Abstract
Rationale
Cigarette smokers smoke in part because nicotine helps regulate attention. Prepulse inhibition (PPI) of the startle reflex is a measure of early attentional gating that is reduced in abstinent smokers and in groups with attention regulation difficulties. Attention difficulties are found in people with posttraumatic stress disorder (PTSD).
Objectives
The aim of this study is to assess whether smoking and abstinence differentially affect the startle response and PPI in smokers with and without PTSD.
Methods
Startle response and PPI (prepulses at 60, 120, or 240 ms) were measured in smokers with (N=39) and without (N=61) PTSD, while smoking and again while abstinent.
Results
Participants with PTSD produced both larger magnitude and faster latency startle responses than controls. Across groups, PPI was greater when smoking than when abstinent. The PTSD and control group exhibited different patterns of PPI across prepulse intervals when smoking and when abstinent. Older age was associated with reduced PPI, but only when abstinent from smoking.
Conclusions
The effects of PTSD on startle magnitude and of smoking on PPI replicate earlier studies. The different pattern of PPI exhibited in PTSD and control groups across prepulse intervals, while smoking and abstinent suggests that previous research on smoking and PPI has been limited by not including longer prepulse intervals, and that nicotine may affect the time course as well as increasing the level of PPI. The reduced PPI among older participants during abstinence suggests that nicotine may play a role in maintaining attention in older smokers, which may motivate continued smoking in older individuals.
Keywords: Smoking, Abstinence, Prepulse inhibition, Attention, Acoustic startle reflex, Posttraumatic stress disorder, Aging
Cigarette smoking is a major public health problem in the US and around the world. Smoking tends to be especially prevalent among people with comorbid psychopathology: between 50 and 80 % of those suffering from a mental illness are smokers, compared to fewer than 40 % of those who have never had mental illness (Lasser et al. 2000). Smoking is prevalent among people diagnosed with posttraumatic stress disorder (PTSD), with population-based estimates suggesting that the smoking rate among people diagnosed with PTSD is 45 %, compared to the rate of 23 % found in the general population (Lasser et al. 2000). The smoking rate is as high as 60 % among patients who seek help for PTSD (Beckham et al. 1997; Beckham et al. 1995).
There are many potential reasons for the higher prevalence of smoking among individuals with PTSD. For example, like virtually all smokers (Kassel et al. 2003), patients with PTSD report that smoking cigarettes reduces their anxiety (Beckham et al. 2007). Compared to non-PTSD smokers, negative affect and PTSD symptoms are significant antecedents to smoking among PTSD smokers (Beckham et al. 2005). Furthermore, smoking urges, negative affect, and PTSD symptoms are increased in response to trauma-related cues, and distressing symptoms are decreased in smokers with and without PTSD after smoking a cigarette with or without nicotine (Beckham et al. 2007).
Another possible reason is that nicotine helps to manage the cognitive and attentional deficits found in PTSD. PTSD has been associated with neuropsychological and attentional deficits (Beckham et al. 1998; Vasterling et al. 1998). Nicotine’s attention-enhancing properties are well-known and extensively studied in both humans and animals (Bushnell et al. 2000). Cognitive and attention deficits have been documented as well in psychiatric disorders such as schizophrenia (Levin et al. 1996) and attention deficit hyperactivity disorder (Ashare and Hawk Jr. 2012). Further, nicotine withdrawal may exacerbate cognitive and attentional problems, which may contribute to smoking relapse (Heishman 1998; 1999).
For over 40 years, prepulse inhibition (PPI) of the acoustic startle reflex response has been used to evaluate sensorimotor gating of attention in humans and other animals (Graham 1975). The startle response occurs to an intense and sudden-onset stimulus, and PPI refers to a reduction in the magnitude of that response when a weaker stimulus (prepulse) occurs between 30–500 ms before the startling stimulus. Inhibition of the startle reflex is thought to reflect protection of the prepulse information, so that it can be adequately processed; thus, prepulse inhibition is considered an indication of early automatic attention regulation of environmental stimuli (Braff et al. 2001). Persons with difficulty regulating early attentional processes, such as persons with schizophrenia, exhibit reduced PPI (Braff et al. 2001).
The literature clearly implicates nicotine in PPI modulation. Although some sex differences in the effect of nicotine have been reported (Ashare and Hawk Jr. 2012; Della Casa et al. 1998), smokers generally exhibit greater PPI after smoking than when abstinent (Ashare and Hawk Jr. 2012; Duncan et al. 2001; Hutchinson et al. 2000; Kumari et al. 1996), and a nicotine injection increases PPI in healthy nonsmokers and smokers (Kumari et al. 1997; Postma et al. 2006). Further, smokers who are more dependent on nicotine (as measured by the Fagerstrom test for nicotine dependence) exhibit less PPI during smoking withdrawal than less-dependent smokers (Kumari and Gray 1999), implicating nicotine use in moderating sensorimotor gating deficits among smokers.
A diagnostic characteristic of PTSD is hypervigilance and an exaggerated startle response (American Psychiatric Association 1994). The evidence for larger magnitude startle responses in those with PTSD is mixed, with findings of larger (Butler et al. 1990; Grillon et al. 1998a; Holstein et al. 2010), smaller (Ornitz and Pynoos 1989), or equivalent (Grillon et al. 1996; Jovanovic et al. 2009; Lipschitz et al. 2005) startle responses in people with PTSD compared to control groups and a significant but small meta-analytic effect size (ES=.13) (Pole 2007). The evidence for diminished PPI in people with PTSD is also mixed. Ornitz and Pynoos (1989) found PPI deficits in children with PTSD. Grillon and colleagues (1996; 1998b) found deficits in Vietnam combat veterans, and more severe PTSD symptoms were correlated with reduced PPI. Two other studies (Holstein et al. 2010; Lipschitz et al. 2005) found reduced PPI in a group with PTSD compared to a non-PTSD group, though these results were not statistically significant. Studies that have not found reduced PPI in a PTSD sample have generally employed a trauma-exposed control (Butler et al. 1990) or fearful stimuli (Grillon et al. 1998a; 1998b), thus it might be trauma exposure or stress that is responsible for diminished PPI, a speculation supported by animal studies (Bakshi et al. 2007). A recent review stated that “drawing conclusions about PPI in patients with PTSD is not possible yet” (Kohl et al. 2013).
It may be that people with deficits in automatically gating out sensory stimuli benefit more from nicotine than do others. Individuals with chronic schizophrenia have difficulty with sensorimotor gating, manifested in reduced PPI, among other measures (Braff and Geyer 1990). In a naturalistic experiment, individuals with schizophrenia who smoked right before having their PPI measured had greater PPI than nonsmoking individuals with schizophrenia, or individuals with schizophrenia who smoked but were abstinent immediately before the PPI measurement (Kumari et al. 2001). In a more controlled study, smoking abstinence reduced PPI, and smoking reinstated PPI, to a much greater extent in smokers with schizophrenia than in controls (George et al. 2006). Smokers with schizophrenia had PPI comparable to nonschizophrenic smokers, whereas among nonsmokers, individuals with schizophrenia exhibited a PPI deficit compared to nonschizophrenics (Woznica et al. 2009). An fMRI study suggested that brain circuits regulating PPI were more sensitive to a nicotine injection in patients with schizophrenia than nonpatient controls (Postma et al. 2006). Thus, it appears that smoking has a greater effect on PPI among smokers with schizophrenia than those without schizophrenia, raising the possibility that the smoking rate among individuals with schizophrenia is due in part to its enhancement of sensorimotor gating mechanisms. It is not known, whether the sensorimotor gating deficits in people with PTSD would be similarly improved by smoking.
Individuals with PTSD, like individuals with schizophrenia, have attention regulation deficits, exhibit diminished PPI, and smoke cigarettes at a higher rate than the general population. However, it is not known whether, like smokers with schizophrenia, smokers with PTSD are more sensitive to the sensorimotor gating effects of nicotine than smokers without PTSD. This study assessed startle response magnitude and latency and PPI of startle response magnitude in smokers with and without a current diagnosis of PTSD. All participants were tested under smoking and abstinent conditions. Because men generally have greater PPI than women (Braff et al. 2001; Swerdlow et al. 1999), and sex differences in the effect of nicotine on PPI have been reported (Ashare and Hawk Jr. 2012; Della Casa et al. 1998), participant sex was also investigated. It was hypothesized that participants would exhibit greater PPI after smoking than while abstinent; and further, this effect would be more prominent in smokers with PTSD than in smokers without PTSD.
Method
Participants
Thirty-nine smokers (24 women) with PTSD and 61 smokers (26 women) with no current PTSD diagnosis completed two startle assessment sessions as part of a larger study on PTSD and smoking cessation (Beckham et al. 2012). All participants provided informed consent before participating in this institutional review board-approved study. Study eligibility criteria included smoking at least ten cigarettes daily for the past year, willingness to make a smoking cessation attempt, and age between 18 and 65. Participants who met the criteria for current alcohol or other substance abuse or dependence, a current psychotic disorder (including schizophrenia), or bipolar disorder with active manic symptoms were excluded from both the PTSD and control groups. Additionally, potential participants were excluded from either group if they used noncigarette forms of nicotine (e.g., cigars, pipes, and chewing tobacco), had major unstable medical problems or major respiratory disorders, or used bupropion or benzodi-azepines (Grillon and Baas 2003). Trauma history and PTSD diagnosis were evaluated using the clinician-administered PTSD scale (Blake et al. 1995) and the Trauma Life Events Questionnaire (Kubany et al. 2000). The structured clinical interview for DSM-IV diagnosis (First et al. 1994) was administered to assess other Axis I disorders (kappa across 14 raters for diagnoses was .94).
Procedure
Following the screening session, participants completed two identical startle reflex response and PPI assessments separated by a week of electronic diary monitoring and two smoking cessation counseling sessions based on the NCI Fresh start program (Lando et al. 1990). A mean of 24.5 days (range 12–106) separated the two assessments. The second assessment occurred on the participant’s smoking quit date, with overnight abstinence (from 10 p.m. the night before) verified by a reduction of expired carbon monoxide based on a formula that takes into account baseline CO levels (Rose and Behm 2004). Each PPI session included 24 total startle trials: 6 startle-alone trials and 6 trials each with the startle preceded by a prepulse at 60, 120, and 240-ms stimulus onset interval. Each session began with a startle-alone trial and proceeded in one of three pseudorandom orders, with the constraint that no condition was repeated more than twice in a row, and each type of trial was represented roughly equally in the first and the second set of 12 trials in each of the three orders. Intertrial intervals averaged 25 s (range=17–51 s).
Assessment of acoustic startle response
Electromyographic (EMG) activity of the orbicularis oculi muscle was used as an index of the startle response. EMG was collected from Ag/AgCl electrodes filled with isotonic gel placed on the skin above the right orbicularis oculi, directly under the pupil and 10 mm lateral. A ground electrode was attached to the forehead. The signal was online-amplified (×1,000) and bandpass-filtered (5 kHz to 1.0 Hz). Signals were digitized at 250 Hz, passed to a PC-based BIOPAC MP100 data acquisition workstation, and saved to disk. The startle (100 dB (A), 50 ms) and prepulse stimuli (70 dB (A), 20 ms) were broadband white noise with instantaneous rise time presented binaurally through Sony model MDR-V600 headphones. Ambient noise was measured at roughly 55 dB (A) through the headphones. Signal levels were calibrated to a standardized tone prior to each session with a sound pressure meter fitted with a headphone adapter.
Data reduction and analyses
Raw EMG data was integrated (root mean square method) prior to scoring. Responses were considered valid if (1) the trial was scorable (stable baseline, no artifact), and (2) a deflection was observed beginning within 20–120 ms of startle onset. Response magnitude was calculated for each trial by subtracting mean baseline EMG for the 25 ms prior to startle onset from response peak. For trials with a valid response, response latency was calculated in millisecond as the difference between the beginning of the startle stimulus to the initiation of the response. For valid trials (stable baseline, no artifact) on which no response occurred, response magnitude was scored as zero, and latency was coded as missing. Mean response magnitude and latency were calculated separately for each startle condition (startle only, and 60, 120, and 240 ms prepulses intervals) in each session. In order to be included in data analyses, a participant needed to have a minimum of two valid data points in each cell of the startle condition × session design. This left 91 (34 PTSD) participants for response magnitude analyses. Because a zero response, which is common in PPI studies, results in missing latency data, there are more participants with fewer than two valid observations in each cell, resulting in 84 (33 PTSD) participants in the response latency analyses. PPI of response magnitude at each prepulse condition was calculated with the formula ((A−B)/A) × 100, where A = the startle only mean and B = the PPI condition mean, so that larger numbers indicate “more” prepulse inhibition. Maximum magnitude PPI is 100 %. To eliminate outliers, PPI values more than two standard deviations below the mean (−300 %) were coded as missing data.
The startle-alone magnitude and latency data were each analyzed using an ANOVA with session (smoking, abstinent) × PTSD status (PTSD, control) × gender. Magnitude data were log-transformed to normalize before analysis, but raw means are used for presentation purposes. Percent PPI data were analyzed with a PPI condition (60, 120, and 240 ms) × session (smoking, abstinent) × PTSD status (PTSD, control) × gender ANOVA. Because startle measures vary across the adult age range (Ellwanger et al. 2003; Ludewig et al. 2003), participant age was included as a covariate in all analyses. Greenhouse–Geisser corrected p values and epsilons are reported for effects of PPI condition to protect against sphericity.
Results
Participant characteristics
Characteristics of the participants at baseline are presented in Table 1. The groups did not differ on demographic variables (age, gender, minority status, education, employment status, marital status, and military veterans) or on baseline CO reading. However, compared to the group without PTSD, the group with PTSD reported more previous traumas, negative affect, past substance abuse or dependence, current depression and anxiety disorder diagnoses, and higher Davidson trauma scale and Fagerstrom test for nicotine dependence scores.
Table 1.
Demographics and clinical characteristics
| PTSD (n=39) | W/out PTSD (n=61) | Test statistic | |
|---|---|---|---|
| Gender (female) | 61.5 % (24) | 42.6 % (26) | χ2=3.40, p=.06, ns |
| Age (years) | 41.7 (11.3) | 42.3 (10.4) | t=−.31, p=.76, ns |
| Minority | 59.0 % (23) | 70.5 % (43) | χ2=1.40, p=.24, ns |
| Married | 18.0 % (7) | 14.8 % (9) | χ2=0.18, p=.67, ns |
| Education | 12.6 (1.9) | 12.9 (2.9) | t=.56, p=.57, ns |
| Employed | 51.3 % (20) | 55.7 % (34) | χ2=0.19, p=.66, ns |
| Veteran | 25.6 % (10) | 23.0 % (14) | χ2=.09, p=.76, ns |
| # Trauma types with fear, helplessness, or horror | 9.3 (3.4) | 3.8 (3.4) | t=−7.82, p<.0001 |
| Current MDD diagnosis | 30.8 % (12) | 1.6 % (1) | χ2=17.8, p=.001 |
| Current other anxiety disorder | 30.8 % (12) | 6.6 % (4) | χ2=10.38, p=.001 |
| Past substance abuse or dependence | 92 % (36) | 67.2 % (41) | χ2=8.4, p=.004 |
| FTND score | 6.1 (2.1) | 5.2 (2.0) | t=−2.02, p=.05 |
| Davidson trauma scale | 66.8 (25.0) | 19.1 (33.3) | t=−7.7, p=.0001 |
| Negative affect | 23.8 (8.7) | 15.7 (5.5) | t=−5.2, p=.0001 |
| Baseline CO reading | 25.5 (13.3) | 24.4 (11.3) | t=−0.44, p=.66, ns |
Startle-alone
Magnitude of response to the startle-alone stimuli was larger for participants with PTSD (M=0.045, SE=0.010) than for the control group (M=0.028, SE=0.008), F(1, 86)=7.51, p=.007, η2=.080. The PTSD × session interaction revealed a trend, F(1,86)=2.46, p=.121, η2=.028, that showed the difference in startle magnitude between the PTSD and control group was greater in the first (smoking) than the second (abstinence) session. In order to explore whether smoking differentially affected people with and without PTSD, the difference between smoking and abstinence sessions were explored for each group. Whereas the PTSD group showed slightly larger startle magnitude in the smoking compared to the abstinence session (M=.050 vs. .041), the control group exhibited slightly smaller startle magnitude in the smoking compared to the abstinence session (M=.027 vs. .031). No other effect of smoking session was significant, all Fs<0.5. Finally, younger participants had larger startle magnitude than older participants, Age F(1, 86)=5.04, p=.027, η2=.055, β=−0.012.
Matching the response magnitude results, latency of response onset to the startle-alone stimuli was faster for participants with PTSD (M=50 ms, SE=0.002) than for the control group (M=57 ms, SE=0.002), F(1, 80)=7.04, p=.010, η2=.082. A marginal age effect, F(1, 80)=3.04, p=.085, η2=.038, β=.0005, indicated that younger age was associated with shorter startle latency. No smoking session effects were significant, all Fs<1.5.
Prepulse inhibition
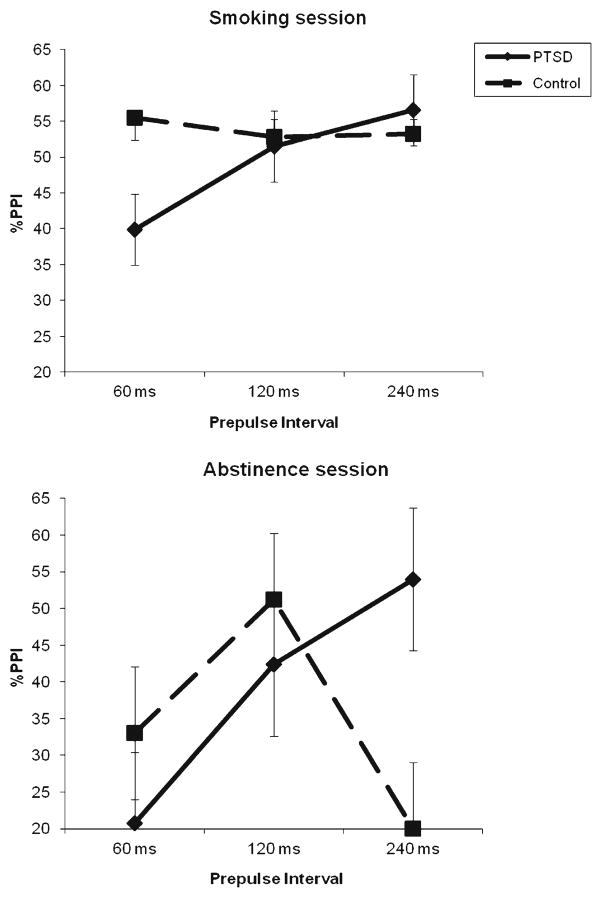
As expected, there was greater prepulse inhibition of startle magnitude in the smoking session (M=53.0 %, SE=6.63) than in the abstinence session (M=37.5 %, SE=6.8), session F(1, 71)=4.22, p=.044, η2=.056. The PTSD and control groups displayed different patterns of PPI across the three PPI conditions, PPI condition × PTSD status F(2, 142)=3.76, p=.026, ε=.992, η2=.050. The control group showed the pattern typically found in PPI studies, with maximum PPI at the 120 ms prepulse position, and the PTSD group exhibited linearly increasing PPI with longer prepulse–startle stimulus intervals, PPI condition × PTSD status linear F(1, 71)=6.75, p=.011, η2=.087. In order to explore whether this effect was influenced by smoking, separate PPI condition × PTSD status analyses of each session were conducted (see Fig. 1). These analyses revealed that the interaction was significant only for the abstinence session, F(2, 142)=3.96, p=.025, ε=.908, η2=.053. Follow-ups found the PTSD group exhibited nonsignificantly less PPI at 60 and 120 ms and significantly greater PPI at the 240 ms prepulse position. This pattern of results produced a trend in the session × PPI condition × PTSD status interaction, F(2, 142)=1.63, p=.201, ε=.941, η2=.022.
Fig 1.
Prepulse inhibition mean (and SE) during smoking and abstinence for the PTSD and the control group
Gender, like PTSD, interacted with the PPI pattern across sessions, session × PPI condition × gender F(2, 142)=3.41, p=.039, ε=.941, η2=.046, though the pattern was less easily explicable (see Table 2). Although men have greater PPI in nearly all conditions, as is typically found (Swerdlow et al. 1999), this difference is reversed at the 120 ms interval in the abstinence condition, resulting in significant (p<.03) quadratic trends, when each session was analyzed separately.
Table 2.
Mean (and SE) percent prepulse inhibition separately by gender, prepulse interval, and smoking session, with age controlled
| PPI interval | Males
|
Females
|
mean | ||
|---|---|---|---|---|---|
| Smoking | Abstinent | Smoking | Abstinent | ||
| 60 ms | 49.0 (11.5) | 41.4 (15.0) | 46.0 (10.7) | 9.8 (13.8) | 36.6 (6.5) |
| 120 ms | 71.2 (13.7) | 44.1 (11.2) | 37.0 (12.7) | 52.0 (10.3) | 51.1 (6.7) |
| 240 ms | 61.5 (11.8) | 47.5 (12.3) | 53.2 (10.9) | 30.0 (11.4) | 48.1 (6.5) |
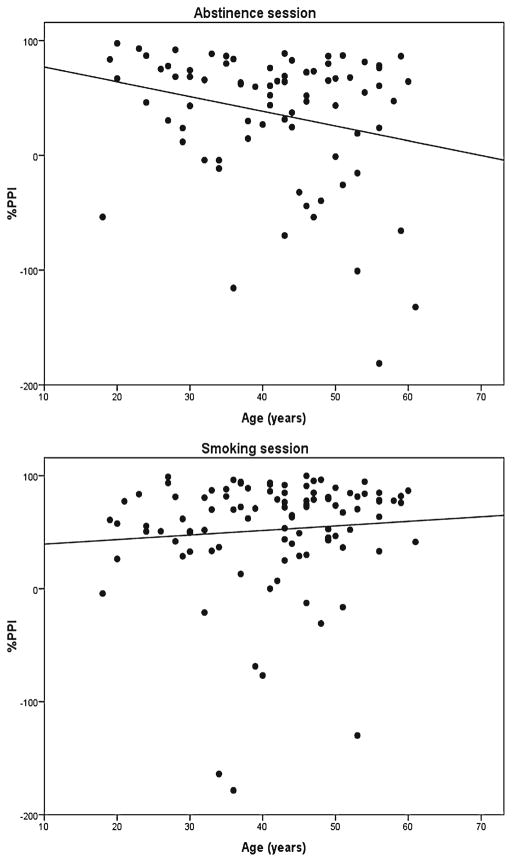
Figure 2 shows that the relationship between age and PPI differed depending on session, session × age F(1, 71)=6.65, p=.012, η2=.086. In the smoking session, there was a slight and nonsignificant trend toward more PPI with increasing age, F=0.72; however, in the abstinence session, PPI decreased with increasing age, F(1, 71)=5.51, p=.022, η2=.072, β=−1.37.
Fig 2.
The relationship between prepulse inhibition and age during smoking and abstinence
Discussion
The main purpose of this study was to determine whether there are sensorimotor gating differences (as measured by prepulse inhibition) between smokers with and without PTSD, and whether smoking differentially affects gating in individuals with PTSD. Results found that, when smoking, there is little difference in prepulse inhibition between individuals with and without PTSD, though those with PTSD have nonsignificantly less PPI (see left panel, Fig. 1). When abstinent from smoking, there is the expected PPI reduction for both groups, demonstrating nicotine withdrawal’s effect on sensorimotor gating. During abstinence, the group diagnosed with PTSD, however, had lower PPI at 60 and 120 ms prepulse latencies but significantly greater PPI at the 240 ms latency (see right panel, Fig. 1), suggesting that their sensorimotor gating mechanism is slower than the group without PTSD. In addition, the startle response in participants with PTSD was both larger in magnitude and faster in latency to initiate than the startle response in smokers without PTSD, an effect consistent with the diagnostic criteria of hypervigilance for PTSD (American Psychiatric Association 1994), and with a growing literature showing a small but consistent startle augmentation for those with PTSD (Pole 2007).
Despite the status of exaggerated startle response as a diagnostic criterion for PTSD and its significance in a meta-analytic comparison, greater startle response in a PTSD group compared to controls has not been consistently detected (Pole 2007). Larger startle response for the PTSD group compared to the control group was found in this study, with a small effect size consistent with the Pole (2007) meta-analysis; furthermore, shorter response latency, another measure of startle response augmentation that has rarely been reported in PTSD research, was also exhibited in the PTSD group. Increased startle responses are associated with hypervigilance to the environment and are seen in negative affective contexts (Vrana et al. 1988), and it has been suggested that the exaggerated startle seen in people with PTSD is due to their increased sensitivity to stressful environmental contexts (Grillon et al. 1998b).
Smoking tends to decrease startle magnitude compared to abstinence and sometimes results in significantly smaller startle response (Ashare and Hawk Jr. 2012), and this was the trend found in the control group in this study. It is notable that, contrary to the control group and to the general trend in the literature, the smokers with PTSD in this study sample exhibited slightly (though nonsignificantly) greater startle magnitude while smoking than when abstinent. This finding is consistent with our earlier data that, although people with PTSD smoke in response to negative affect and PTSD symptoms (Beckham et al. 2005), and report that smoking reduces their anxiety (Beckham et al. 2007), nicotine may actually increase negative arousal and be ineffective in reducing negative affect and PTSD symptoms (Beckham et al. 2007). Alternatively, it may be that, specifically for people with PTSD, startle magnitude was increased by contextual anxiety in the first (smoking) session, and this contextual anxiety (and thus startle magnitude) decreased by the second (abstinence) session (Grillon et al. 1998b).
Across the sample, prepulse inhibition was greater when smoking than when abstinent, consistent with the attention-enhancing properties of nicotine and the literature on smoking and prepulse inhibition (Duncan et al. 2001; Hutchinson et al. 2000; Kumari et al. 1996). Although smoking condition was confounded with session order in this study, with the smoking session always preceding the abstinence session, this effect has been consistently found, even when nicotine conditions are counterbalanced (Kumari et al. 1997). Further, there is little evidence of habituation of PPI across sessions (Duncan et al. 2001; Postma et al. 2001).
There was little evidence to support the hypotheses that people with PTSD would exhibit less PPI than controls and would be more susceptible to nicotine’s enhancing effect on PPI. The PTSD group displayed less PPI than the control group at all three prepulse intervals in the smoking session and two out of three prepulse intervals in the smoking abstinence session; however, these effects were very small and did not reach statistical significance either individually or overall. On the other hand, in the longest (240 ms) prepulse interval of the abstinence session, the PTSD group displayed significantly greater PPI than the control group. Greater PPI for people with PTSD has been found inconsistently (Pole 2007), and only with a control group that has not experienced trauma (Holstein et al. 2010; Lipschitz et al. 2005). Members of the control group in this study all reported trauma exposure, with an average of 3.8 different trauma types. Thus, the finding of nonsignificantly greater PPI in the control group compared to the PTSD group in five of six conditions is consistent with the existent literature.
Instead of finding the hypothesized less PPI and greater susceptibility to nicotine among people with PTSD than controls, we unexpectedly found a difference between the groups in the pattern of PPI over the 60, 120, and 240 ms prepulse intervals (see Fig. 1). Maximum PPI tends to occur at around the 120-ms interval, with slightly reduced PPI at the 240-ms interval (Braff et al. 2001). In this study, both the PTSD and control groups maintained or increased PPI through the 240-ms interval while smoking; whereas when abstinent, the PTSD group exhibited increased PPI from 120 to 240 ms, and the control group showed a sharp drop in PPI at 240 ms. Unfortunately, no other studies could be found examining the effect of nicotine on PPI intervals longer than 120 ms; furthermore, it should be noted that this pattern of data was not supported by a statistically significant three-way interaction. More research is needed to determine whether nicotine affects the time course as well as the overall level of PPI, and whether the increased PPI through 240 ms in the PTSD group involves a sensorimotor gating response that is slower than normal. This latter possibility is particularly intriguing, in that it may mean that people with PTSD need more time to process any new stimulus to assure that it is not a threat—stimuli with an affective content require more processing and produce PPI at longer intervals (Bradley et al. 1993). The longer processing time that people with PTSD take to process the neutral prepulse may mean that they automatically interpret any new stimulus as a threat.
Consistent with previous studies of startle response and other measures of neural speed and function during aging (Ellwanger et al. 2003; Kok 2000; Ludewig et al. 2003), the startle reflex response diminished in magnitude and latency in older participants. Prepulse inhibition did not diminish with age in the smoking session, replicating other studies of PPI in healthy adults across a similar age range (Ellwanger et al. 2003; Ludewig et al. 2003). However, PPI did decrease with age in the abstinence session. Thus, while healthy adults may not consistently show reduced sensorimotor gating with increased age, there may be a group that does exhibit decreased attentional gating capacity, and they may smoke in part because abstinence reduces this capacity (Kleykamp and Heishman 2011). Older unsuccessful quitters may be the smokers who see the greatest decline in their cognitive abilities and automatic stimulus processing when they make a quit attempt, or they find cessation more difficult, as they face an age-related decline in cognitive capacities.
Many studies of startle magnitude and PPI in people with PTSD have sampled only or primarily men, and this study expanded on previous work in examining men and women in relatively equal numbers. One limitation in this regard, however, is that no information was collected from female participants on the current phase of their menstrual cycle, with women exhibiting a reduction of PPI in the luteal versus follicular phase of the menstrual cycle (Swerdlow et al. 1997). Since the majority of female participants were premenopausal (median age 42; 90 % were below 51, the average age of a woman having her last period), this could have affected PPI data in unknown ways. Another sampling factor to consider is that all participants in this study were willing to make a smoking cessation attempt. This may be a particular issue in the smoking versus abstinence comparison, since the abstinence session occurred for all participants on the first day of a cessation attempt. In this respect, it is reassuring that the effects of abstinence on PPI in this study were consistent with the effects found in studies of nontreatment seeking smokers.
In summary, exaggerated startle responses were found for smokers with PTSD when compared to without PTSD, consistent with previous research and the disorder’s diagnostic criterion, and smoking increased prepulse inhibition of the startle response, consistent with previous work on the attention-enhancing properties of nicotine. Smokers with PTSD did not exhibit reduced PPI compared to control participants, and smoking did not differentially improve PPI in the PTSD group. However, when abstinent, the PTSD and control groups did exhibit a different pattern of PPI across prepulse intervals. The control group showed a significant reduction in PPI at the 240-ms prepulse interval when abstinent compared to when smoking, whereas the PTSD group did not. This suggests that previous research on smoking and PPI has been limited by not including longer prepulse intervals, and that nicotine may affect the time course as well as increasing the level of prepulse inhibition. Finally, PPI was reduced in older subjects during the abstinent condition, suggesting that nicotine may play a role in maintaining attention in older smokers, and this may be a factor that motivates continues smoking in older individuals.
Acknowledgments
This work was supported primarily by the National Institutes of Health grants 2R01CA081595, 2K24DA016388 and the Department of Veterans Affairs Office of Research and Development, Clinical Science. We would like to thank the participants who volunteered to participate in this study. The authors also wish to thank Terry Blumenthal for consultation on scoring and interpreting the startle response and PPI results. The views expressed in this presentation are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
Footnotes
Conflict of interest The authors have no competing interests to report.
Contributor Information
Scott R. Vrana, Virginia Commonwealth University, Richmond, VA, USA
Patrick S. Calhoun, Durham Veterans Affairs Medical Center, 508 Fulton St. (116 B), Durham, NC 27705, USA. Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham Veterans Affairs Medical Center, Durham, NC 27705, USA. Veterans Affairs Mid-Atlantic Region Mental Illness Research, Education, and Clinical Center, Durham, NC 27705, USA. Veterans Affairs Center for Health Services Research in Primary Care, Durham, NC 27705, USA
F. Joseph McClernon, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham Veterans Affairs Medical Center, Durham, NC 27705, USA.
Michelle F. Dennis, Durham Veterans Affairs Medical Center, 508 Fulton St. (116 B), Durham, NC 27705, USA. Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham Veterans Affairs Medical Center, Durham, NC 27705, USA
Sherman T. Lee, Durham Veterans Affairs Medical Center, 508 Fulton St. (116 B), Durham, NC 27705, USA
Jean C. Beckham, Email: jean.beckham@va.gov, beckham@duke.edu, Durham Veterans Affairs Medical Center, 508 Fulton St. (116 B), Durham, NC 27705, USA. Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham Veterans Affairs Medical Center, Durham, NC 27705, USA. Veterans Affairs Mid-Atlantic Region Mental Illness Research, Education, and Clinical Center, Durham, NC 27705, USA
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Ashare R, Hawk L., Jr Effects of smoking abstinence on impulsive behavior among smokers high and low in ADHD-like symptoms. Psychopharmacol. 2012;219:537–547. doi: 10.1007/s00213-011-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi V, Alsene K, Roseboom P, Conners E. Enduring sensorimotor gating abnormalities following predator exposure or corticotrophin-releasing factor in rats: a model for PTSD-like information-processing deficits? Neuropharmacology. 2007;62:737–748. doi: 10.1016/j.neuropharm.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, Levin ED, Rose JE, Fairbank JA. Smoking in Vietnam combat veterans with posttraumatic stress disorder. J Trauma Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Davidson JRT, Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addict Behav. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Beckham J, Moore S, Feldman M, Kirby A, Hertzberg M, Fairbank J. Self-report and physician-rated health in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1998;155:1565–1569. doi: 10.1176/ajp.155.11.1565. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A, Clancy CP, Rose JE. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: a preliminary study. Exp Clin Psychopharmacol. 2005;13:218–228. doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addict Behav. 2007;32:2900–2915. doi: 10.1016/j.addbeh.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Johnson YC, Dedert EA. Predictors of lapse in the first week of smoking abstinence in posttraumatic stress disorder and non-posttraumatic stress disorder smokers. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts252. Epub ahead of print [Nov. 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered posttraumatic stress disorder scale. J Trauma Stress. 1995;8:75–80. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bradley M, Cuthbert B, Lang P. Pictures as prepulse: attention and emotion in startle modification. Psychophysiology. 1993;30:541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow N. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacol. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Levin ED, Marrocco RT, Sarter MF, Strupp BJ, Warburton DM. Attention as a target of intoxication: insights and methods from studies of drug abuse. Neurotoxicol Teratol. 2000;22:487–502. doi: 10.1016/s0892-0362(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch J, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Della Casa V, Hofer I, Weiner I, Feldon J. The effects of smoking on acoustic prepulse inhibition in healthy men and women. Psychopharmacol. 1998;137:362–368. doi: 10.1007/s002130050631. [DOI] [PubMed] [Google Scholar]
- Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, Gonzenbach S, Angrist B, Rotrosen J. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacol. 2001;156:266–272. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- Ellwanger J, Geyer M, Braff D. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biol Psychol. 2003;62:175–195. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research Department; New York: 1994. [Google Scholar]
- George T, Termine A, Sacco K, Allen T, Reutenauer E, Vessicchio J, Duncan E. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: involvement of nicotinic receptor mechanisms. Schizophr Res. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Graham F. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davison M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatr Res. 1996;64:169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan C, Davis M, Southwick S. Effect of darkness on acoustic startle in Vietnam veterans with PTSD. Am J Psychiatry. 1998a;155:812–817. doi: 10.1176/ajp.155.6.812. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatr. 1998b;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and cognitive effects of smoking: relationship to nicotine addiction. Nicotine Tob Res. 1999;1(suppl 2):S143–S147. doi: 10.1080/14622299050011971. [DOI] [PubMed] [Google Scholar]
- Holstein D, Vollenweider F, Jäncke L, Schopper C, Csomor P. P50 suppression, prepulse inhibition, and startle reactivity in the same patient cohort suffering from posttraumatic stress disorder. J Affect Disord. 2010;126:188–197. doi: 10.1016/j.jad.2010.02.122. [DOI] [PubMed] [Google Scholar]
- Hutchinson K, Niaura R, Swift R. The effects of smoking high nicotine cigarettes on prepulse inhibition, startle latency, and subjective responses. Psychopharmacol. 2000;150:244–252. doi: 10.1007/s002130000399. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm S, Sakoman A, Esterajher S, Kozaric-Kovacic D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. Int J Psychophysiol. 2009;71:264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Heishman SJ. The older smoker. J Am Med Assoc. 2011;306:876–877. doi: 10.1001/jama.2011.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders—apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking withdrawal, nicotine dependence, and prepulse inhibition of the acoustic startle reflex. Psychopharmacol. 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley W, Gray JA. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacol. 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Checkley SA, Gray JA. Effect of acute subcutaneous nicotine on prepulse inhibition of the acoustic startle inhibition of the acoustic startle reflex in healthy male non-smokers. Psychopharmacol. 1997;132:389–395. doi: 10.1007/s002130050360. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Hum Psychopharmacol Clin Exp. 2001;16:321–326. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- Lando HA, McCovern PG, Barrios FX. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. Am J Public Health. 1990;80:554–559. doi: 10.2105/ajph.80.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine–haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Lipschitz DS, Mayes LM, Rasmusson AM, Anyan W, Billingslea E, Gueorguieva R, Southwick SM. Baseline and modulated acoustic startle response in adolescent girls with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:807–814. doi: 10.1097/01.chi.0000166379.60769.b6. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer M, Vollenweider F. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biol Psychol. 2003;63:311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Pynoos RS. Startle modulation in children with posttraumatic stress disorder. Am J Psychiatry. 1989;146:866–870. doi: 10.1176/ajp.146.7.866. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Postma P, Kumari V, Sharma T, Hines M, Gray JA. Startle response during smoking and 24 h after withdrawal predicts successful smoking cessation. Psychopharmacol. 2001;156:360–367. doi: 10.1007/s002130100829. [DOI] [PubMed] [Google Scholar]
- Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, Zachariah E, Hines M, Williams SCR, Kumari V. A behavioral and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacol. 2006;184:189–199. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: pharmacologic and behavioral treatments. Nicotine Tob Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuro-psychiatric disorders. Biol Psychiatr. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Geyer M, Hartman P, Sprock J, Auerback P, Cadenhead K, Perry W, Braff D. Sex differences in sensorimotor gating of the human startle reflex: all smoke? Psychopharmacol. 1999;146:228–232. doi: 10.1007/s002130051111. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? J Abnorm Psychol. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Woznica A, Sacco K, George T. Prepulse inhibition deficits in schizophrenia are modified by smoking status. Schizophr Res. 2009;112:86–90. doi: 10.1016/j.schres.2009.04.016. [DOI] [PubMed] [Google Scholar]