol. 2007;166:246–254. doi: 10.1093/aje/kwm066. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Qiu L-X, Wang Z-H, Wang J-L, He S-S, et al. NAT2 polymorphisms combining with smoking associated with breast cancer susceptibility: a meta-analysis. Breast Cancer Res Treat. 2010;123:877–883. doi: 10.1007/s10549-010-0807-1. [DOI] [PubMed] [Google Scholar]
- 42.Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, et al. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15:535–546. doi: 10.1097/01.fpc.0000165904.48994.3d. [DOI] [PubMed] [Google Scholar]
- 43.Qiu L-X, Yao L, Mao C, Yu K-D, Zhan P, et al. Lack of association of CYP1A2-164 A/C polymorphism with breast cancer susceptibility: a meta-analysis involving 17,600 subjects. Breast Cancer Res Treat. 2010;122:521–525. doi: 10.1007/s10549-009-0731-4. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, et al. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of colorectal cancer: a hospital-based case-control study in Japan. Scand J Gastroenterol. 2009;44:952–959. doi: 10.1080/00365520902964721. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, et al. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of stomach cancer: a hospital-based case-control study in Japan. Gastric Cancer. 2009;12:198–205. doi: 10.1007/s10120-009-0523-x. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Morris JS, Li Y, Doll MA, Hein DW, et al. Interaction of the cytochrome P4501A2, SULT1A1 and NAT gene polymorphisms with smoking and dietary mutagen intake in modification of the risk of pancreatic cancer. Carcinogenesis. 2008;29:1184–1191. doi: 10.1093/carcin/bgn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin A, Shrubsole MJ, Rice JM, Cai Q, Doll MA, et al. Meat Intake, Heterocyclic Amine Exposure, and Metabolizing Enzyme Polymorphisms in Relation to Colorectal Polyp Risk. Cancer Epidemiol Biomarkers Prev. 2008;17:320–329. doi: 10.1158/1055-9965.EPI-07-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha R, Caporaso N. Diet, genetic susceptibility and human cancer etiology. J Nutr. 1999;129:556S–559S. doi: 10.1093/jn/129.2.556S. [DOI] [PubMed] [Google Scholar]
- 49.Felton JS, Fultz E, Dolbeare FA, Knize MG. Effect of microwave pretreatment on heterocyclic aromatic amine mutagens/carcinogens in fried beef patties. Food Chem Toxicol. 1994;32:897–903. doi: 10.1016/0278-6915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 50.Salmon CP, Knize MG, Felton JS. Effects of marinating on heterocyclic amine carcinogen formation in grilled chicken. Food Chem Toxicol. 1997;35:433–441. doi: 10.1016/s0278-6915(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 51.Tran NL, Salmon CP, Knize MG, Colvin ME. Experimental and simulation studies of heat flow and heterocyclic amine mutagen/carcinogen formation in pan-fried meat patties. Food Chem Toxicol. 2002;40:673–684. doi: 10.1016/s0278-6915(01)00126-0. [DOI] [PubMed] [Google Scholar]
- 52.Keating GA, Sinha R, Layton D, Salmon CP, Knize MG, et al. Comparison of heterocyclic amine levels in home-cooked meats with exposure indicators (United States) Cancer Causes Control. 2000;11:731–739. doi: 10.1023/a:1008935407971. [DOI] [PubMed] [Google Scholar]
- 53.Alexander J, Reistad R, Hegstad S, Frandsen H, Ingebrigtsen K, et al. Biomarkers of exposure to heterocyclic amines: approaches to improve the exposure assessment. Food Chem Toxicol. 2002;40:1131–1137. doi: 10.1016/s0278-6915(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 54.Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol. 2011;24:1169–214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bessette EE, Yasa I, Dunbar D, Wilkens LR, Le Marchand L, Turesky RJ. Biomonitoring of carcinogenic heterocyclic aromatic amines in hair: a validation study. Chem Res Toxicol. 2009;22:1454–1463. doi: 10.1021/tx900155f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magagnotti C, Orsi F, Bagnati R, Celli N, Rotilio D, et al. Effect of diet on serum albumin and hemoglobin adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Int J Cancer. 2000;88:1–6. doi: 10.1002/1097-0215(20001001)88:1<1::aid-ijc1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 57.Dingley KH, Curtis KD, Nowell S, Felton JS, Lang NP, et al. DNA and protein adduct formation in the colon and blood of humans after exposure to a dietary-relevant dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Epidemiol Biomarkers Prev. 1999;8:507–512. [PubMed] [Google Scholar]